Distribution, Abundance and Population Ecology of Ashtoret lunaris (Forskel, 1775) and Matuta planipes Fabricius, 1798 from the Sonmiani Bay (Lagoon), Pakistan
Distribution, Abundance and Population Ecology of Ashtoret lunaris (Forskel, 1775) and Matuta planipes Fabricius, 1798 from the Sonmiani Bay (Lagoon), Pakistan
Noor Us Saher1, Zunaira Amanat2, M. Asif Gondal3 and Naureen Aziz Qureshi4,*
1Centre of Excellence in Marine Biology, University of Karachi, Karachi 75270, Pakistan.
2Department of Zoology, Federal Urdu University of Arts, Science and Technology, University Road, Karachi, Pakistan.
3Department of Biosciences, COMSATS Institute of Information Technology, Park Road, Islamabad, Pakistan
4Government College Women University, Faisalabad, Pakistan
ABSTRACT
Matuta planipes and Ashtoret lunaris were collected as a part of by catch of gillnet deployed for shrimps from April 2005 to March 2007 in Sonmiani Bay waters (Miani Hor lagoon). M. planipes was found abundantly in Sep. 2005, Nov. - Dec. 2005 and Dec. 2006 (93.3%, 94.7%, 92.3% and 87 % respectively). A. lunaris was found in abundance during May 2005 (85.7%) and Aug. 2005 (81.8%). Male specimens of both species (M. planipes: χ2 = 245.4, P < 0.005) and (A. lunaris: χ2 = 99.4, P<0.005) were significantly more than females throughout the study period. There was a significant difference in size of male and female crabs within sexes; (M. planipes: t = -4.93, P < 0.005) and (A. lunaris: t = -2.92, P<0.005). Various morphometrical relationships were demonstrated that the males of M. planipes and A. lunaris were larger in size and heavier than females (4.34g ± 0.9g; 14.86 ± 6.8g and 3.87g ± 0.6g; 11.66 g ± 5.4 g) respectively. There was a negative allometry for both male and females of M. planipes and A. lunaris for length and weight relationship. The estimated carapace size at onset of sexual maturity was 3.2 cm and 3.3 cm for males and females of A. lunaris whereas, males and females of M. planipes had sexual maturity carapace size 3.1 cm and 3.2 cm in lagoon waters of Sonmiani bay.
Article Information
Received 03 July 2013
Revised 11 December 2013
Accepted 03 September 2016
Available online 31 January 2017
Authors’ Contributions
NUS designed the study, performed the experiments and wrote the article. MAG helped in field and lab work. ZA helped in statistical analysis. NAQ helped in preparation of manuscript.
Key words
Matuta planipes, Ashtoret lunaris, Sonmiani Bay, Sexual maturity, Population biology.
* Corresponding author: naureenaziz.qureshi@gmail.com; noorusaher@yahoo.com
0030-9923/2016/0004-1161 $ 8.00/0
Copyright 2016 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.455.465
INTRODUCTION
Matuta planipes Fabricius, 1798 (reticulated moon crab) and Ashtoret lunaris (Forskal, 1775) (flower moon crab) are two commonly occurring species of sub-family Matutinae (De Haan, 1835) in Pakistani waters belonging to family Calappidae (Tirmizi and Kazmi, 1986). Galil and Clark (1994) revised the genus Matuta (Weber, 1795) and re-designated the Matuta lunaris as Ashtoret lunaris.
These two species are common inhabitant of the surf zone of tropical sandy shores and have a widespread distribution which extends from the Red Sea to South Africa, Asia, and Australia (Chappgar, 1957; Sankarankutty, 1962; Guinot, 1966; Vannini, 1976; Perez, 1990). Despite their common occurrence, there have been very few published studies on the ecology and biology of these crabs (Perez, 1990; Varadharajan et al., 2012). They are nocturnal, found in sandy shallow benthic zone (2-4 m). However, Richmond (1997) has reported them from deep waters also. They feed on small shellfish, worms and other animals e.g. small crabs at night (Tan and Ng, 1988; Hazlett, 1997). Some investigators declared them as a predator of flat fishes (Thomassin, 1974; Hossain et al., 2002; Fatima, 2003; Saitoh et al., 2003).
These crabs are not the real mangrove crabs but can be found in open areas of sandy to muddy tidal flats in front of the mangroves and often associated with them (Shukla et al., 2013). These crabs are found from the intertidal zone to 10-15 m in depth widely in the Indo-Pacific region (Sakai, 1965) and play an imperative role in the ecology of sandy shores. These crabs are also commercially important and used as food in many tropical and subtropical countries but in Pakistan, the fishermen usually throw back in the waters when these crabs caught in a net or included in the trash.
Growth in animals is often accompanied by changes in proportion as well as in size, i.e. some body parts grow at a different rate than others. This phenomenon is called relative or allometric growth. Due to the rigid exoskeleton and consequent discontinuous growth, crustaceans have been extensively used for the relative growth analyses (Hartnoll, 1972). The relative growth in crustaceans has been widely studied by numerous authors, mainly because the rigid exoskeleton of these animals allows accurate measurements (Huxley and Richards, 1931; Weymouth and Mackay, 1936; Pinheiro and Fronsozo, 1998). Review of literature shows no previous work on the biology and the ecology of Matuta and Ashtoret species along the coast of Pakistan except for a little work related to the length-weight relationship of Matuta species from the Karachi coast (Fatima, 2003). This is the first attempt to study the population biology and ecology of commonly occurring species of family Matutidae from the coastal areas of Pakistan. Due to the ecological importance of Matuta species, this study was aimed to determine the morphometric relationships within species along the coast of Pakistan.
MATERIALS AND METHODS
Study site
The Sonmiani Bay (locally called as Miani Hor) is a lagoon and an important marine fishing center along the coast of Pakistan. It remains the site of brisk fishing activities on the Makran coast throughout the year, evidently due to its nearness to Karachi and also because marine lagoons are generally characterized by high productivity. It is situated 90 km away from Karachi on the eastern most part of Balochistan coast. It is located at the latitude of 25° 27’ 431’’ North, and the longitude of 66° 33’ 700’’ East, approximately (Fig. 1). It is a 60 km long and 7 km wide tortuous body of water, which is connected to the sea by 4 km wide mouth. The seasonal rivers, Porali and the Windor, enter into the bay near the study area. In Balochistan, the mangroves are restricted to three localities e.g., Miani Hor, Kalmat Khor and Gawader Bay. The only significant area that contains extensive mangrove stands is a Sonmiani Bay in Balochistan (Saifullah and Rasool, 1995; Rasool et al., 2002).
