Eco-Friendly Control of Culex quinquefasciatus Say (Diptera: Culicidae) through Botanical Insecticides and Predatory Insects
Eco-Friendly Control of Culex quinquefasciatus Say (Diptera: Culicidae) through Botanical Insecticides and Predatory Insects
Mirza Gul1*, Muhammad Zahid1, Ikram Ilahi2, Hazrat Ali2*, Fida Hussain3 and Muhammad Anwar Sajad3
1Department of Zoology, Islamia College Peshawar, Peshawar 25000, Khyber Pakhtunkhwa, Pakistan
2Department of Zoology, University of Malakand, Chakdara 18800, Dir Lower, Khyber Pakhtunkhwa, Pakistan
3Department of Botany, Islamia College Peshawar, Peshawar 25000, Khyber Pakhtunkhwa, Pakistan
ABSTRACT
The present study aims to evaluate the efficacy of n-hexane extracts of two medicinal plants, Artemisia scoparia and Anisomeles indica against larvae, pupae and adults of Culex quinquefasciatus. The study also evaluated the predatory effects of the diving beetle, Agabus cybister, against various instar larvae of Cx. quinquefasciatus. Bioassay of whole-plant extracts was performed following WHO methods, with slight modifications. LC50 values for A. scoparia and A. indica against early fourth instar larvae were 360.4 and 971.1 ppm, respectively. LC50 values for pupae were 1665 and 2838 ppm for A. scoparia and A. indica extracts, respectively. Percent knockdown after 1 h exposure was 49.0 for A. scoparia. KDT50 and KDT90 values for A. scoparia were 69.7 and 763.5 min, respectively. LC50 values for A. scoparia and A. indica against adult mosquitoes were 0.266 and 3.364 per cent respectively. A linear relationship was found between extract concentration and mosquitocidal activity. Regarding predatory control, it was found that during a 12-hour laboratory study, A. cybister consumed 10 exposed larvae. Under field conditions, introduction of predator decreased the larval density from 141.7 to 71 in 15 days. In conclusion, these plants and predator may be useful in controlling mosquito populations in an eco-friendly way.
Article Information
Received 07 June 2017
Revised 13 June 2018
Accepted 10 November 2020
Available online 07 October 2021
(early access)
Published 25 January 2022
Authors’ Contribution
MZ and II conceived and designed the study. MG collected the materials and conducted the experiments. MG, HA, FH and MAS analysed the data and wrote the manuscript. MZ supervised the research work.
Key words
Biocontrol, Botanical insecticides, Culex quinquefasciatus, Environmental pollution, Plant products
DOI: https://dx.doi.org/10.17582/journal.pjz/20170607200629
* Corresponding author: hazratali@uom.edu.pk, hazrataliuom@gmail.com, mirzagul90@gmail.com
0030-9923/2022/0002-0873 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
Being vector of the deadly diseases of filariasis and West Nile Virus (WNV), globally millions of people die just because of Culex quinquefasciatus. Being a blood sucking insect and disease vector, it is seriously needed to control populations of this mosquito.
Insecticides of synthetic origin are commonly used for vector control. Although these insecticides control the growth and populations of mosquitos, they also kill and adversely affect the useful insects and other non-target organisms. On the other hand, the development of resistance to these chemical insecticides such as that observed in Cx. quinquefasciatus (Karunaratne and Hemingway, 2001) has promptly created the need for the development and utilization of eco-friendly alternative approaches for mosquito control. Mosquito control through chemicals of plant origin and their biological control through natural predators may be very effective in this regard. Plants are found to be the likely source of bioactive chemicals and are generally free from harmful effects (Das et al., 2007). Various products of plant origin such as plant essential oils (Zhu and Tian, 2011), ethyl acetate extract (Rawani et al., 2010), methanol extract (Pavela, 2008), acetone extract (Ramkumar et al., 2015), and nanoparticles (Muthukumaran et al., 2015; Santhosh et al., 2015; Govindarajan et al., 2016) have been documented as effective bioactive agents for controlling mosquito vectors. Various studies have reported the mosquitocidal potential of plant n-hexane extracts against mosquito vectors (Kamaraj et al., 2009; Cheah et al., 2013). Similarly reports on the bio-control efficacy of different species of odonate nymphs (Mandal et al., 2008; Akram and Ali-Khan, 2016) and on the dytiscid beetles (Chandra et al., 2008; Culler and Lamp, 2009) against mosquitoes are also available. However, such reports on these plants and predator species are limited. Hence, investigation of the insecticidal potential of the n-hexane extracts of these plants and the biocontrol efficacy of the naturally occurring predators against mosquito vectors is of great importance.
