Dietary Effect of Zinc on Growth Performance, Carcass Characteristics and Immune Response in Hubbard Broilers Raised under Hot Climate
Research Article
Dietary Effect of Zinc on Growth Performance, Carcass Characteristics and Immune Response in Hubbard Broilers Raised under Hot Climate
Safdar Hassan1, Shaukat Ali Bhatti1, Fawwad Ahmad1, Asad Ullah Hyder1, Muhammad Arslan2, Ashar Mehfooz3, Ijaz Saleem3, Muhammad Umar Yaqoob4,5, Mushrraf Nazir1, Muhammad Sharif1*
1Institute of Animal and Dairy Sciences, University of Agriculture Faisalabad, Pakistan; 2Department of Poultry Science, Cholistan University of Veterinary and Animal Sciences, Bahawalpur, Pakistan; 3Department of Clinical Medicine and Surgery, University of Agriculture, Faisalabad; 4Provincial Key Agricultural Enterprise Research Institute of King Techina, Hangzhou King Techina Feed Co., Ltd., Hangzhou 311107, China; 5College of Animal Science, Zhejiang University, Hangzhou 310058, China.
Abstract | Broilers may not have same dietary zinc requirements under temperate climate conditions. This experiment was conducted to evaluate the effect of super dosing of dietary Zn (above National Research Council recommended: 40 mg/kg) on broilers’ growth performance, carcass characteristics, and immune response under raised temperature. A basal corn-soy diet (Zn-40, crude protein (CP): 20%; metabolizable energy (ME) : 3000 Kcal/Kg) was formulated to contain 40 mg/kg Zn. The basal diet was supplemented with three super doses viz; 25, 50, or 75% of NRC recommended (1994) level and then these diets (Zn-50; Zn-60; Zn-70) contained 50, 60, and 70 mg/kg Zn, respectively. Two hundreds day-old broiler chicks were used for this study. Each diet was fed to a group of 50 broilers (Hubbard, BW 40±3 g, mixed male and female) divided into 5 replicates from day 1 to 35 for their life, raised under the temperature of 34 ± 1.3 °C. Results revealed that FCR was reduced (P=0.006) in Zn-50 than in Zn-70, but it was similar to other groups during the starter phase. The highest feed intake (P=0.007) and body weight gain (P=0.013) were observed in Zn-70. Overall data showed that body weight gain was significantly (P=0.006) improved in all Zn-supplemented groups than in the control group. Significantly higher (P=0.027) dressing percentage and lower (P=0.028) abdominal fat rate were observed in Zn-50 than the control group. Results showed that weight of thymus and bursa were increased with supplementation of Zn (70 mg/kg) in diet. Optimal broilers’ performance requires a higher dietary Zn (70 mg/kg) concentration than NRC recommended under high-temperature climatic conditions.
Keywords | Broiler, Zinc, Growth performance, ND titer, Carcass characteristics, High temperature zone.
Received | February 05, 2024; Accepted | March 11, 2024; Published | March 20, 2024
*Correspondence | Muhammad Sharif, Institute of Animal and Dairy Sciences, University of Agriculture Faisalabad, Pakistan; Email: drsharifuaf@gmail.com
Citation | Hassan S, Bhatti SA, Ahmad F, Hyder AU, Arslan M, Mehfooz A, Saleem I, Yaqoob MU, Nazir M, Sharif M (2024). Dietary effect of zinc on growth performance, carcass characteristics and immune response in hubbard broilers raised under hot climate. J. Anim. Health Prod. 12(1): 78-84.
DOI | http://dx.doi.org/10.17582/journal.jahp/2024/12.1.78.84
ISSN | 2308-2801

Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
In 1958 zinc (Zn) was reported as an essential mineral, because it s deficiency caused different problems like retarded growth leg abnormalities, and poor feathering in broilers (Nielsen, 2012).
It has a critical role to support the growth and immune functions (Hassan et al., 2020), and skeletal improvement (Tomaszewska et al., 2017) in broilers. NRC recommended 40 mg/kg as an optimum dietary concentration of Zn for broilers (Hidayat et al., 2020). At the same time, other reports suggested that higher Zn concentration (> 40mg/kg) could help strengthen the immune system in broilers without any harmful effects on growth performance (Sajadifar et al., 2011). However, another study suggested that a dietetical Zn concentration of 40mg/kg from either organic or nano sources is sufficient for broilers’ growth requirements under high environmental temperature (Akhavan-Salamat and Ghasemi, 2019).
But, in tropical countries, broiler production remains sub-optimal as shown by decreased weight gains, higher FCR, poor immune responses and high death numbers, apparently this is all because of high environmental temperature and humidity present in these areas. It shows that zinc supplementation may be more significant in the high environmental temperature zones in order to curtail the negative effects related with such condition (Hidayat et al., 2020).