Field study
M. planipes and A. lunaris crabs were collected from the Apr. 2005 to Mar. 2007 as bycatch during the gill net fishing for fisheries survey project in Sonmiani bay. In Sonmiani, the fishermen throw them a side or fling back to water during sorting of catch. The gill net collections were made fortnightly during every month. The net was deployed from the commercial fishing boat with the help of commercial fishermen. Three hauls of twenty to twenty five minutes each, were made at every sampling event. The crabs trapped in each haul were immediately weighted, placed separately in polythene bags and kept on ice for transfer to the laboratory for further analysis. Samples for the hydrographic parameters (salinity, temperature and pH) were also collected at each visit. Salinity was measured with a refractometer (ATAGO, S-mill USA), temperature and pH, with a field pH meter (Hanna 8314) at each sampling event.
Laboratory study
The collected crab’s samples were brought to the laboratory. Later the crabs were sorted, identified up to species level by using the identification keys of Tirmizi and Kazmi (1986) and Galil and Clark (1994). Crabs of both species were sexed into male and female crabs by the shape of their abdominal flaps. The morphometric measurements of different parts of the body, carapace width (CW), chelae length (Ch. L) and total body (Carapace length) length (TL) of crabs were measured to the nearest 0.1 cm (Fig. 2) and total body wet weight was measured up to 0.01g.
Statistical analysis
Analysis of variance (ANOVA) with nested treatment arrangement was carried out by using the statistical package Minitab (Version 15.0) for differences between seasons in the abundance of crabs. Test of significance was accepted as significant at alpha = 0.05 for statistical analyses. Monthly data were grouped into seasons following Saher and Qureshi (2011), wherein December, January and February are defined as northeast (NE) monsoon, March, April and May are defined as pre-monsoon period. June, July and August are defined as the south west (SW) monsoon period, September, October and November as post-monsoon period, to observe the seasonal variability.
Chi Square (χ2) test was calculated to study the sex ratio at Sonmiani Bay with the help of following equation:
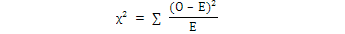
Where, O was the observed value, and E was the expected value of that species.
All Matuta crabs were sorted into four groups i.e., young males, adult males, young females and adult females. Young and adult crabs were sorted according to the examination of secondary sexual characters, such as pleopod morphology, free abdomen and distinct cheliped development in adult male crabs as compared to young male and convex abdomen (forming an incubator chamber) in adult females. The relative frequency of all males, non-ovigerous females and ovigerous females (%) in each size class was plotted and fitted as a sigmoid curve following the result of the logarithmic equation.
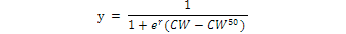
Where, CW50 was the carapace width at which 50% of the individuals attain sexual maturity, and r was the slope of the curve. The adjusted equation was fitted by the least squares regression method (Vazzoler, 1996; Bertini et al., 2001; Saher and Qureshi, 2011; Amanat and Qureshi, 2011).
The relative growth was studied by using power function Y = aXb and was the equation linearized by logrithmic transformation.
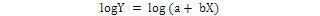
The CW and TL were used as independent variables (X) and whereas, all other body dimensions were considered as the slope “b” of the equation is the allometric constants that express the analogy between two variables, then it is used to determine a growth coefficient for males and females. The pattern of the growth established for length and weight relationships was characterized as positive allometry when b > 3, negative allometry b < 3 and isometry b = 3 (Hartnoll, 1982). The best fit linear regression equation was calculated using least square method to determine the relationship between morphometric variables (Teissier, 1960; Hartnoll, 1982; Qureshi and Kazmi, 1999) and growth was considered isometric when 0.9 < b < 1.1 (Castiglioni and Negreiros-Fransozo, 2004; Saher and Qureshi, 2011).
RESULTS
Hydrographic parameters
Seasonal variations in air temperature, water temperature, salinity and pH were observed and presented in Table I. The mean air temperature (28.8 ± 3.14oC) with ranges from (22-34oC) and mean water temperature (25.9 ± 4.1) with ranges from (17-31oC) were observed during the study period. The highest temperature was recorded during the summer months (May - Jul) and the lowest temperature during winter months (Nov - Jan). The salinity was observed in the range of 30 - 47 ppt, and pH of 6.8 - 9.0. The seawater surface salinity and pH were found high (40.5 o/oo) and 8.5, respectively (Table II).
Distribution and abundance
The catch sizes varied with the months and showed a seasonal pattern (Fig. 3). Monthly percent distribution showed that both species were found throughout the study period except in the months of May, Jun. and Sep. 2006. Whereas in the month of July (2005, 2006) only A. lunaris species were found. Monthly percent distribution showed that the highest number of M. planipes was found from Aug. 2005 to Feb. 2006 and Dec. 2006 to Feb. 2007, whereas, A. lunaris abundance was observed from March to August and again in the month of October throughout the study period.
