Effect of Nano-Se Particles Supplemented Sunflower Meal Based Diets on Mineral Absorption and Carcass Composition of Cirrhinus mrigala Fingerlings
Effect of Nano-Se Particles Supplemented Sunflower Meal Based Diets on Mineral Absorption and Carcass Composition of Cirrhinus mrigala Fingerlings
Nisar Ahmad1, Syed Makhdoom Hussain2,*, Azhar Rasul2, M. Mudassar Shahzad3, Arshad Javid4, Hamda Azmat5, M. Zubair ul Hassan Arsalan2, Sadia Tabassum2 and Bilal Ahmad2
1Department of Zoology, DG Khan Campus, University of Education, Dera Ghazi Khan, Pakistan
2Department of Zoology, Government College University, Faisalabad
3Department of Zoology, Division of Science and Technology, University of Education, Township, Lahore
4Department of Wildlife and Ecology, Faculty of Fisheries and Wildlife, University of Veterinary and Animal Sciences, Lahore
5Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore
ABSTRACT
The research was conducted to estimate the efficacy of Se nanoparticles on mineral absorption and carcass composition of C. mrigala fingerlings fed nano-Se particles supplemented sunflower meal based diets. The experiment was consisted on seven test diets on the basis of supplementation of nano Se graded levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg/kg). Chromic oxide was added as an inert marker. Fingerlings were fed at the rate of 5% of their live wet weight. Maximum improvement in mineral absorption (Ca, Na, K, Cu, P and Al) was observed at test diet with 1.5 mg/kg supplementation of Se nanoparticles. Maximum Fe, Mn and Cr absorption was noted at 2 mg/kg supplementation of nano Se. The highest absorption of Mg and Zn was found in the fingerlings fed at test diet with 1 mg/kg supplementation of nano Se particles. The best results in regard of body composition (CP; 61% and EE; 14%) were noted in fingerlings when fed 1.5 mg/kg levels of Se-nano particles based diets. It was concluded from the results of current study that supplementation of Se NPs (1.5 mg/kg) in sunflower meal based diet improves the mineral absorption and body composition of C. mrigala fingerlings.
Article Information
Received 21 April 2019
Revised 15 July 2019
Accepted 02 February 2021
Available online 07 May 2021
(early access)
Published 14 February 2022
Authors’ Contribution
NA conducted the trial and collected data. SMH planned and supervised the research and provided all materials for the research. NA prepared manuscript while AR and MMS helped him. AJ helped in statistical analysis. HA proffread the manuscript. MZH and ST helped in feed analysis and compiling the results. BA helped in chemical analysis of fish.
Key words
Nano-Se particles, Mineral absorption, Carcass composition, C. mrigala.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190421160425
* Corresponding author: drmakhdoom90@gmail.com
0030-9923/2022/0003-1103 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
Nanomaterials show novelty in high surface activity leading to absorption efficiency (Pelyhe and Miklos, 2013). Selenium is an important micronutrient (Dare et al., 2001). Se improves animal health and productivity and its deficiency causes great losses in livestock (Xun et al., 2012). Selenium is necessary for functioning of body and metabolism (Hamilton, 2004). Se improves animal health and productivity (Xun et al., 2012). Growth is regularized by Se and it also plays an important role in immune system activities (Pelyhe and Miklos, 2013). se improves RBCs count and the immunity of fish (Sadeghian et al., 2012; Le et al., 2013). A number of studies suggest the efficiency of Se-NPs as a dietary source (Wang et al., 2007; Zhou et al., 2009). Animals consuming a Se deficient diet face pneumonia, infertility and oxidative stress (Pelyhe and Miklos, 2013).
Fish farming is one of the best industries to explore the nano products but unfortunately a very minute work has been done in this regard. The nanomaterials hold different properties from larger molecules regarding absorption (Albrecht et al., 2006; Wang et al., 2007). Nanoparticles can be used to improve feed quality. Nanoparticles improve the absorption of feed molecules (Bunglavan et al., 2014). Nanoparticles may increase the bioavailability of nutrients. Nanoform of supplementation also increases the absorption and utilization of minerals (Vijayakumar and Balakrishnan, 2014).
C. mrigala is one of the most important fish with a maximum market demand and it contributes along with Catla catla and Labeo rohita about 67% of total freshwater fish production in South India (Krishnaveni et al., 2013). Sunflower meal contains about 45-48% crude protein (Mushtaq et al., 2006). It is used in feed formulations as it contains endogenous proteolytic enzymes to digest proteins for fish (Kocher et al., 2000). In Pakistan it has lowest cost among protein sources (Khan et al., 2006). It is important to formulate nutritionally balanced and highly palatable feed which results in maximum growth of fish (Afzal et al., 2004; Tahir et al., 2008).
In under developed countries human population is being increased day by day resulting in an alarming increase in demand of food and quality nutrition. Aquaculture is one of the best industries to fulfil such huge demand but it also requires proper feeds for fish in tanks and ponds. Though aquaculture is a very advance industry but a lot is required to produce cost effective and environment friendly feeds for aquatic organisms as a challenge. About 50-60% of the total cost of a fish form is subjected to feed (Essa et al., 2004). Fish meal is an excellent protein source for fish feed because it contains required amino acids profile and all other nutrients but it is very costly (Zhou et al., 2004). Due to high cost, uneven supply and increasing demand of fish meal it has become necessary to search for alternative protein sources (Pham et al., 2008). This is the reason we search alternative sources of protein for fish feed and sunflower is one of the best protein source from plant by-products as they are easily available round the year. These plant by-products are being used by scientists from last two decades in fish feed (Wang et al., 2015). Due to all these above said reasons we selected sunflower meal as alternative of fish meal for current study.