Biological control which uses living organisms against pests to reduce reliance on chemical insecticides may be more effective for mosquito control and is also eco-friendly. Biological control has thus received worldwide attention in recent years. Over the last few years, a wide variety of living organisms such as bacteria (Mani et al., 2015), fungi (Mohanty and Prakash, 2008), invertebrate and vertebrate animals including fishes (Bhattacharjee et al., 2009), tadpoles (Bowatte et al., 2013) and flatworms (Tranchida et al., 2009) have been reported to possess predatory potential against mosquitoes. Diving beetle is a beneficial insect as it possesses biocontrol efficacy against mosquitoes.
Keeping in mind the current interest in biological control of mosquitos through their natural predators and in developing botanical insecticides as an alternative to chemical insecticides, this study was conducted in an attempt to investigate the biocontrol efficacy of Agabus cybister and the insecticidal activity of n-hexane extracts from Artemisia scoparia and Anisomeles indica against the medically important mosquito vector Cx. quinquefasciatus. The results of the present study will be beneficial and may pave the way for the search and application of natural enemies of mosquitoes and for the development of plant-based bioactive agents for mosquito control.
MATERIALS AND METHODS
Plant collection and extraction
The study plants, Artemisia scoparia and Anisomeles indica were collected from Khairabad, District Swat (34°47′ N, 72°17′ E) and Ouch Khairabad, District Dir Lower (34°43′ N, 72°1′ E) areas of Khyber Pakhtunkhwa, Pakistan, respectively. The taxonomic identification of Artemisia scoparia was confirmed by Dr. Nasrullah Khan, Assistant Professor at Department of Botany, University of Malakand while that of Anisomeles indica was confirmed by Dr. Gul Rahim, Subject Specialist in Biology at GHSS Ouch, Dir Lower. Dust free and shade-dried plant materials were ground to fine powder in electric blender. Hexane extract from powdered whole plant was obtained by soaking it in n-hexane for three days. The soaked plant material was filtered through Whatman filter paper no.42 and afterward the filtrate was evaporated on a rotary evaporator under reduced pressure at 45°C.
Collection of predators
The study predator was collected using larval dipper from shallow water near a spring located in the area of Ouch Khairabad, District Dir Lower (34°43′ N, 72°1′ E), Khyber Pakhtunkhwa, Pakistan. The taxonomic identification was confirmed by Dr. Syed Basit Rasheed, Assistant Professor at Department of Zoology, University of Peshawar.
Rearing and maintenance of mosquitoes
Laboratory colonies of Cx. quinquefasciatus were reared and maintained in Entomological Research Laboratory under controlled conditions at 28 ± 2°C and 70-75% relative humidity inside mosquito cages (45 × 45 × 45 cm). Larvae were fed with finely ground brewer’s yeast and dog biscuits at 1:3 ratios as nutrient. After feeding with 10% glucose solution for three days after emergence, the adult mosquitoes were fed periodically with the blood of rabbits for egg production.