Table I.- Seasonal variations (mean± SD) and range of air temperature, water temperature, Salinity, pH and density of A. lunaris and M. planipes collected from Sonmiani waters during April, 2005 to March, 2007.
| Season | N |
Air Temp. (oC) (min-max) |
Water Temp. (oC) (min- max) |
Salinity (o/oo) (min-max) |
pH (min-max) |
Ashtoret lunaris (min- max) |
Matuta planipes (min- max) |
| Pre Monsoon (2005) | 3 |
29.33±1.52 (28.0-31.0) |
27.80±2.44 (25.0-29.5) |
34.2±3.8 (30-37.5) |
7.5±0.55 (7.2-8.2) |
13.3± 12.8 (4-28) |
4.7±4.6 (0-12) |
| SW Monsoon (2005) | 4 |
30.80±2.88 (28.4-34.0) |
30.73±1.41 (29.2-32.0) |
33±3.61 (33-37) |
8.3±0.86 (7.3-9.0) |
15.5± 13.6 (2-30) |
11.5±18 (0-38) |
| Post Mons oon (2005) | 3 |
30.66±1.15 (30.0-32.0) |
27.66±1.52 (26.0-29.0) |
35±1.73 (34-37.) |
8.5±0.17 (8.3-8.6) |
8.7±8.3 (2-18) |
2±2 (0-4.0) |
| NE Monsoon (2005-06) | 5 |
26.16±0.76 (25.5-27.0) |
19.67±2.31 (17.0-21.0) |
39±1.0 (38-40) |
8.3±0.33 (8.1-8.7) |
21.7± 16.5 (2-43) |
35.3± 40.4 (2-94) |
| Pre Monsoon (2006) | 2 |
31.50±0.70 (31.0-31.5) |
27.25±4.60 (24.0-30.5) |
37.5±0.7 (37-38) |
7.4±0.06 (7.3-7.4) |
201± 9.9 (194-208) |
125±24 (108- 142) |
| SW Monsoon (2006) | 2 |
26.55±2.62 (24.7-28.4) |
27.85±1.20 (27.0-28.7) |
36±1.41 (35-37) |
6.8±0.06 (6.8-6.9) |
4.0±5.6 (0-8) |
19.0± 24.0 (2-36) |
| Post Monsoon (2006) | 3 |
27.00±3.00 (24.0-30.0) |
27.20±5.63 (21.0-32.0) |
41.3±5.1 (37-47) |
7.6±0.24 (7.3-7.8) |
64.0± 50.9 (28-100) |
42.0± 53.7 (4-80) |
| NE Monsoon (2006-07) | 3 |
22.57±1.91 (21.0-24.7) |
23.33±4.51 (19.0-28.0) |
41.0±2.6 (39-44) |
7.8±0.23 7.5-8.0) |
60.7± 18.1 40-74) |
33.3± 30.4 (6-66) |
| Pre Monsoon (2007) | 1 |
30.50 (30.5-30.5) |
31.60 (31.6-31.6) |
38.00 (38- 38) |
8.0 (8.0-8.0) |
18.0 (18-18) |
20.0 (20-20) |
Table II.- Monthly total percent abundance and distribution of male and female individuals of Matuta planipes and Ashtoret lunaris caught from Sonmiani Bay, (April, 2005 to March, 2007).
| Month |
Percent Matuta planipes |
Percent Ashtoret lunaris |
Matuta planipes |
|
Ashtoret lunaris |
||
|
% Female |
% Male |
% Female |
% Male |
||||
| Apr. 05 | 30.0 | 70.0 |
33.3 |
66.7 |
|
28.6 |
71.4 |
| May. 05 | 14.3 | 85.7 |
0.0 |
100.0 |
|
0.0 |
100.0 |
| Jun. 05 | 38.7 | 61.3 |
10.5 |
89.5 |
|
41.7 |
58.3 |
| Jul. 05 | NC | 100.0 |
NC |
NC |
|
0.0 |
100.0 |
| Aug. 05 | 70.7 | 29.3 |
24.1 |
75.9 |
|
25.0 |
75.0 |
| Sep. 05 | 93.3 | 6.7 |
NC |
NC |
|
14.3 |
85.7 |
| Oct. 05 | 42.9 | 57.1 |
NC |
100.0 |
|
75.0 |
25.0 |
| Nov. 05 | 94.7 | 5.3 |
NC |
NC |
|
NC |
100.0 |
| Dec. 05 | 92.3 | 7.7 |
NC |
NC |
|
NC |
100.0 |
| Jan. 06 | 63.2 | 36.8 |
16.4 |
83.6 |
|
30.8 |
69.2 |
| Feb. 06 | 76.5 | 23.5 |
25.6 |
74.4 |
|
25.0 |
75.0 |
| Mar. 06 | 40.6 | 59.4 |
18.3 |
81.7 |
|
29.8 |
70.2 |
| Apr. 06 | 35.8 | 64.2 |
66.7 |
33.3 |
|
41.2 |
58.8 |
| May. 06 | NC | NC |
NC |
NC |
|
NC |
NC |
| Jun. 06 | NC | NC |
NC |
NC |
|
NC |
NC |
| Jul. 06 | NC | 100.0 |
NC |
NC |
|
100.0 |
NC |
| Aug. 06 | 18.2 | 81.8 |
0.0 |
100.0 |
|
11.1 |
88.9 |
| Sep. 06 | NC | NC |
NC |
NC |
|
NC |
NC |
| Oct. 06 | 25.9 | 74.1 |
50.0 |
50.0 |
|
10.0 |
90.0 |
| Nov. 06 | 50.0 | 50.0 |
20.0 |
80.0 |
|
50.0 |
50.0 |
| Dec. 06 | 87.0 | 13.0 |
10.0 |
90.0 |
|
33.3 |
66.7 |
| Jan. 07 | 72.5 | 27.5 |
91.9 |
8.1 |
|
0.0 |
100.0 |
| Feb. 07 | 50.7 | 49.3 |
38.2 |
61.8 |
|
66.7 |
33.3 |
| Mar. 07 | 47.4 | 52.6 |
11.1 |
88.9 |
|
10.0 |
90.0 |
NC, not collected.