The major goal of the current study was to estimate the effects of Se nano-particles (optimum level) on mineral absorption and carcass composition of C. mrigala fingerlings.
Materials and methods
C. mrigala fingerlings were purchased from Government Fish Seed Hatchery Faisalabad. Fingerlings were acclimatized for 15 days in Fish Nutrition Laboratory, GCUF and were fed on the basal diet once in a day (Allan and Rowland, 1992).
Analysis of feed ingredients and Cr-nanoparticles
The feed ingredients were analysed by following standard methods (AOAC, 1995). Se nenoparticles (NPs) were purchased from market (sigma-Aldrich), to confirm their pure crystalline structure and size, they were analysed by XRD and TEM (TEM-JEOL2100-20171206), respectively (Ramamurthy et al., 2013; Iqbal et al., 2014).
Formation of pellets
All the feed ingredients were grinded until they passed through 0.5 mm sized sieve. All ingredients were mixed for 5 min, fish oil was gradually added. Finally water was added slowly to make suitable dough and pellets were formulated thereafter with the help of pelleting machine by following Lovell (1989).
Preparation of NP stock solution
Preparation and confirmation of stock solutions of NPs was carried out according to Federici et al. (2007). Stock solution of 100% pure NPs dry powder was made by sonication method (for 6-8 h) and from this stock solution further dilutions were made to ensure our required levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg/kg) of Se NPs.
Addition of NPs to basal diets
The diluted Se solutions were sonicated further for 15 min just before spraying on basal diets according to Ramsden et al. (2009). One Kg feed was placed in a commercial food mixer and gradually sprayed with the appropriate dilution. The seven test diets were formulated by mixing graded levels (0, 0.5, 1, 1.5, 2, 2.5 and 3 mg/kg) of nano Se. NPs immediately coated the feed pellets. The feed pellets were allowed to dry then ultimately were stored in air tight containers for further use.
Feeding protocol and sample collection
C. mrigala fingerlings were fed for 90 days on prescribed diets mentioned above at the rate of 5% of their live wet weight. Three replicates were used for each diet and 15 fingerlings were stocked in each replicate. Their feces were collected by alternative opening and closing of valve-I and valve-II following Hussain et al. (2018).
Chemical analysis of feed and feces
The samples of experimental diets and feces were homogenized using mortar and pestle and analysed following standard methods (AOAC, 1995). Diets and feces samples were digested in boiling nitric acid and perchloric acid mixture (2:1) by following standard methods (AOAC, 1995). After appropriate dilution, mineral contents such as calcium (Ca), magnesium (Mg), iron (Fe), copper (Cu), zinc (Zn) and manganese (Mn) were estimated using Atomic Absorption Spectrophotometer (Hitachi Polarized Atomic Absorption Spectrometer, Z-8200). Calibrated standards for mineral estimation were prepared from commercially available standards (AppliChem® Gmbh Ottoweg4, DE-64291 Darmstadt, Germany). The estimation of Na and K was done through flame photometer (Jenway PFP-7, UK). Phosphorus (P) was analysed colorimetrically (UV/VIS spectrophotometer) using ammonium molybdate as reagent at 720 nm absorbance through standard methods (AOAC, 1995). Chromic oxide contents in diets and feces were estimated after oxidation with molybdate reagent using a UV-VIS 2001 Spectrophotometer at 370 nm absorbance (Divakaran et al., 2002).
Calculation of apparent digestibility coefficient (ADC)
Apparent mineral digestibility coefficients (ADC) of test diets were calculated by the standard formula (NRC, 1993).
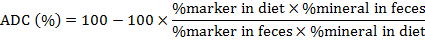
Table I.- Chemical composition (%) of feed ingredients.
|
Ingredients |
Fish meal |
Rice polish |
Wheat flour |
Sunflower |
|
Dry matter (%) |
91.67 |
94.06 |
92.4 |
93.80 |
|
Crude protein (%) |
49.03 |
11.87 |
09.73 |
40.81 |
|
Crude fat (%) |
6.93 |
12.69 |
2.24 |
3.69 |
|
Crude fiber (%) |
1.23 |
11.91 |
2.73 |
1.94 |
|
Ash (%) |
23.15 |
11.32 |
1.99 |
09.96 |
|
Gross energy (kcal/g) |
2.49 |
3.41 |
3.06 |
3.64 |
|
Carbohydrates |
19.66 |
52.21 |
82.21 |
43.6 |
Chemical analysis of fish whole body
At the end of 90 days trial four fish from each tank were sacrificed randomly and dried at room temperature. Moisture contents of carcass were calculated after oven-drying of homogenised samples at 105oC for 12 h. Micro kjeldahl apparatus (InKjel Mbehr Labor Technik GmbH D-40599 Dusseldorf) was used to determine the crude protein (CP) (N × 6.25) whereas soxhlet system (Soxhlet Extraction Heating Mantels, 250 ml 53868601) was used to check the amount of crude fat by the help of petroleum ether extraction (EE) method. Crude fiber contents were calculated as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH whereas ash was determined by ignition at 650oC for 12 h in electric furnace (Naberthern B170) to constant weight. Total carbohydrates (N-free extract) were determined by difference, i.e., total carbohydrates % =100- (EE % + CP % + Ash % + CF %). Oxygen bomb calorimeter was used to estimate the gross energy.
Statistical analysis
One-way analysis of variance was applied to data of mineral absorption and carcass composition of fish (Steel et al., 1996). Tukey’s Honesty Significant Difference Test (p<0.05) was used to compare the differences among various levels (Snedecor and Cochran, 1991). For statistical analysis CoStat Computer Package (Version 6.303, PMB 320, Monterey, CA, 93940 USA) was used.