Larval and pupal bioassay of plant extracts
Larval and pupal bioassay of plant extracts was performed by following the WHO (1996) standard guidelines, with slight modifications. From prepared stock solution of 4000 ppm, experimental concentrations of 50 ml volume ranging from 125 to 1500 ppm concentrations in dechlorinated tap water were prepared in 250 ml separate disposable plastic cups. Twenty-five early fourth instar larvae and pupae were put into each of these cups. The control was set up under similar conditions. For each concentration, three replicates were run simultaneously. Larval and pupal mortality was recorded after 24 h of exposure period. Control mortality was zero percent. Therefore, Abbot’s formula was not applied. Percentage mortality was calculated by using the formula as under,
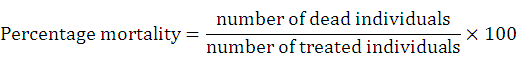
Larval consumption by predator in laboratory
Larval bio-assay of predator was performed using plastic boxes measuring (28 × 19 × 9 cm) in size. Sixty, one hundred thirty-two and one hundred ninety-two larvae of Cx. quinquefasciatus ranging from 2nd to 4th instar (20, 44 and 64 of each of that instar) were put separately into each of those boxes filled 1.5 cm with dechlorinated tap water. After a fasting period of 6 h, seven individuals of Agabus cybister were then transferred into each of these boxes. The boxes were tightly covered with mosquito net to prevent their escape. For each of these larval numbers, three replicates were set up at a time. Larval consumption was recorded after 12 h of exposure period. To evaluate the effect of water depth on larval consumption of predator, two boxes of the same size (28 × 19 × 9 cm) were filled with water at two different depths (1.5 and 3 cm). Into each of these boxes, 160 larvae of 2nd to 4th instar were introduced. Percentage of larval consumption was calculated by using the following formula:
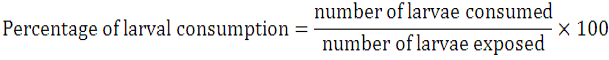
Predation experiment in the field
To evaluate the predation efficacy of Agabus cybister against mosquito larvae in field condition, 220 individuals of the predator were introduced at different places into a pond (11 feet length, 6 feet width and 1 foot depth), a habitat rich in Cx. quinquefasciatus larvae. To determine the predatory effect, number of larvae in the dipper samples before and after the introduction of predator were counted.
Adulticidal bioassay of plant extracts
Adulticidal activity was evaluated at five different concentrations (0.075, 0.15, 0.31, 0.62, and 1.25 %). Adulticidal bioassay was conducted by applying WHO standard procedure (WHO, 1981). Four ml from each of the aforesaid concentrations was impregnated on Whatman no. 1 filter paper (size 12 × 15 cm2) making concentrations of 0.017, 0.03, 0.06, 0.13 and 0.27 mg/cm2 respectively. Control papers were treated with acetone only under similar conditions. Through aspirator, twenty female mosquitoes (2-5 days old glucose fed, blood starved) from the mosquito rearing cages were transferred into a plastic holding tube. The mosquitoes were exposed for 1 h to test paper after acclimatization period of 1 h in the tube. At the end of exposure period, the mosquitoes were transferred back to the holding tube and laid 24 h for recovery period. The tubes were tightly covered with a net cloth and a pad of cotton soaked with 10% glucose solution was provided in the tube as a food source. Three replicates for each tested concentration, as well as for control were set up at a time. Mortality of the mosquitoes was determined at the end of 24-h recovery period. Control mortality was less than five percent. Therefore, Abbot’s formula was not applied.
Statistical analyses
The values of LC50, LC90, and their 95% confidence limits of upper confidence limit and lower confidence limit were determined by using the SPSS Statistical Software Package 16.0 version, while the values of Regression equation were determined using Excel 2010. Results with P < 0.05 were considered to be statistically significant.