Table III.- The descriptive statistics for Matuta planipes and Ashtoret lunaris for various morphometrical variables (Sonmiani Bay, April, 2005 to March, 2007).
| Variable | Sex | No |
Mean + SD |
Range |
| Matuta planipes | ||||
| CW (cm) | F | 93 |
3.8 + 0.6 |
2.6-5.3 |
| M | 358 |
4.3 + 0.9 |
2.1-11.5 | |
| TL (cm) | F | 93 |
2.7 + 0.4 |
1.8-3.7 |
| M | 358 |
3.1 + 0.5 |
1.2-6.5 | |
| Wt. (g) | F | 93 |
9.1 + 4.2 |
2.4-21.6 |
| M | 358 |
12.9 + 5.3 |
1.3-30.9 | |
| Ch. L (cm) | F | 93 |
1.8 + 0.5 |
1.1-4.9 |
| M | 358 |
2.1 + 0.5 |
0.9-6.0 | |
| Ashtoret lunaris | ||||
| CW (cm) | F | 151 |
4.2 + 1.0 |
1.7-5.9 |
| M | 318 |
4.5 + 0.8 |
2.2-6.9 | |
| TL (cm) | F | 151 |
3.4 + 2.0 |
1.1-21.6 |
| M | 318 |
3.1 + 0.5 |
1.5-4.70 | |
| Wt. (g) | F | 151 |
11.6 + 5.4 |
2.6-27.1 |
| M | 318 |
14.8 + 6.8 |
1.7-39.9 | |
| Ch. L (cm) | F | 151 |
1.9 + 0.3 |
0.7-3.0 |
| M | 318 |
2.1 + 0.4 |
0.8-4.4 |
CW, carapace width; Ch. L, cheliped length; TL, total length of carapace; Wt. wet weight.
The significant variations were observed in the seasonal distribution of M. planipes (F3,23 = 5.76, P=0.004) but A. lunaris (F3,23= 2.62, P=0.075) showed no significant variations among seasons.
Sex ratio
In both species M. planipes and A. lunaris, males were collected in higher numbers as compared to females throughout the study period except in a few months (Table III). Total 451 specimens of M. planipes were collected in which 93 specimens were females and 358 males, whereas, 469 specimens of A. lunaris in which 151 were females and 318 were male individuals. The comparison of male and females showed that the sex ratio showed significant deviations from 1:1 ratio for the M. planipes (χ2 = 245.4, P < 0.005, DF = 15) and for A. lunaris (χ2 = 99.4, P < 0.005, DF = 18), respectively.
Size variations
In the present study two species of Matutidae showed size variations in different body parts within the species and sexes. The males of M. planipes and A. lunaris (12.9 ± 5.3g; 14.8 ± 6.8g, respectively) were heavier than the females (9.09 ± 4.2g; 11.6 ± 5.4 g, respectively). There was a significant difference in the size of male and female crabs of M. planipes (t = -4.93, P < 0.005, DF =169) and A. lunaris (t = -2.92, P < 0.005, DF = 228), respectively. In M. planipes, the total length of carapace (measured along the mid line vertically from the tip of rostrum to posterior margin of the carapace) in males (3.06 + 0.5 cm) were greater as compare to females (2.75±0.4 cm), whereas in A. lunaris males were smaller (3.16±0.5 cm) than females (3.42 ± 2.0 cm).
Length-weight relationship
The relative growth between TL and its relationship with wet wt. of the body for M. planipes and A. lunaris is presented in Table IV. In M. planipes the relative growth showed the higher value of slope ‘b’ for the male (b = 2.70) as compared to female (b = 2.64), whereas A. lunaris showed the higher value of slope ‘b’ for females (b = 2.84) as compared to males (b = 2.75). M. planipes and A. lunaris showed negative allometry for both males and females. The other morphometrical characters (CW, Ch.L) also showed the highest values in males as compared to females in both M. planipes and A. lunaris species (Table IV).
Size-weight relationship
The size and weight relationship between CW and wet wt. of the body showed negative allometry for both (M. palnipes and A. lunaris) species. The higher value of slope ‘b’ for A. lunaris was observed in females (b = 2.48) as compared to males (b = 2.32), whereas in M. palnipes higher value of slope ‘b’ were observed in males (b = 2.61) as compared to females (b = 2.35) (Table IV).
Carapace-Chela length relationship
The size-length relationship between CW and Ch.L of A. lunaris and M. planipes are presented in Table IV. The relative growth showed the negative allometry for A. lunaris and isometric allometry for M. planipes. The size-length relationship showed no difference within the sexes of A. lunaris (b = 0.84; b = 0.87) and M. planipes (b = 1.00; b = 0.91), the value of slope ‘b’ were almost same for male and female, respectively. The length-length relationship between TL and Ch.L of both species showed that the relative growth had an isometric allometry for A. lunaris and M. planipes species with higher value of slope ‘b’ in males (b = 1.07; b = 1.06) as compare to females (b = 0.98; b = 0.97), respectively. These relationships shows no difference within species ‘b’ values of males and females of both species and were nearly same.
Sexual maturity
The growth curve in juvenile and adult phases differed in all the regressions. The size at which 50% of the population of M. planipes was morphologically mature is shown in Figure 4 and of A. lunaris in Figure 5. The result of the logistic equation of A. lunaris showed that approximately 50% of male crabs were sexually mature at the size of carapace width 3.2 cm, whereas for females this proportion was found in the size of carapace width 3.3 cm. Thus, the estimated size at onset of sexual maturity was 3.2 cm and 3.3 cm
Table IV.- The regression analysis of different morphometrical parameters of two Matutidae species of Sonmiani Bay (April, 2005 to March, 2007).
| Relationship |
Species |
Sex |
N |
LogY=loga+logbX |
R2 |
F |
Allometry |
|
Length-weight TL vs. Wt.
|
Ashtoret lunaris
Matuta planipes |
F M F M |
151 318 93 358 |
= -0.298 + 2.84 xlog = -0.243 + 2.75 xlog = -0.237 + 2.64 xlog = -0.228 + 2.70 xlog |
85.6 82.1 69.6 85.8 |
742.02 1461.81 213.04 2150.61 |
-ve -ve -ve -ve |
|
Size-weight CW vs. Wt.
|
Ashtoret lunaris
Matuta planipes |
F M F M |
151 318 93 358 |
= -0.567 + 2.48 xlog = -0.405 + 2.32 xlog = -0.469 + 2.35 xlog = -0.572 + 2.61 xlog |
83.7 74.4 72.7 84.2 |
613.02 882.58 213.26 1768.08 |
-ve -ve -ve -ve |
|
Length-Length TL vs. Ch.L
|
Ashtoret lunaris
Matuta planipes |
F M F M |
151 318 93 358 |
= -0.176 + 0.984 xlog = -0.214 + 1.07 xlog = -0.184 + 0.976 xlog = -0.196 + 1.06 xlog |
83.3 73.6 72.1 83.0 |
591.75 817.23 196.22 1505.42 |
0 0 0 0 |
|
Size-Length CW vs. Ch.L
|
Ashtoret lunaris
Matuta planipes |
F M F M |
151 318 93 358 |
= -0.277 + 0.871 xlog = -0.233 + 0.841 xlog = -0.294 + 0.915 xlog = -0.315 + 1.00 xlog |
77.2 57.3 76.0 77.4 |
397.03 392.48 240.54 1057.30 |
-ve -ve 0 0 |
for males and females, respectively. In M. planipes the results of the logistic equation showed that approximately 50 % males were sexually matured at the size of the carapace width reached 3.1 cm and in females, the size was 3.2 cm. Thus the estimated size of M. planipes at the onset of sexual maturity was 3.1 cm and 3.2 cm for males and females, respectively.