Results
Results of morphological analysis of the Se-NPs by using transmission electron microscope (TEM) are shown in Figure 1. TEM confirms the shape and size of Se NPs, as magnified form depicted in Figure 1A and normal TEM form in Figure 1B. In these samples, the scale bar was set to 10 nm in case of magnified TEM image and 50 nm of scale bar in terms of normal form TEM image. TEM image justify the spherics form of Se NPs with about 8–10 nm diameter almost homogenous structure format. The above results of TEM confirm that Se NPs used in experimental diets of our study contain size less than 100nm about 10nm. It confirms that they are pure nano particles in their nature.
The crystal structure and the phase composition of selenium nanoparticles were confirmed by using X-Ray Diffraction (XRD) techniques shown in Figure 2. The XRD pattern confirms very clearly that the sample is nano-crystalline in nature as it matches very well with that of the standard selenium powder of selenium nano-particles.
Analysis showed that there was balanced amount of all the minerals in the control and test diets formulated by sunflower meal based diet supplemented with Se NPs at 0, 0.5, 1, 1.5, 2, 2.5 and 3 mg/kg level. Amount of minerals such as Ca, Na, K, Fe, Cu, Zn, Mn, P, Mg, Al and Cr were statistically (p<0.05) similar in control and Se nano supplemented test diets whereas Ba, Cd, Co and Ni were found lowest from the range (<0.0001) in diets.
Mineral absorption was lowest at control diet (0 mg/kg Se NPs level based diet) after that it started to increase from 0.5 mg/kg level and reached its maximum at 1.5 or 2 mg/kg level. It was also noted that further increase in Se NPs supplementation decreased absorption of minerals (Table III). Maximum mineral absorption i.e. Ca (70%), Na (74%), K (70%), Cu (66%), P (75%) and Al (61%) were found at 1.5 mg/kg level diet. Whereas highest absorption value of Fe (67%), Mn (71%) and Cr (57%) was noted at 2 mg/kg level diet. On the other side Mg (64%) and Zn (62%) were absorbed at 1 mg/kg level diet. These values were statistically higher (p<0.05) when compared with control and other Se-nano supplemented test diets. It was also observed that some of the minor minerals such as Ba, Cd, Co and Ni were very low (<0.0001) in diet and could not be analysed when absorption was calculated.
It was concluded that supplementation of Se-nano particles in sunflower meal based diets played a significant role in improving mineral absorption. These reduced minerals in water will be helpful to control water pollution. Furthermore, Se NPs supplementation in fish feed may also decrease feed cost because there will be no need of extra mineral supplementation.
Similarly maximum values of CP (61%) and EE (14%) were observed in fish fed at 1.5 mg/kg Se-nano diet. The least CP (54%) contents in fish body was observed when fed test diet T7, 3 mg/kg Se-nano and the least EE (9%) contents in fish body was observed when fed test diet T2, 0.5 mg/kg Se-nano. From results (Table IV) it was found that improvement in protein and fat contents for C. mrigala fingerlings was started from 0.5 mg/kg Se-nano diet and reached its maximum in fingerlings fed 1.5 mg/kg level of Se-nano supplemented sunflower meal based diet. Further increase in Se-nano supplementation could not enhance nutrient contents in fish body.
Analysis showed minimum amount of carbohydrates (11%) and crude fiber (1%) contents in fish fed at 1 and 1.5 mg/kg Se-nano level based diets. However maximum values of carbohydrates (18%) and crude fibre (1%) were observed in fish fed at 0 mg/kg Se-nano level based diet (control diet). Minimum ash contents (8%) and moisture contents (5%) were recorded in fish fed at 1.5 mg/kg Se-nano level based diet that were significantly (p<0.05) different from the fish which fed at other Se-nano supplemented diets and without supplemented sunflower meal based diet (control diet). However maximum moisture (7%) and ash (10%) contents were found in the fish fed at control diet and 3 mg/kg Se-nano level based diet, respectively. It was found that 1.5 mg/kg Se-nano supplementation in diet is the best level for the maximum deposition of protein and lipids in fish; these nutrients are essential for fish performance. Moreover, almost lowest carbohydrates, ash, crude fiber as well as moisture contents were observed in C. mrigala fed 1.5 mg/kg Se-nano diet.
Table II.- Ingredients composition (%) oilseed meal based test diets.
|
Ingredients |
Test diet-I |
Test diet-II |
Test diet-III |
Test diet-IV |
Test diet-V |
Test diet-VI |
Test diet-VII |
|
Nanoparticles (mg/kg) |
0 |
0.5 |
1 |
1.5 |
2 |
2.5 |
3.0 |
|
Sunflower meal |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|
Fish meal |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
14.5 |
|
Wheat flour* |
13 |
13 |
13 |
13 |
13 |
13 |
13 |
|
Rice polish |
11 |
11 |
11 |
11 |
11 |
11 |
11 |
|
Fish oil |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
7.5 |
|
Vitamin premix** |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Minerals premix*** |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Ascorbic acid |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Chromic oxide |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
*Nano-particles will be added on the cost of wheat flour. ** Vitamin premix/kg: Vitamin D3, 3,000,000 IU; Vitamin A, 15,000,000 IU; Vitamin E, 30000 IU; Vitamin B1, 3000 mg; Vitamin B6, 4000 mg; Vitamin B12, 40mg; Vitamin B2, 7000 mg; Vitamin C, 15,000 mg; Vitamin K3, 8000 mg; Folic acid, 1500 mg; Calcium pantothenate, 12,000mg; Nicotinic acid, 60,000 mg. ***Mineral premix/kg: Mn (manganese), 2000 mg; Ca (calcium), 155 gm; Zn (zinc), 3000 mg; Cu (copper), 600 mg; Co (cobalt), 40 mg; I (iodine), 40 mg; P (phosphorous), 135 gm; Fe (iron), 1000 mg; Mg (magnesium), 55 gm; Se (selenium), 3 mg; Na (sodium), 45 g.