RESULTS
The results of the study are presented in Tables I-V. The 24 h LC50 for larvae was 360.4 ppm for the n- hexane extract of A. scoparia and 971.1 ppm for A. indica while the corresponding LC90 values of these plant extracts were 1328 and 4791 ppm (Table I). The LC50 values of A. scoparia and A. indica against pupae were 1665 and 2838 ppm respectively while the corresponding LC90 values were 83670 and 109600 ppm (Table I). Percent knockdown at the end of 1 h exposure was 49.0 for A. scoparia. The KDT50 and KDT90 values for A. scoparia were 69.7 and 763.5 minutes respectively (Table II). LC50 values for A. scoparia and A. indica against adult Cx. quinquefasciatus were 0.266 and 3.364 per cent respectively while corresponding LC90 values were 1.257 and 33.58 per cent respectively (Table III). Direct correlation was observed between concentration and toxicity. Regression equations are given in Tables I-III, which show that concentration is the factor responsible for determining the mosquitocidal activity of plant extracts.
Regarding predatory control, results of the laboratory study clearly showed that diving beetle, A. cybister, mostly preferred and consumed 2nd instar larvae (Table IV). After 12 h exposure period, larvae consumption by seven individuals of A. cybister was 49 larvae out of 60, 79 out of 132 and 85 out of 192 (Table IV). Results of the study also showed that predation increased with increasing number (density) of larvae and decreased with increasing depth of the water (Table IV). When applied in the field, results of the study revealed a decrease in larval density in three dipper samples from 141.7 to 71.0, 15 days after the introduction of the predator and increase in larval density in dipper samples from 71.0 to 126.3 after 15 days of the removal of the predator (Table V).
DISCUSSION
Besides the development of insect resistance to conventional synthetic insecticides, potential risk posed by these chemicals to the environment has paved the way for the development of an alternative control strategy. As a result of rich source of bioactive compounds, currently the use of plants for developing environment friendly insecticides has got worldwide attention. Botanical insecticides may be an effective agent for controlling mosquito vectors as they are relatively safe and are also effective in terms of resistance development compared to synthetic insecticides. Nzelibe and Chintem (2013) have reported the application of oil-rich ethnobotanicals as mosquitocides due to extraction of non-polar compounds by n-hexane. The results of our study revealed the toxicity of whole-plant n-hexane extracts of these plants against early fourth instar larvae, pupae and adult Cx. quinquefasciatus. In previous studies (Kumar et al., 2012; Warikoo et al., 2012), hexane extracts of different plants have been reported with remarkable mosquitocidal activity.
Table I. Effect of whole-plant n-hexane extracts against 4th instar larvae and pupae of Culex quinquefasciatus.
|
Plant |
Concentration (ppm) |
Regression equation |
LC50, LC90 (95 % confidence limits) |
||||||||
|
125 |
250 |
500 |
1000 |
1500 |
LC50 (LCL-UCL) |
LC90 (LCL-UCL) |
|||||
|
Larval mortality % (Mean ±SD) |
|||||||||||
|
Artemisia scoparia |
21.3 ±2.3 |
26.7 ±20.1 |
65.3 ±16.2 |
74.7 ± 6.1 |
100.0 ±0.0 |
y = 0.06x + 20.0 |
360.4 (267.3- 469.8) |
1328.0 (922.3-2483.2) |
|||
|
Anisomeles indica |
4.0 ±6.9 |
6.7 ±11.5 |
46.7 ±6.1 |
48.0 ± 8.0 |
58.7 ±14.0 |
y = 0.04x + 6.4 |
971.1 (709.4-1561.7) |
4791.0 (2543.3-19100.6) |
|||
|
Pupal mortality %(Mean ±SD) |
|||||||||||
|
Artemisia scoparia |
16.0 ±4.0 |
33.3 ±12.2 |
34.7 ± 6.1 |
38.7 ±16.6 |
50.7 ± 4.6 |
y = 0.01x + 21.5 |
1665 (941.6-7525.7) |
83670 (13650.1- 29840000) |
|||
|
Anisomeles indica |
16.0 ±12 |
18.7 ±6.1 |
28.0 ± 4.0 |
37.3 ± 6.1 |
42.7 ±10.1 |
y = 0.01x + 15.1 |
2838 (1448.1-17796.4) |
109600 (17583.0-28230000) |
|||
Table II. Knock down effect of whole-plant n-hexane extracts against Culex quinquefasciatus.