DISCUSSION
This study showed the monthly distribution and abundance of M. planipes and A. lunaris in the Sonmiani Bay (Miani Hor). The two species were found almost throughout the study period varying in number in each month and showed significant seasonal variations in the distribution. Monthly and seasonal variations in species abundance may be related to oceanographic changes in the lagoon and the temperature was found to be a significant factor influence on the distribution of both species. Variations in temperature determine the spatial and temporal patterns resulting in distribution and abundance of species around the world (Thomas et al., 2000; Delaney, 2003). The temporal or spatial abundance pattern of these two co-occurring species varies among populations likely as a result of temperature changes. There was an expressive reduction in the temperature during winter that may have influenced the both species distributions, agreed with the study of De Carvalho et al. (2010), as related to the distribution of Persephona lichtensteinii and P. punctata (Brachyura, Leucosiidae) was associated with the temperature in the Brazil region. The biochemical and physiological kinetics determine the survival, growth, and reproduction of any species, which remain in conjunction of environmental temperature and probably this interactive phenomenon determines that when and where a certain species can survive and thrive (Wethey, 1983; 1984; Thomas et al., 2000; Hochachka and Somero, 2002).
The overall sex ratio was female-biased. The variations from the 1:1 ratio resulted from sexual differences in the spatial and temporal distribution and mortality of organisms (Wada et al., 2000), also dependant on differential life span, migration, longevity of each sex, food restriction, utilization of different habitats, differential production of gametes and growth rates (Wenner, 1972). Emmerson (1994) and Lardies et al., (2004) suggest that deviations from the 1:1 ratio can internally regulate the size of a population by affecting its reproductive potential. Sexual dimorphism was also observed in the present study. The males of both species attain larger sizes than females, agrees with the study of De Carvalho et al. (2010). Males attain larger size as compared to females in Persephona lichtensteinii and P. punctata. Lopez Greco et al. (2000) and Mantellato et al. (2003) suggested that females may have reduced somatic growth, compared to males because they concentrate on their energy budget for gonad development. Moreover, males may reach larger sizes for successful competition for copulation with more than one female since larger male Ocypodid crabs may have greater chances of obtaining females for copulation and win more intraspecific fights (Henmi, 1992).
In relative growth of crabs, carapace width is one of the key measurements used as an independent variable, it showed significant physiological changes that occur throughout their lifespan (Castiglioni and Negreiros-Fransozo, 2004). The relative growth in crustaceans has been studied extensively using the morphometric data (Hartnoll, 1982). In the present study CW and TL were used as an independent variable in length-weight relationship (TL - Wt.), size-weight relationship (CW-Wt.), length-length relationship (TL- Ch.L) and size-length relationship (CW- Ch.L) showed no significant difference within the sexes for both species. The slope of regression lines showed lesser difference for males and females in both species. The length-weight and length-length relationship showed positive allometry for two species except female of M. planipes that showed negative length-weight relationship, whereas size-length and size-weight relationship showed positive allometry for both male and female crabs of M. planipes whereas for male and females of A. lunaris showed negative size-length relationship. Can et al. (2007) reported a negative allometric growth pattern both in males and females of C. aestuarii. The presented length-weight and size-weight morphometrics will enable biologists to accurately estimate the wet weight and size for both species based on a single measured value of the carapace of the specimen. Weight-size relationships can provide useful information about the increase in weight of a population and this parameter could also be important for comparative studies between populations (Mori et al., 1990; Kocak et al., 2011).
Both growth and reproduction are life history mechanisms that compete for energy resources. Increase in a component is associated with a decrease in the other, characterizing the relationship known as a trade-off (Hartnoll, 1985; Haefner and Spaargaren, 1993; Llodra, 2002; Begon et al., 2007). Consequently, the resource distribution for growth and reproduction should be optimized over the life so it has a higher reproductive success. The lack of significant difference in maturation size between the sexes in both species reported in this study was also observed in Persephona species by De Carvalho et al. (2010) and P. mediterranea by Bertini et al. (2010). However, differences in W50 between males and females were recorded in several brachyuran crabs. Some species such as Callinectes ornatus (Ordway, 1863) (Mantelatto and Fransozo, 1996; Baptista et al., 2003), C. danae (Smith, 1869) (Branco and Masunari, 2000) and Goniopsis cruentata (Latreille, 1803) (Moura and Coelho, 2004), males acquire the sexual maturity at larger body size than that of females. However, Hepatus pudibundus (Herbst, 1785) (Reigada and Negreiros-Fransozo, 1999), Necora puber (Linnaeus, 1767) (González-Gurrirán and Freire, 1994) and Maja squinado (Herbst, 1788) (Sampedro et al., 1999), were not recorded for evident differences in maturity size between the sexes. Females can reach maturity at a smaller size than males when they invest more energy to egg production and males allocate more energy for somatic tissue production (Mantelatto and Fransozo, 1996).
Small changes in W50 can be caused by individual genetic variations, food availability, the presence of competitors, predators and many other factors that exist in the habitat. In contrast, the occurrence of significant variations between sexes and congeneric species may be due to differences in reproductive strategy of organisms (Hartnoll, 1985; Llodra, 2002; Begon et al., 2007).