Table III.- Apparent mineral digestibility (%) of C. mrigala fingerlings fed graded levels of Se-nano supplemented sunflower meal based diets.
|
Diets |
Se-nano (mg/kg) |
Concentrations (%) |
||
|
Diet |
Feces |
Digestibility |
||
|
Digestibility of Ca |
||||
|
Test diet – I (control diet) |
0 |
0.87 |
0.48 a |
49.27 e |
|
Test diet – II |
0.5 |
0.88 |
0.47 a |
50.47 e |
|
Test diet – III |
1 |
0.89 |
0.38 bc |
59.95 c |
|
Test diet – IV |
1.5 |
0.87 |
0.29 c |
69.52 a |
|
Test diet – V |
2 |
0.88 |
0.31 c |
68.00 b |
|
Test diet – VI |
2.5 |
0.88 |
0.44 ab |
55.05 d |
|
Test diet – VII |
3 |
0.88 |
0.50 a |
49.61 e |
|
PSE |
0.0385 |
0.018 |
0.309 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Digestibility of Na |
||||
|
Test diet – I (control diet) |
0 |
0.008 |
0.004a |
50.26 c |
|
Test diet – II |
0.5 |
0.008 |
0.004ab |
52.05 c |
|
Test diet – III |
1 |
0.008 |
0.003bc |
59.40 b |
|
Test diet – IV |
1.5 |
0.008 |
0.002d |
73.95 a |
|
Test diet – V |
2 |
0.008 |
0.002d |
72.11 a |
|
Test diet – VI |
2.5 |
0.008 |
0.003c |
60.59 b |
|
Test diet – VII |
3 |
0.008 |
0.003bc |
60.74 b |
|
PSE |
0.0002 |
0.0001 |
0.406 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Digestibility of K |
||||
|
Test diet – I (control diet) |
0 |
1.403 |
0.822a |
46.52e |
|
Test diet – II |
0.5 |
1.390 |
0.753ab |
50.14d |
|
Test diet – III |
1 |
1.400 |
0.500c |
66.80b |
|
Test diet – IV |
1.5 |
1.386 |
0.457c |
70.24a |
|
Test diet – V |
2 |
1.396 |
0.505c |
67.57b |
|
Test diet – VI |
2.5 |
1.393 |
0.717b |
53.13c |
|
Test diet – VII |
3 |
1.393 |
0.759ab |
51.45d |
|
PSE |
0.026 |
0.015 |
0.329 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Digestibility of P |
||||
|
Test diet – I (control diet) |
0 |
2.023 |
1.044a |
52.89d |
|
Test diet – II |
0.5 |
2.026 |
0.870b |
60.51c |
|
Test diet – III |
1 |
2.014 |
0.660c |
69.52b |
|
Test diet – IV |
1.5 |
2.017 |
0.565d |
74.67a |
|
Test diet – V |
2 |
2.010 |
0.650c |
70.97b |
|
Test diet – VI |
2.5 |
2.020 |
0.867b |
60.92c |
|
Test diet – VII |
3 |
2.020 |
0.921b |
59.33c |
|
PSE |
0.026 |
0.011 |
0.492 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Diets |
Se-nano (mg/kg) |
Concentrations (%) |
||
|
Diet |
Feces |
Digestibility |
||
|
Digestibility of Fe |
||||
|
Test diet – I (control diet) |
0 |
0.046 |
0.027a |
46.03f |
|
Test diet – II |
0.5 |
0.047 |
0.026a |
48.74e |
|
Test diet – III |
1 |
0.046 |
0.021bcd |
58.24c |
|
Test diet – IV |
1.5 |
0.048 |
0.018cd |
64.66b |
|
Test diet – V |
2 |
0.047 |
0.017d |
67.13a |
|
Test diet – VI |
2.5 |
0.047 |
0.023abc |
56.13cd |
|
Test diet – VII |
3 |
0.047 |
0.023ab |
55.44d |
|
PSE |
0.002 |
0.0009 |
0.439 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Digestibility of Cu |
||||
|
Test diet – I (control diet) |
0 |
0.005 |
0.0031 a |
47.40 c |
|
Test diet – II |
0.5 |
0.005 |
0.0023 b |
62.79 b |
|
Test diet – III |
1 |
0.005 |
0.0021 b |
64.92 a |
|
Test diet – IV |
1.5 |
0.005 |
0.0021 b |
66.02 a |
|
Test diet – V |
2 |
0.005 |
0.0023 b |
62.10 b |
|
Test diet – VI |
2.5 |
0.005 |
0.0035 a |
42.71 d |
|
Test diet – VII |
3 |
0.005 |
0.0037 a |
41.45 d |
|
PSE |
0.0002 |
0.0001 |
0.375 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Digestibility of Zn |
||||
|
Test diet – I (control diet) |
0 |
0.042 |
0.023ab |
49.10d |
|
Test diet – II |
0.5 |
0.041 |
0.021abc |
51.17c |
|
Test diet – III |
1 |
0.041 |
0.017c |
61.87a |
|
Test diet – IV |
1.5 |
0.042 |
0.018bc |
60.72a |
|
Test diet – V |
2 |
0.042 |
0.020abc |
56.98b |
|
Test diet – VI |
2.5 |
0.043 |
0.024a |
47.77de |
|
Test diet – VII |
3 |
0.043 |
0.025a |
46.45e |
|
PSE |
0.002 |
0.001 |
0.364 |
|
|
P-value |
0.99 NS |
0.0008*** |
0.00*** |
|
|
Digestibility of Mn |
||||
|