|
Plant species |
Percentage of mosquito knock down KD ± SD |
Regression equation |
KDT50 (LCL-UCL) |
KDT90 (LCL-UCL) |
|||
|
15 min |
30 min |
45 min |
60 min |
||||
|
Artemisia scoparia |
21.7 ±18.4 |
30.7 ±18.9 |
39.7 ±23.7 |
48.7 ±29.3 |
y = 0.6x +12.66 |
69.7 (51.5-141.1) |
763.5 (277.3-52583.4) |
Table III. Adulticidal activity of whole-plant n-hexane extracts against adult Culex quinquefasciatus.
|
Plant species |
Mortality (%) (mean ± SD) at different concentrations |
Regression equation |
LC50, % (LCL-UCL) |
LC90, % (LCL-UCL) |
||||
|
0.075 % |
0.15 % |
0.31 % |
0.62 % |
1.25 % |
||||
|
Artemisia scoparia |
20.0 ±5.0 |
35.0 ±5.0 |
43.3 ±7.6 |
71.7 ±7.6 |
96.7 ±5.8 |
y= 62.53x +23.25 |
0.266 (0.214-0.329) |
1.257 (0.891-2.120) |
|
Anisomeles indica |
0.0 ±0.0 |
6.7 ±5.8 |
8.3 ±7.6 |
20 ±10 |
26.7 ±7.6 |
y= 21.49x +1.99 |
3.364 (1.723-17.053) |
33.588 (8.817-1059.608) |
Table IV. Predatory effect (Mean ± SD, %) of the aquatic insect, Agabus cybister on different larval stages of Culex quinquefasciatus.
|
No. of larvae exposed |
Mosquito life stages |
% of larval consumption at water depth |
|||
|
2nd instar |
3rd instar |
4th instar |
1.5 cm |
3 cm |
|
|
60 |
100.0± 0.0 |
80.0± 10.0 |
65.0± 20.0 |
75.6 |
70.0 |
|
132 |
93.2± 11.8 |
87.9± 11.4 |
78.8± 7.3 |
||
|
192 |
86.4± 12.6 |
77.3± 8.2 |
56.8± 9.9 |
||
Table V. Predatory effect of the aquatic insect, Agabus cybister on larvae of Culex quinquefasciatus in the field.
|
Experimental observation |
Average number of larvae in dipper samples (n = 3) |
|
Before the introduction of predator |
141.7 |
|
15 days after the introduction of predator |
71.0 |
|
15 days after the removal of predator |
126.3 |
The data of the present study displayed in Tables I-III show that, the studied plants possess mosquitocidal activity. This activity of the plants may be due to various compounds present in them including saponins, alkaloids, terpenoids, steroids and flavonoids etc. The values of LC50 (0.266 and 3.364 percent) obtained in the present study for A. scoparia, and A. indica respectively against adult female mosquito were too much higher than the LC50 (148.86 and 231.59 ppm) reported by Govindarajan and Rajeswary (2015) for the leaf and seed hexane extracts of Albizia lebbeck against adult Cx. quinquefasciatus. Percent knockdown at the end of 1 h exposure was 49.0 for A. scoparia. In a similar observation, Kamaraj et al. (2010), working with insecticidal and larvicidal activities of leaf and rhizome extracts of eight plants, reported 100% knock down in 1h for the n-hexane extract of Zingiber zerumbet.