CONCLUSIONS
The pronounced seasonal variations were observed in the distribution and abundance of M. planipes and A. lunaris and the change of temperature observed the key influential factor for these changes. The maximum size of the male of both species was larger than females, the males of both species, attain the earlier sexual maturity than females crabs as evident in most of the Brachyuran crabs. The size in which the sexual maturity is reached and how it is determined are important aspects of the biological cycle of decapods and it can be determined through the study of reproductive aspects, whereas morphometric techniques can indicate allometric changes in size related to the external morphological maturity. Crustacean would be able to mate when attain such size. Traditionally two criteria have been utilized to determine such size: the first was directly related to reproduction of gonad development, spermatheca analysis, presence of ovigerous females and spermatophores in males and the other was based on morphometric data. It was assumed by various authors that only one technique is not enough to define the size at the sexual maturity, which is basically determined by genetic information of each species and influenced by environmental factors in its distribution area.
ACKNOWLEDGEMENT
The authors are thankful for the present study as part of (Agricultural Linkage Programme-Pakistan Agriculture Research Council) ALP–PARC funded project “Status of shrimp fishery in Sonmiani Bay (Miani Hor) (Lagoon)” to NAQ that also helped to study the biodiversity of the lagoon.
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Amanat, Z. and Qureshi, N.A., 2011. Ovarian maturation stages and size at sexual maturity of Penaeus indicus (H. Milne Edwards, 1937) in the lagoon waters of Sonmiani Bay, Balochistan. Pakistan J. Zool., 43: 447-459.
Baptista, C., Pinheiro, M.A.A., Blankensteyn, A., and Borzone, C.A., 2003. Estrutura populacional de Callinectes ornatus Ordway (Crustacea, Portunidae) no Balneário Shangri-Lá, Pontal do Paraná, Paraná, Brasil. Rev. Bras. Zool., 20: 661-666. https://doi.org/10.1590/S0101-81752003000400018
Begon, M., Townsend, C.R. and Harper, J.L., 2007. Ecologia: de indivíduos a ecossistemas. 4th ed. Artmed, Porto Alegre.
Bertini, G., Fransozo, A. and Costa, R.C., 2001. Ecological distribution of three species of Persephona (Brachyura, Leucosiidae) in the Ubatuba region, São Paulo, Brazil. Nauplius, 9: 31- 42.
Bertini, G., Teixeira, G.M., Fransozo, V. and Fransozo, A., 2010. Reproductive period and size at the onset of sexual maturity of mottled purse crab Persephona mediterranea (Herst, 1794) (Brachyura: Leucosioidea) on the southeastern Brazilian coast. Inverteb. Reprod. Devel., 54: 7-17. https://doi.org/10.1080/07924259.2010.9652311
Branco, J.O. and Masunari, S., 2000. Reproductive Ecology of the blue crab, Callinectes danae Smith, 1869 in the Conceição Lagoon system, Santa Catarina Isle, Brasil. Rev. Bras. Biol., 60: 17-27. https://doi.org/10.1590/S0034-71082000000100004
Campbell, A. and Eagles, M.D., 1983. Size at maturity and fecundity of rock crabs, Cancer irroratus, from the Bay of Fundy and southwestern Nova Scotia. Fish. Bull., 81: 357-362.
Campbell, G.R. and Fielder, D.R., 1986. Size at sexual maturity and occurrence of ovigerous females in three species of commercially exploited portunid crabs in S.E. Queensland. Proc. R. Soc. Queens, 97: 79-87.
Can, E., Tiraşin, E.M., Cihangier, B. and Yilmaz, U., 2007. Weight- carapace width relationship of the Mediterranean green crab (Carcinus aestuarii, Nardo 1847) in Çakalburnu Lagoon, İzmir Bay. Rapp. Com. int. Mer Médit., 38: 443.
De Caravalho, De F.L., De Caravalho, E.A.S. and Couto, E.C.G., 2010. Comparative analysis of the distribution and morphological sexual maturity of Persephona lichtensteinii and P. punctata (Brachyuran: Leucosiidae) in Ilheus, BA, Brazil. Nauplius, 18: 109-115
Castiglioni, D.S. and Negreiros-Fransozo, M.L., 2004. Comparative analysis of the mudflat crab Uca rapax (Smith, 1870) (Brachyura: Ocypodidae) from two mangroves in Sao Pauolo, Brazil. Rev. Bras. Zool., 21: 137-144. https://doi.org/10.1590/S0101-81752004000100023
Chapgar, B.F., 1957. On the marine crabs (Decapoda: Brachyura) of Bombay State. J. Bombay Nat. Hist. Soc., 54: 399-439.
Conan, G.Y. and Comeau, M., 1986. Functional maturity and terminal molt of male snow crab, Chionoecetes opilio. Can. J. Fish. aquat. Sci., 43: 1710-1719. https://doi.org/10.1139/f86-214
Delaney, M., 2003. Effects of temperature and turbulence on the predator–prey interactions between a heterotrophic flagellate and a marine bacterium. Microb. Ecol., 45: 218–225. https://doi.org/10.1007/s00248-002-1058-4
Emmerson, W.D., 1994. Seasonal breeding cycles and sex ratios of eight species of crabs from Mgazana, a mangrove estuary in Transkei, Southern Africa. J. Crust. Biol., 14: 568–578. https://doi.org/10.1163/193724094X00137
Fatima, M., 2003. Length Weight Study of Two Species of Crabs Matuta planipes and Matuta lunaris from Karachi, Pakistan. Pak. J. biol. Sci., 6: 397-398. https://doi.org/10.3923/pjbs.2003.397.398
Fernández, L., Gonzalez-Gurriarán, E. and Freire, J., 1991. Population biology of Liocarcinus depurator (Brachyura: Portunidae) in Mussel Raft culture areas in the Ria de Arousa (Galicia, NW Spain). J. Mar. Biol. Assoc. U.K., 71: 375-390. https://doi.org/10.1017/S0025315400051651
Freire, J., Muiño, R., Fernández, L. and Gonzalez-Gurriarán, E., 1991. Life cycle of Liocarcinus arcuatus (Brachyura: Portunidae) in the Ria de Arousa (Galicia, NW Spain): role of beach and mussel raft culture areas. Mar. Ecol., 12: 193-210. https://doi.org/10.1111/j.1439-0485.1991.tb00253.x
Galil, B.S. and Clark, P.F., 1994. A revision of the genus Matuta Weber, 1795 (Crustacea: Brachyura: Calappidae). Zool. Verh., 294: 1-55.