Test diet – I (control diet) |
0 |
0.024 |
0.014a |
45.09e |
|
Test diet – II |
0.5 |
0.023 |
0.013ab |
47.41d |
|
Test diet – III |
1 |
0.024 |
0.009abc |
62.83c |
|
Test diet – IV |
1.5 |
0.025 |
0.008bc |
69.82a |
|
Test diet – V |
2 |
0.024 |
0.007c |
70.94a |
|
Test diet – VI |
2.5 |
0.025 |
0.009abc |
64.99b |
|
Test diet – VII |
3 |
0.025 |
0.010abc |
62.29c |
|
PSE |
0.002 |
0.001 |
0.255 |
|
|
P-value |
0.99 NS |
0.006** |
0.00*** |
|
|
Digestibility of Mg |
||||
|
Test diet – I (control diet) |
0 |
0.009 |
0.004bc |
56.30c |
|
Test diet – II |
0.5 |
0.009 |
0.004bcd |
57.16 c |
|
Test diet – III |
1 |
0.009 |
0.003d |
64.37 a |
|
Test diet – IV |
1.5 |
0.009 |
0.003cd |
62.16 b |
|
Test diet – V |
2 |
0.009 |
0.004bc |
57.38 c |
|
Test diet – VI |
2.5 |
0.009 |
0.005ab |
50.97 d |
|
Test diet – VII |
3 |
0.009 |
0.005a |
48.31 e |
|
PSE |
0.0002 |
0.0001 |
0.374 |
|
|
P-value |
0.99 NS |
0.00*** |
0.00*** |
|
|
Diets |
Se-nano (mg/kg) |
Concentrations (%) |
||
|
Diet |
Feces |
Digestibility |
||
|
Digestibility of Cr |
||||
|
Test diet – I (control diet) |
0 |
0.028 |
0.016a |
46.38d |
|
Test diet – II |
0.5 |
0.027 |
0.017a |
42.36e |
|
Test diet – III |
1 |
0.027 |
0.015a |
45.57d |
|
Test diet – IV |
1.5 |
0.028 |
0.015a |
51.55c |
|
Test diet – V |
2 |
0.027 |
0.013a |
57.23a |
|
Test diet – VI |
2.5 |
0.026 |
0.013a |
55.41b |
|
Test diet – VII |
3 |
0.026 |
0.013a |
54.69b |
|
PSE |
0.002 |
0.001 |
0.327 |
|
|
P-value |
0.99 NS |
0.153 NS |
0.00*** |
|
|
Digestibility of Al |
||||
|
Test diet – I (control diet) |
0 |
0.0006 |
0.0004a |
36.30 e |
|
Test diet – II |
0.5 |
0.0006 |
0.0003bcd |
51.66 c |
|
Test diet – III |
1 |
0.0006 |
0.0002d |
60.43 a |
|
Test diet – IV |
1.5 |
0.0006 |
0.0002cd |
61.13 a |
|
Test diet – V |
2 |
0.0006 |
0.0003bcd |
56.09 b |
|
Test diet – VI |
2.5 |
0.0006 |
0.0003bc |
47.43 d |
|
Test diet – VII |
3 |
0.0006 |
0.0003ab |
46.54 d |
|
PSE |
2.211 |
1.756 |
0.242 |
|
|
P-value |
0.98 NS |
0.0001*** |
0.00*** |
|
|
Digestibility of Cd, Co, Ni and Ba |
||||
|
Test diet – I (control diet) |
0 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – II |
0.5 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – III |
1 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – IV |
1.5 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – V |
2 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – VI |
2.5 |
<0.0001 |
<0.0001 |
<0.0001 |
|
Test diet – VII |
3 |
<0.0001 |
<0.0001 |
<0.0001 |
|
PSE |
<0.0001 |
<0.0001 |
<0.0001 |
|
|
P-value |
<0.0001 |
<0.0001 |
<0.0001 |
|
PSE, pooled SE = √MSE/n (where MSE is mean-squared error).
Table IV.- Proximate composition (%) of C. mrigala carcass fed graded levels of Se-nano supplemented Sunflower meal based diets.
|
Diets |
Se-nano (mg/kg) |
Protein |
Fat |
Ash |
Moisture |
Crude fibre |
Carbohydrates |
|
Test diet – I (control diet) |
0 |
54.93 de |
9.27 d |
9.37 a |
7.16 a |
1.26 a |
18.02 a |
|
Test diet – II |
0.5 |
55.41 d |
9.23 d |
9.34 a |
7.10 a |
1.26 a |
17.66 a |
|
Test diet – III |
1 |
60.74 a |
13.01 ab |
8.26 bc |
6.14 b |
1.06 a |
10.78 c |
|
Test diet – IV |
1.5 |
61.37 a |
13.72 a |
7.76 c |
5.17 c |
1.02 a |
10.97 c |
|
Test diet – V |
2 |
58.28 b |
12.82 b |
8.42 b |
5.50 c |
1.21 a |
13.77 b |
|
Test diet – VI |
2.5 |
57.29 c |
11.73 c |
9.44 a |
6.40 b |
1.24 a |
13.90 b |
|
Test diet – VII |
3 |
54.29 e |
11.73 c |
9.60 a |
6.40 b |
1.24 a |
16.73 a |
|
PSE |
0.193 |
0.150 |
0.125 |
0.118 |
0.058 |
0.287 |
|
|
P Value |
0.00*** |
0.00*** |
0.00*** |
0.00*** |
0.046* |
0.00*** |
|
Means within columns having different superscripts are significantly different at p< 0.05. Data are means of three replicates. PSE, pooled SE = √MSE/n (where MSE is mean-squared error).