Present study showed that after 24 h of exposure, n-hexane extract from A. scoparia and A. indica had LC50 values of 360.4 and 971.1 ppm against early 4th instar larvae. In a similar observation, Singh et al. (2006) reported lower LC50 value of 96.11 ppm for the leaf hexane extract of Momordica charantia while Younoussa et al. (2016) reported higher LC50 value of 3394.9 ppm for the n-hexane fraction of leaf methanol extract of Boswellia dalzielii against 4th instar larvae of Cx. quinquefasciatus. LC50 values of 1665 and 2838 ppm obtained in the present investigation for the n-hexane extract from A. scoparia and A. indica against pupae were higher than that observed by Modise and Ashafa (2016). Results of regression analyses confirmed direct correlation between concentration and mosquitocidal potential of the extract. Similar trend has also been reported by Barik et al. (2016) working with mosquito larvicidal activity of solvent extracts of fruits of Acacia auriculiformis against Japanese encephalitis vector group.
Regarding predatory control, negative correlation between prey consumption and water depth but positive correlation between prey consumption and the density of prey was observed. Similar trend has been reported by Chandra et al. (2008) working with biocontrol of larval mosquitoes by Acilius sulcatus (Coleoptera: Dystisciae), and also by Saha et al. (2012) working with predation potential of two larval odonates on mosquito larvae. Furthermore, similar to the observation of Venkatesh and Tyagi (2015), results of the study showed that diving beetle mostly targeted and preyed on smaller larvae. During a period of 12-h, a diving beetle was found to consume (mean value of three observations) 10 larvae. This is consistent with Mandal et al. (2008) who reported 25 larvae for Coenagrion kashmirum odonate nymph within a period of 24 h working with biocontrol efficiency of five coexisting odonate nymphs against larvae of the mosquito Cx. quinquefasciatus. In the field experiment, a decrease in larval density in dipper samples from 141.7 to 71.0 was observed 15 days after the introduction of the predator. Similar result has also been obtained by Chatterjee et al. (2007) testing the biocontrol potential of the dragonfly Brachytron pratense against larvae of the mosquito Anopheles subpictus.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Nasrullah Khan, Assistant Professor at Department of Botany, University of Malakand and Dr. Gul Rahim, Subject Specialist in Biology at GHSS Ouch, Dir Lower, for their help in plant identification and to Dr. Syed Basit Rasheed, Assistant Professor at Department of Zoology, University of Peshawar, for his help in the insect identification.
Statement conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Akram, W. and Ali-Khan, H.A., 2016. Odonate nymphs: Generalist predators and their potential in the management of dengue mosquito, Aedes aegypti (Diptera: Culicidae). J. Arthropod-Borne Dis., 10: 252-257.
Barik, M., Rawani, A. and Chandra, G., 2016. Mosquito larvicidal activity of solvent extracts of fruits of Acacia auriculiformis against Japanese encephalitis vector Culex vishnui group. J. Mosq. Res., 6: 1-8. https://doi.org/10.5376/jmr.2016.06.0013
Bhattacharjee, I., Aditya, G. and Chandra, G., 2009. Laboratory and field assessment of the potential of larvivorous, air-breathing fishes as predators of culicine mosquitoes. Biol. Contr., 49: 126-133. https://doi.org/10.1016/j.biocontrol.2008.12.014
Bowatte, G., Perera, P., Senevirathne, G., Meegaskumbura, S. and Meegaskumbura, M., 2013. Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol. Contr., 67: 469-474. https://doi.org/10.1016/j.biocontrol.2013.10.005
Chandra, G., Mandal, S.K., Ghosh, A.K., Das, D., Banerjee, S.S. and Chakraborty, S., 2008. Biocontrol of larval mosquitoes by Acilius sulcatus (Coleoptera: Dytiscidae). BMC Infect. Dis., 8: 138-145. https://doi.org/10.1186/1471-2334-8-138
Chatterjee, S.N., Ghosh, A. and Chandra, G., 2007. Eco-friendly control of mosquito larvae by Brachytron pratense nymph. J. environ. Hlth., 69: 44-48.