González-Gurriarán, E. and Freire, J., 1994. Sexual maturity in the velvet swimming crab Necora puber (Brachyura, Portunidae): morphometric and reproductive analyses. ICES J. Mar. Sci., 51: 133-145. https://doi.org/10.1006/jmsc.1994.1015
Guinot, D., 1966. Les Crabes comestibles de l’Indo-Pacifique. In: Expédition française sur les récifs coralliens de la Nouvelle-Calédonie. Volume préliminaire, 2: 1-145, pls. 1-10. (Editions de la Fondation Singer-Polignac, Paris). [1966 volume, published 1967]
Haefner, P.A. and Spaargaren, D. H., 1993. Interactions of ovary and hepatopancreas during the cycle of Crangon crangon (L.) I. Weight and volume relationships. J. Crust. Biol., 13: 523-531. https://doi.org/10.2307/1548792
Haefner, P.A., 1990. Morphometry and size at maturity of Callinectes ornatus (Brachyura, Portunidae) in Bermuda. Bull. Mar. Sci., 46: 264-286.
Hartnoll, R.G., 1972. The biology of burrowing crab, Coryetes cassivelaunus. Bijdr. Dierkd., 42: 139-155
Hartnoll, R.G., 1982. Growth In: The biology of Crustacea (ed. L. G. Abele) 2: 111-196 Academic Press, New York.
Hartnoll, R.G., 1985. Growth, sexual maturity and reproductive output, pp. 101-128. In: Crustacean Issues 3: factors in adult growth (ed. A. M. Wenner). Rotterdam, Balkema. 362p.
Hazlett, B.A., 1997. The organisation of behaviour in hermit crabs: Responses to variation in stimulus strength. Behaviour, 134: 59-70. https://doi.org/10.1163/156853997X00278
Henmi, Y., 1992. Mechanism of cross shore distribution pattern of the intertidal mud crab Macrophthalmus japonicus. Ecol. Res., 7: 71–78. https://doi.org/10.1007/BF02348599
Hines, A. H., 1982. Allometric constraints and variables of reproductive effort in Brachyuran crabs. Mar. Biol., 69: 309-320. https://doi.org/10.1007/BF00397496
Hochachka, P.W. and Somero, G.N., 2002. Biochemical adaptation: Mechanism and process in physiological evolution. Oxford University Press, New York, pp. 1- 450
Hossain, M.A.R., Tanaka, M. and Masuda, R., 2002. Predator-prey interaction between hatchery-reared Japanese flounder juvenile, Paralichthys olivaceus, and sandy shore crab, Matuta lunaris: daily rhythms, anti-predator conditioning and starvation. J. exp. mar. Biol. Ecol., 267: 1-14. https://doi.org/10.1016/S0022-0981(01)00340-9
Huxley, J.S. and Richards, O.W., 1931. Relative growth of the abdomen and the carapace of the shore crab, Carcinus meanas. J. mar. Biol. Assoc. U.K., 17: 1001-1015. https://doi.org/10.1017/S0025315400052085
Koçak, C., Acarli, D., Katağan, T. and Özbek, M., 2011. Morphometric characters of the Mediterranean green crab (Carcinus aestuarii Nardo, 1847) (Decapoda, Brachyura), in Homa Lagoon, Turkey. Turk. J. Zool., 35: 551-557.
Lardies, M.A., Rojas, J.M. and Wehrtman, I.S., 2004. Breeding biology and population structure of the intertidal crab Petrolisthes laevigatus (Anomura: Porcellanidae) in central-southern Chile. J. nat. Hist., 38: 375–388. https://doi.org/10.1080/0022293021000016543
Llodra, E.R., 2002. Fecundity and life-history strategies in marine invertebrates. Adv. mar. Biol., 43: 88-170.
Lopez Greco, L.S., Hernandez, J.E., Bolanos, J.R. and Hernandea, G., 2000. Population features of Microphrys bicornutus Latreille, 1825 (Brachyura, Majidae) from Isla Margarita, Venezuela. Hydrobiologia, 439: 151–159. https://doi.org/10.1023/A:1004130621093
Mantelatto, F.L.M. and Fransozo, A., 1996. Size at sexual maturity in Callinectes ornatus (Brachyura, Portunidae) from the Ubatuba Region (SP), Brazil. Nauplius, 4: 29-38.
Mantelatto, F.L.M., Faria, F.C.R. and Garcia, R.B., 2003. Biological aspects of Mithraculus forceps (Brachyura: Mithracidae) from Anchieta Island, Ubatuba, Brazil. J. mar. Biol. Assoc. U. K., 83: 789–791. https://doi.org/10.1017/S0025315403007811h
Mori, M., Manconi, R. and Fanciulli, G., 1990. Notes on the reproductive biology of Carcinus aestuarii Nardo (Crustacea, Decapoda) from the Lagoon of Dan Teodoro (Island of Sardinia, Italy). Rivist. Idrobiol., 29: 763-774.
Moura, N.F.O. and Coelho, P. A., 2004. Maturidade sexual fisiologica em Goniopsis cruentata (Latreille) (Crustacea, Brachyura, Grapsidae) no estuario do Paripe, Pernambuco, Brasil. Rev. Bras. Zool., 21: 1011.1015.
Perez, O. S., 1990. Reproductive biology of the sandy shore crab Matuta lunaris (Brachyura: Calappidae). Mar. Ecol. Prog. Ser., 59: 83-89. https://doi.org/10.3354/meps059083
Pinheiro, M.A.A. and Fransozo, A., 1998. Sexual maturity of speckled swimming crab Arenaeus cribrarius (Lamark, 1818) (Decapoda, Brachyura, Portunidae), in the Ubatuba litoral, São Paulo State, Brazil. Crustac. Int. J. Crust. Res.,71:434-452.