Discussion
Our study demonstrates that Se NPs increase intestinal uptake of minerals. These findings are coinciding with the results of Srinivasan et al. (2016) who found that optimum concentration of nano-particles increased the absorption of Cu, Zn, Ca, Mg, Na and K minerals in test prawns (Macrobrachium rosenbergii post-larvae). The above said results were on supplementation of Fe2O3 nano particles at the rate of 20 mg/kg while the others levels were 10, 30, 40 and 50 mg/kg. Similarly an increase in iron has been reported in L. rohita fed with ferrous oxide NPs supplemented diets (Behera et al., 2014) and in M. rosenbergii fed with Zn and Cu nano sized forms (Muralisankar et al., 2014, 2016).
Differing from our results Zaboli et al. (2013) found no effects on composition of blood minerals (Ca, P, Fe, Cu and Zn) of Markhoz goat kids when fed to zinc oxide (ZnO) and nano zinc oxide (nZnO) supplemented diets. Zinc was administered at daily doses of zero, 20 and 40 ppm in both ZnO and nZnO groups by adding to their basal diets. Our results are antagonistic to the findings of Sirirat et al. (2012) who found that supplementation of Nano Cr Pic did not affect the absorption of Cu, Zn, Fe and Mn. Our findings are also opposed by the results of different studies who concluded that dietary Zn supplementation showed no affects on the Zn levels of blood (Droke et al., 1998; Eryavuz et al., 2002; Salama et al., 2003; Spears et al., 2004). The result may be differing due to difference in basal diet, experimental species, nature, level and shape of nanoparticles.
Our results are also parallel to the findings of Bunglavan et al. (2014), that nanoparticles improved the minerals bioavailability. Similarly, lien et al. (2009) concluded that nano Cr pic group significantly enhanced the Cr digestibility in rats.
The results of current study proved that nano particles supplementation is very important to improve the carcass composition of C. mrigala fingerlings. These results are similar to the findings of Srinivasan et al. (2016) who found that supplementation of Fe2O3 NPs significantly improved carcass parameters as compared to control diets in giant fresh water prawn. Wang and Xu (2004) reported that supplementation of Cr-Nanoparticles has beneficial effects on carcass composition, pork quality and individual skeletal muscle weight. Dietary Cr-Nano supplementation increased (p<0.05) the lean ratio of carcass of pigs by 14.06%. This improvement is due to the special metabolism pathway and deposition mechanism of NPs in carps due to which soluble proteins can interact with nanoparticles to form halo (crona). Nano-protein cronas can interfere with protein folding and can enhance protein cross linking (Zhou et al., 2009; Onuegbu et al., 2018). When concentration of NPs crosses the optimum levels then feed starts to lose palatability which may be the possible reason of decrease of carcass parameters on higher levels of supplementation (Onuegbu et al., 2018).
On the other hand, Ashouria et al. (2015) reported that proximate composition of fish was not affected by the dietary treatment after 8-weeks of culture, indicating that the carp muscle proximate composition is not sensitive to dietary Se treatments. Similar observation was also reported by Le et al. (2013) for juvenile yellowtail king fish. Wang et al. (2015) studied the effects of Cu NPs and soluble Cu on carcass composition of juvenile Epinephelus coioides. The fish were exposed in triplicate to control, 20 or 100μg CuL-1 as either copper sulphate (CuSO4) or Cu-NPs for 25 days. With an increase in CuSO4 and Cu-NPs dose, crude protein and crude lipid decreased while ash and moisture increased ultimately causing decrease in growth performance of fish.
Conclusion
This study provides sufficient evidence that supplementation of the Se nano particles are helpful for the improvement of mineral absorption and carcass composition C. mrigala fingerlings fed sunflower meal based diets. It was also concluded that 1.5 mg/kg supplementation levels of Se-nanoparticles is the optimum level for the improvement of all above said factors and higher supplementations could not cause further improvement.
Acknowledgements
The authors would like to acknowledge HEC Pakistan for providing funds for NRPU Projects # 20-4892/NRPU/R&D/HEC/14/1145 and 5649/Punjab/NRPU/R&D/HEC/2016 to conduct this research work.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Afzal, K.M., Jafri, A.K. and Chadha, N.K., 2004. Growth and body composition of rohu, Labeo rohita (Hamilton), fed compound diet: Winter feeding and rearing to marketable size. J. appl. Ichthyol., 20: 265-270. https://doi.org/10.1111/j.1439-0426.2004.00550.x
Albrecht, M.A., Evans, C.W. and Raston, C.L., 2006. Green chemistry and the health implications of nanoparticles. Green Chem., 8: 417–432. https://doi.org/10.1039/b517131h
Allan, G.L. and Rowland, S.J., 1992. Development of an experimental diet for silver perch (Bidyanus bidyanus). Austasia Aquacul., 3: 39-40.
Ashouria, S., Keyvanshokooh, S., Salatia, A.P., Joharib, S.A. and Zanoosic, H.P., 2015. Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture, 446: 25–29. https://doi.org/10.1016/j.aquaculture.2015.04.021
AOAC, 1995. Official methods of analysis, 15th ed. Association of Official Analytical Chemists, Washington, D.C. USA, pp. 1094.