Cheah, S.-X., Tay, J.-W., Chan, L.-K. and Jaal, Z., 2013. Larvicidal, oviposition, and ovicidal effects of Artemisia annua (Asterales: Asteraceae) against Aedes aegypti, Anopheles sinensis, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res., 112: 3275-3282. https://doi.org/10.1007/s00436-013-3506-0
Culler, L.E., Lamp, W.O., 2009. Selective predation by larval Agabus (Coleoptera: Dytiscidae) on mosquitoes: Support for conservation-based mosquito suppression in constructed wetlands. Freshw. Biol., 54: 2003-2014. https://doi.org/10.1111/j.1365-2427.2009.02230.x
Das, N.G., Goswami, D. and Rabha, B., 2007. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J. Vector Borne Dis., 44: 145-148.
Govindarajan, M. and Rajeswary, M., 2015. Ovicidal and adulticidal potential of leaf and seed extract of Albizia lebbeck (L.) Benth. (Family: Fabaceae) against Culex quinquefasciatus, Aedes aegypti, and Anopheles stephensi (Diptera: Culicidae). Parasitol. Res., 114: 1949-1961. https://doi.org/10.1007/s00436-015-4384-4
Govindarajan, M., Rajeswary, M., Veerakumar, K., Muthukumaran, U., Hoti, S.L., Mehlhorn, H., Barnard, D.R. and Benelli, G., 2016. Novel synthesis of silver nanoparticles using Bauhinia variegata: A recent eco-friendly approach for mosquito control. Parasitol. Res., 115: 723-733. https://doi.org/10.1007/s00436-015-4794-3
Kamaraj, C., Bagavan, A., Rahuman, A.A., Zahir, A.A., Elango, G. and Pandiyan, G., 2009. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol. Res., 104: 1163-1171. https://doi.org/10.1007/s00436-008-1306-8
Kamaraj, C., Rahuman, A.A., Mahapatra, A., Bagavan, A., Elango, G., 2010. Insecticidal and larvicidal activities of medicinal plant extracts against mosquitoes. Parasitol. Res., 107: 1337-1349. https://doi.org/10.1007/s00436-010-2006-8
Karunaratne, S.H.P.P. and Hemingway, J., 2001. Malathion resistance and prevalence of the malathion carboxylesterase mechanism in populations of mosquito vectors of disease in Sri Lanka. Bull. World Hlth. Org., 79: 1060-1064.
Kumar, S., Wahab, N., Mishra, M. and Warikoo, R., 2012. Evaluation of 15 local plant species as larvicidal agents against an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Front. Physiol., 3: 104-109. https://doi.org/10.3389/fphys.2012.00104
Mandal, S.K., Ghosh, A., Bhattacharjee, I. and Chandra, G., 2008. Biocontrol efficiency of odonate nymphs against larvae of the mosquito, Culex quinquefasciatus Say, 1823. Acta Trop., 106: 109-114. https://doi.org/10.1016/j.actatropica.2008.02.002
Mani, C., Thirugnanasambantham, K., Sundarapandian, S. and Poopathi, S., 2015. Identification and characterization of a novel marine Bacillus cereus VCRC-B540 for mosquito control. BioControl, 60: 71-79. https://doi.org/10.1007/s10526-014-9605-8
Modise, S.A. and Ashafa, A.O.T., 2016. Larvicidal, pupicidal and insecticidal activities of Cosmos bipinnatus, Foeniculum vulgare and Tagetes minuta against Culex quinquefasciatus mosquitoes. Trop. J. Pharm. Res., 15: 965-972. https://doi.org/10.4314/tjpr.v15i5.10
Mohanty, S.S. and Prakash, S., 2008. Laboratory and field evaluation of the fungus Chrysosporium lobatum against the larvae of the mosquito Culex quinquefasciatus. Parasitol. Res., 102: 881-886. https://doi.org/10.1007/s00436-007-0843-x
Muthukumaran, U., Govindarajan, M. and Rajeswary, M., 2015. Mosquito larvicidal potential of silver nanoparticles synthesized using Chomelia asiatica (Rubiaceae) against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res., 114: 989-999. https://doi.org/10.1007/s00436-014-4265-2
Nzelibe, H.C. and Chintem, D.G.W., 2013. Larvicidal potential of leaf extracts and purified fraction Ocimum gratissimum against Culex quinquefasciatus mosquito larva. Int. J. Sci. Res., 4: 2254-2258.