Prasad, P.N. and Neelakantan, B., 1990. Size at maturity in the male crab Scylla serrata as determined by chela allometry and gonad condition. Fish. Tech., 21: 25-29.
Qureshi, N.A. and Kazmi, Q.B., 1999. Allometric relationship of Morphometric characters in Scizophrys pakistanensis, Tirmizi and Kazmi, 1995 (Decapoda: MAjidae) from Karachi coast, Pakistan. Pak. J. mar. Sci., 8: 171-182.
Rasool, F., Tunio, S., Hasnian, S.A. and Ahmad, E., 2002. Mangrove conservation along the coast of Sonmiani, Balochistan, Pakistan. Pakistan J. Zool., 16: 213-217. https://doi.org/10.1007/s00468-001-0151-5
Reigada, A.L.D. and Negreiros-Fransozo, M. L., 1999. Maturidade sexual em Hepatus pudibundus (Decapoda, Brachyura, Calappidae). Iheringia, Série Zoologia, Porto Alegre., 86: 159-164.
Richmond, M.D., 1997. A guide to the seashores of Eastern Africa and the Western Indian Ocean Islands. Sida / Department for Research Cooperation, SAREC. Zanzibar, Tanzania. pp. 448.
Saher, N.U. and Qureshi, N. A., 2011. Relative growth and morphological sexual maturity of Ilyoplax frater (Brachyura: Ocypodoidea: Dotillidae) from mangrove area of Korangi creek. Pakistan J. Zool., 43: 133-140.
Saifullah, S.M. and Rasool, F., 1995. A preliminary survey of mangroves of Balochistan. WWF Pakistan Project Report. pp. 1-99.
Saitoh, K., Takagaki, M. and Yamashita, Y., 2003. Detection of Japanese flounder-specific DNA from gut contents of potential predators in the field. Fish. Sci., 69: 473-477. https://doi.org/10.1046/j.1444-2906.2003.00647.x
Sakai, T., 1965. On two genera and five species of xanthid crabs from the collections of His Majesty the Emporer of Japan made in Sagami Bay. Crustac. Int. J. Crust. Res., 8: 97-106.
Sampedro, M.P., González-Gurriarán, E., Freire, J. and Muino, R., 1999. Morphometry and sexual maturity in the spider crab Maja squinado (Decapoda: Majidae) in Galicia, Spain. J. Crust. Biol., 19: 578-592. https://doi.org/10.2307/1549263
Sankarankutty, C., 1962. On Decapoda Brachyura from the Andaman and N~cobar Islands 3. Families: Calappidae, Leucosiidae, Parthenopldae. Maiidae and Geocarcinidae. J. mar. Boil. Assoc. India, 4: 151-164.
Shukla, M.L., Patel, B.K., Trivedi, J.N. and Vachhrajani, K.D. 2013. Brachyuran crabs diversity of Mahi and Dhadhar Estuaries, Gujarat, India. Res. J. mar. Sci., 1: 8-11.
Tan, L.W.H. and Ng, P.K.L., 1988. A guide to seashore life. The Singapore Science Centre, Singapore, pp. 160.
Teissier, G., 1960. Relative growth. In: The Physiology of the Crustacea (ed. T.H. Waterman). Academic Press, New York, pp. 357-560. https://doi.org/10.1016/b978-0-12-395628-6.50022-1
Thomas, C.W., Crear, B.J. and Hart, P.R., 2000. The effect of temperature on survival, growth, feeding and metabolic activity of the southern rock lobster, Jasus edwardsii. Aquaculture, 185: 73-84. https://doi.org/10.1016/S0044-8486(99)00341-5
Thomassin, B.A., 1974. Soft bottom carcinological fauna (sensu lato) on Tulear coral reef complexes (S.W. Madagascar): Distribution, importance, roles played in trophic food chains and in bottom deposits. Proc. Sec. into Cor. Reef Symp., I: 297-300.
Tirmizi, N.M. and Kazmi, Q.B., 1986. Marine fauna of Pakistan: 4 Crustacea: Brachyura (Dromiacea, Archaeobrachyura, Oxystomata, Oxyryncha). Publication 1. BCCI foundation chair Institute of Marine Sciences, University of Karachi. pp. 1-244.
Vannini, M., 1976. Researches on the coast of Somalia: the shore and dune of Sar Uanle. 10. Sandy beach decapods. Monitore zool. Ital., 8: 255-286.
Varadhranjan, D., Pushparanjan, N. and Soundarapandian, P., 2012. First record of Portunid crabs from Pondicherry coast, South East Coast of India. Int. J. Pharma. Biol. Arch., 3: 1255-1257.
Vazzoler, A.E.A.M., 1996. Reproduction biology of teleostean fishes: theory and practice. Maringa, EDUEM, Brazilian Society of Ichthyology, pp. 169 (In Portuguese).
Wada, S., Ashidate, M., Yoshino, K., Sato, T. and Goshima, S., 2000. Effects of sex ratio on egg extrusion and mating behavior of the spiny king crab Paralithodes brevipes (Decapoda: Lithodidae). J. Crust. Biol., 20: 479–482. https://doi.org/10.1651/0278-0372(2000)020[0479:EOSROE]2.0.CO;2
Wenner, A.M., 1972. Sex ratio as a function of size in marine Crustacea. Am. Nat., 106: 321–350. https://doi.org/10.1086/282774
Wenner, A.M., Fusaro, C. and Oaten, A., 1974. Size at onset of sexual maturity and growth rate in crustacean populations. Can. J. Zool., 52: 74-147. https://doi.org/10.1139/z74-147
Wethey, D.S., 1983. Geographic limits and local zonation: the barnacles Semibalanus (Balanus) and Chthamalus in New England. Biol. Bull., 165: 330–341. https://doi.org/10.2307/1541373
Wethey, D.S., 1984. Sun and shade mediate competition in the barnacles Chthamalus and Semibalanus: a field experiment. Biol. Bull., 167: 176–185. https://doi.org/10.2307/1541346
Weymouth, F. W. and Mackay, D.C.G., 1936. Analysis of the relative growth of the pacific edible crab. Cancer Magist., 106: 257-280.
To share on other social networks, click on any share button. What are these?