Behera, T., Swain, P., Rangacharulu, P.V. and Samanta, M., 2014. Nano-Fe as feed additive improves the hematological and immunological parameters of fish, Labeo rohita H. Appl. Nanosci., 4: 687–694. https://doi.org/10.1007/s13204-013-0251-8
Bunglavan, S.J., Garg, A.K., Dass, R.S. and Shrivastava, S., 2014. Use of nanoparticles as feed additives to improve digestion and absorption in Livestock. J. Livest. Res. Int., 2: 36-47.
Dare, C., Eisler, I., Russell, G., Treasure, J. and Doge, L., 2001. Psychological therapies for adults with anorexia nervosa: randomized controlled trial of out-patient treatments. Br. J. Psychiat., 178: 216-221. https://doi.org/10.1192/bjp.178.3.216
Dawood, M.A., Koshio, S., Ishikawa, M. and Yokoyama, S., 2015. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellf. Immunol., 45: 33-42. https://doi.org/10.1016/j.fsi.2015.01.033
Divakaran, S., Leonard, G.O. and Lan, P.F., 2002. Note on the methods for cetermination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
Droke, E.A., Gengelbach, G.P. and Spears, J.W., 1998. Influence of level and source (inorganic vs organic) of zinc supplementation on immune function in growing lambs. Asian-Australasian J. Anim. Sci., 11: 139–144. https://doi.org/10.5713/ajas.1998.139
Eryavuz, A., Durgan, Z. and Keskun, E., 2002. Effects of ration supplemented with zinc on some rumen and blood parameters, mohair production and quality in faunated and defaunated Angora goats. Turk. J. Vet. Anim. Sci., 26: 753-760.
Essa, A.M., Mabrouk, A.H. and Zaki, A.M., 2004. Growth performance of grass carp, Ctenopharyngodon idella and hybrid grass carp fingerlings fed on different types of aquatic plants and artificial diet in concrete basins. Egypt. J. aquat. Res., 30: 341-348.
Federici, G., Shaw, B.J. and Handy, R.D., 2007. Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol., 84: 415–430. https://doi.org/10.1016/j.aquatox.2007.07.009
Hamilton, S.J., 2004. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ., 326: 1-31. https://doi.org/10.1016/j.scitotenv.2004.01.019
Hussain, S.M, Ahmad, N., Javid, A., Shahzad, M.M., Hussain, M. and Arsalan, M.Z., 2018. Effects of phytase and citric acid supplemented corn gluten (30%) meal based diets on the mineral digestibility of Cirrhinus mrigala fingerlings. Turk. J. Fish. aquat. Sci., 18: 501-507.
Iqbal, M.Z., Fengping, W., Riaz, H., Tahir, I., Israt, A., Yasir, R.M. and Shujjat, A., 2014. Synthesis and characterization of SnO2 nanorods for energy storage applications. Adv. Sci. Engin. Med., 6: 1–6. https://doi.org/10.1166/asem.2014.1563
Khan, S.H., Sardar, R. and Siddique, B., 2006. Influence of enzymes on performance of broilers fed sunflower-corn based diets. Pakistan Vet. J., 26: 109-114.
Kocher, A., Choct, M., Porter, M.D. and Broz, J., 2000. The effects of enzyme addition to broiler diets containing high concentrations of canola or sunflower meal. Poult. Sci., 79: 1767-1774. https://doi.org/10.1093/ps/79.12.1767
Krishnaveni, K., Palanivelu, k. and Velavans, S., 2013. Spiritualizing effect of probiotic and spirulina on growth and biochemical performance in common carp (Catla catla). Int. J. Res. Zool., 3: 27-31.
Le, K.T., Fotedar, R. and Partridge, G., 2013. Selenium and vitamin E interaction in the nutrition of yellowtail kingfish (Seriola lalandi): Physiological and immune responses. Aquacul. Nutr., 20: 303-313. https://doi.org/10.1111/anu.12079
Lien, T.F., Yeh, H.S., Lu, F.Y. and Fu, C.M., 2009. Nanoparticles of chromium picolinate enhance chromium digestibility and absorption. J. Sci. Fd. Agric., 89: 1164–1167. https://doi.org/10.1002/jsfa.3569
Lovell, R.T., 1989. Fish nutrition and feeding. Van Nostrand-Reinhold Co., New York. https://doi.org/10.1007/978-1-4757-1174-5
Muralisankar, T., Bhavan, P.S., Radhakrishnan, S., Seenivasan, C. and Srinivasan, V., 2016. The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachium rosenbergii post larvae. J. Trace Elem. Med. Biol., 34: 39-49. https://doi.org/10.1016/j.jtemb.2015.12.003
Muralisankar, T., Bhavan, P.S., Radhakrishnan, S., Seenivasan, C., Manickam, N. and Srinivasan, V., 2014. Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol. Trace Elem Res., 160: 56-66. https://doi.org/10.1007/s12011-014-0026-4
Mushtaq, T., Sarwar, M., Ahmad, G., Nisa, M.U. and Jamil, A., 2006. The influence of exogenous multi-enzyme preparation and graded levels of digestible lysine in sunflower meal-based diets on the performance of young broiler chicks two weeks posthatching. Poult. Sci., 85: 2180-2185. https://doi.org/10.1093/ps/85.12.2180
NRC, 1993. Nutrient requirements of fish. Committee on Animal Nutrition, Board on Agriculture, National Research Council, National Academy Press, Washington DC, USA.
Onuegbu, U.C., Aggarwal, A. and Singh, B.N., 2018. ZnO nanoparticles as feed supplementation on growth performance of cultured African catfish fingerlings. J. scient. indust. Res., 77: 213-218.