Pavela, R., 2008. Larvicidal effects of various Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol. Res., 102: 555-559. https://doi.org/10.1007/s00436-007-0821-3
Ramkumar, G., Karthi, S., Muthusamy, R., Natarajan, D. and Shivakumar, M.S., 2015. Adulticidal and smoke toxicity of Cipadessa baccifera (Roth) plant extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus. Parasitol. Res., 114: 167-173. https://doi.org/10.1007/s00436-014-4173-5
Rawani, A., Ghosh, A. and Chandra, G., 2010. Mosquito larvicidal activities of Solanum nigrum L. leaf extract against Culex quinquefasciatus Say. Parasitol. Res., 107: 1235-1240. https://doi.org/10.1007/s00436-010-1993-9
Saha, N., Aditya, G., Banerjee, S. and Saha, G.K., 2012. Predation potential of odonates on mosquito larvae: Implications for biological control. Biol. Contr., 63: 1-8. https://doi.org/10.1016/j.biocontrol.2012.05.004
Santhosh, S.B., Yuvarajan, R. and Natarajan, D., 2015. Annona muricata leaf extract-mediated silver nanoparticles synthesis and its larvicidal potential against dengue, malaria and filariasis vector. Parasitol. Res., 114: 3087-3096. https://doi.org/10.1007/s00436-015-4511-2
Singh, R.K., Dhiman, R.C. and Mittal, P.K., 2006. Mosquito larvicidal properties of Momordica charantia Linn (Family: Cucurbitaceae). J. Vector Borne Dis., 43: 88-91.
Tranchida, M.C., Maciá, A., Brusa, F., Micieli, M.V. and García, J.J., 2009. Predation potential of three flatworm species (Platyhelminthes: Turbellaria) on mosquitoes (Diptera: Culicidae). Biol. Contr., 49: 270-276. https://doi.org/10.1016/j.biocontrol.2008.12.010
Venkatesh, A., Tyagi, B., 2015. Bradinopyga geminata (Anisoptera: Libellulidae) as a predator of Aedes aegypti immatures (Diptera: Culicidae). Int. J. Mosq. Res., 2: 98-105.
Warikoo, R., Ray, A., Sandhu, J.K., Samal, R., Wahab, N., Kumar, S., 2012. Larvicidal and irritant activities of hexane leaf extracts of Citrus sinensis against dengue vector Aedes aegypti L. Asian Pac. J. Trop. Biomed., 2: 152-155. https://doi.org/10.1016/S2221-1691(11)60211-6
WHO, 1981. Instructions for determining the susceptibility or resistance of adult mosquitoes to organochlorine, organophosphate and carbamate insecticides. Establishment of the baseline, Geneva.
WHO, 1996. Report of the WHO informal consultation on the evaluation and testing of insecticides. World Health Organization, Geneva, pp. 69.
Younoussa, L., Nukenine, E.N. and Esimone, C.O., 2016. Toxicity of Boswellia dalzielii (Burseraceae) leaf fractions against immature stages of Anopheles gambiae (Giles) and Culex quinquefasciatus (Say) (Diptera: Culicidae). Int. J. Insect Sci., 8: 23-31. https://doi.org/10.4137/IJIS.S37188
Zhu, L. and Tian, Y., 2011. Chemical composition and larvicidal activity of Blumea densiflora essential oils against Anopheles anthropophagus: a malarial vector mosquito. Parasitol. Res., 109: 1417-1422. https://doi.org/10.1007/s00436-011-2388-2
To share on other social networks, click on any share button. What are these?