Pelyhe, C. and Miklós, M., 2013. Myths and facts about the effects of nano selenium in farm animals – Mini-review. Eur. chem. Bull., 2: 1049-1052.
Pham, M.A., Lee, K.J., Dang, T.M., Lim, S.J., Ko, G.Y., EO, J. and Oh, D.H., 2008. Improved apparent digestibility coefficient of protein and phosphorus by supplementation of microbial phytase in diets containing cottonseed and soybean meal for juvenile olive flounder (Paralichthys olivaceus). J. Anim. Sci., 21: 1367-1375. https://doi.org/10.5713/ajas.2008.80053
Ramamurthy, C.H., Sampath K.S., Arunkumar, P., Kumar M.S., Sujatha, V., Premkumar, K. and Thirunavukkarasu, C., 2013. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioproc. Biosyst. Eng., 36: 1131–1139. https://doi.org/10.1007/s00449-012-0867-1
Ramsden, C.S., Smith, T.J., Shaw, B.J. and Handy, R.D., 2009. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): No effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology, 18: 939–951. https://doi.org/10.1007/s10646-009-0357-7
Sadeghian, S., Kojouri, G.A. and Mohebbi, A., 2012. Nanoparticles of selenium as species with stronger physiological effects in sheep in comparison with sodium selenite. Biol. Trace Elem. Res., 146: 302-308. https://doi.org/10.1007/s12011-011-9266-8
Salama, A.A.K., Cajat, G., Albanell, E., Snch, X. and Casals, R., 2003. Effects of dietary supplements of zinc-methionine on milk production, udder health and zinc metabolism in dairy goats. J. Dairy Sci., 70: 9–17. https://doi.org/10.1017/S0022029902005708
Sirirat, N., Lu, J.J., Hung, A.T., Chen, S.Y. and Lien, T.F., 2012. Effects different levels of nanoparticles chromium picolinate supplementation on growth performance, mineral retention, and immune responses in broiler chickens. J. Agric. Sci., 4: 48 -58. https://doi.org/10.5539/jas.v4n12p48
Snedecor, G.W. and Cochran, W.G., 1991. Statistical methods, 8th ed. Iowa State University Press, Americans, USA, pp. 503.
Spears, J.W., Schlegel, P., Seal, M.C. and Lloyd, K.E., 2004. Bioavailability of zinc from zinc sulfate and different organic zinc sources and their effects on ruminal volatile fatty acid proportions. Livest. Prod. Sci., 90: 211-217. https://doi.org/10.1016/j.livprodsci.2004.05.001
Srinivasan, V., Saravana, B.P., Rajkumar, G., Satgurunathan, T. and Muralisankar, T., 2016. Effects of dietary iron oxide nanoparticles on the growth performance, biochemical constituents and physiological stress responses of the giant freshwater prawn Macrobrachium rosenbergii post-larvae. Int. J. Fish. Aquat. Stud., 4: 170-182.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1996. Principles and procedures of statistics. McGraw Hill International Book Co. Inc., New York, USA, pp. 336-352.
Tahir, M.Z.I., Ahmed, I., Mateen, A., Ashraf, M., Naqvi, Z.H. and Ali, H., 2008. Studies on partial replacement of fish meal with oilseeds meal in the diet of major carps. Int. J. Agric. Biol., 10: 455-458.
Vijayakumar, M.P. and Balakrishnan, V.B., 2014. Evaluating the bioavailability of calcium phosphate nanoparticles as mineral supplement in broiler chicken. Indian J. Sci. Tech., 7: 1475–1480. https://doi.org/10.17485/ijst/2014/v7i10.10
Wang, T., Long, X., Cheng, Y., Liu, Z. and Yan, S., 2015. A comparison effect of copper nanoparticles versus copper sulphate on juvenile Epinephelus coioides: Growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int. J. Genom., https://doi.org/10.1155/2015/783021
Wang, H.L., Wang, M.Q., Xu, Z.R., Zha, L.Y. and Lindemann, M.D., 2007. Effects of chromium nanocomposite supplementation on blood metabolites, endocrine parameters and immune traits in finishing pigs. Anim. Feed Sci. Tech., 139: 69-80. https://doi.org/10.1016/j.anifeedsci.2006.12.004
Wang, M.Q. and Xu, Z.R., 2004. Effect of chromium nanoparticle on growth performance, carcass characteristics, pork quality and tissue chromium in finishing pigs. Asian-Australian J. Anim. Sci., 17: 1118-1122. https://doi.org/10.5713/ajas.2004.1118
Xun, W., Shi, L., Yue, W., Zhang, C., Ren, Y. and Liu, Q., 2012. Effect of high-dose nano-selenium and selenium–yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Element Res., 150: 130–136. https://doi.org/10.1007/s12011-012-9452-3
Zaboli, K., Aliarabi, H., Bahari, A.A. and Abbasalipourkabir, A., 2013. Role of dietary nano-zinc oxide on growth performance and blood levels of mineral: A study on in Iranian Angora (Markhoz) goat kids. J. Pharmaceut. Hlth. Sci., 2: 19-26.
Zhou, X., Wang, Y., Gu, Q. and Li, W., 2009. Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture, 291: 78–81. https://doi.org/10.1016/j.aquaculture.2009.03.007
Zhou, Q.C., Tan, B.P., Mai, K.S. and Liu, Y.J., 2004. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture, 241: 441-451. https://doi.org/10.1016/j.aquaculture.2004.08.044
To share on other social networks, click on any share button. What are these?