Corn and soybean meal are the main ingredients used for poultry diet formulation throughout the world, which are also being used by most of the researchers in different poultry nutrition experiments. A corn-soy diet also contains higher levels of an anti-nutritional factor named phytate complex that binds some minerals, including Zn (Hassan, 2024). It makes them biologically unavailable for poultry birds, which suggests super dosing of Zn in such a diet. Higher dietary Zn levels (100-200 mg/kg) have been observed to enhance the humoral immunity in broilers against the Newcastle disease vaccine (Sajadifar et al., 2011; Ezzati et al., 2013). Deficiency of Zn can induce thymic atrophy, decrease adaptive and innate immune responses and antibody secretion of T and B cells (Hidayat et al., 2020). Zinc also metabolizes nutrients like carbohydrates, proteins, and fats and it has a role in the utilization of nutrients (Saenmahayak, 2007; Mohammadi et al., 2015). In broilers fed diet having 90 mg/kg Zn enhanced the weight of immune organs (Feng et al., 2010). However, such a response was observed in broiler breeders fed 100 mg/kg Zn (Soni et al., 2013). Higher dietary Zn levels than recommended by NRC-1994 showed conflicting results regarding production performance and immunity related parameters in broilers. Therefore, there is a need to investigate further the effects of dietary Zn supplementation on immunity-related functions in broilers. The preferred Zn source for broilers’ diet is zinc sulfate due to its low cost and availability (Ezzati et al., 2013). Each dietary nutrient has different response criteria, making it difficult to assess the maximum performance of the animals. It is of great importance to define suitable response parameters. In most previous experiments (Ahmadi et al., 2013; Chitithoti et al., 2012), only growth performance was studied, and scarcely considered immune competence. Therefore, this experiment was conducted to examine the response of broilers on Zn supplemented diets at raised environmental temperature.
MATERIALS AND METHODS
The protocol of this experiment was approved by the Graduate Study and Research Board, University of Agriculture, Faisalabad, Pakistan (DGS/15775-78; dated: 10/03/2016) for ethical use and welfare of experimental birds.
Two hundreds day-old male broiler chicks (Hubbard, BW 40 ± 3.0 g) were divided into four treatments of 5 replicates (having 10 birds per replicate). Bird’s room, drinkers, feeders, and feeding trays were cleaned, disinfected, and fumigated before the arrival of chicks. Chick paper was used in brooding to avoid litter intake. Birds were vaccinated according to the local schedule. Thermometers and hygrometers were installed to observe environmental temperature and humidity. Diets and fresh water were available throughout the experiment al period, and the experiment was conducted for 35 days, under high temperature 34 ± 1.3 °C environment.
A basal diet with crude protein of 20.00 % and metabolizable energy of 3000 Kcal/Kg (Table 1) was formulated. Zinc contents of corn and soybean meal were analyzed before feed formulation. Premix used in diets was free from Zn. The concentration of Zn was maintained at 40 mg/kg in the basal diet (Zn-40) to meet NRC recommendations (1994) (Council, 1994) for broilers. ZnSO4 (mono-hydrate) was used as a source of Zn, and its concentration in the other diets was 50, 60, and 70 mg/kg (Zn-50, Zn-60, and Zn-70). Zinc was analyzed through wet digestion followed by atomic absorption spectrophotometry (Perkin, 1982) in all experimental diets after formulation (Table 2).
The initial body weight of chicks was recorded followed by weekly body weight recording. The amount of feed offered and the refusal was collected to record the amount of feed intake . The corrected feed conversion ratio (FCR) was calculated from weight gain, feed intake, and mortality data. Blood samples (2 birds/replicate) were collected from jugular vein on the 17th and 35th days of experiment to determine antibody titer levels with a standard haemagglutination inhibition test, against Newcastle disease (Allan and Gough, 1974). Data on the carcass, giblet organ, and immune organ weights were recorded by slaughtering two birds from each replicate at 35th day of the experiment. Relative breast, thigh, wings, giblet and immune organ weights were calculated as % of carcass weight. Polythene sheets were placed in pens for excreta collection on the 33rd day of the experiment for determination of Zn retention. Zinc concentration in feed and excreta samples was analyzed through wet digestion followed by Atomic Absorption Spectrophotometry (Hitachi Polarized Zeeman AAS, Z-8200, Japan), (Perkin, 1982). Zn retention was calculated from the data of feed intake, fecal output, and their Zn contents using the following equation:
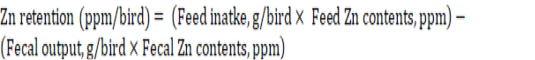
Table 1: Composition of basal diet
| Items | Concentration |
| Ingredients (%) | |
| Maize | 67.10 |
| Soy bean meal | 27.40 |
| Lime-stone | 1.40 |
| Di-calcium phosphate | 1.80 |
|
DL-Methionine |
0.20 |
| L-Lysine HCL | 0.40 |
| Vegetable oil | 1.20 |
| *Premix | 0.50 |
| Total | 100.00 |
| Calculated nutrients (%) | |
| Metabolizeable energy (kcal/kg) | 3000.00 |
| Crude protein | 20.00 |
| Ether extract | 6.10 |
| Crude fiber | 3.40 |
| Available Phosphorous | 0.45 |
| Calcium | 1.07 |
| Sodium | 0.18 |
| Potassium | 0.78 |
|
Chlorine |
0.20 |
| Dig. Lys. | 1.05 |
| Dig. Met | 0.46 |
| Zinc (mg/kg) | 40.00 |
* Zinc free vitamins and minerals premix provides per kg of diet: Vitamins; A 103 IU, E 11.0 IU, K 1.1 mg, D3 1100 IU, Riboflavin (B2) 5 mg, Ca Pantothenate (B5) 12 mg, Cyanocobalamin (B12) 12.1 μg, Pyridoxine (B6) 2.2 mg, Thiamin (B1) 2.2 mg, Nicotinic acid (B3) 44 mg, Folic acid 1.55 mg, Choline chloride 250 mg, d-biotin 0.11 mg. I 0.3mg, Mn 60 mg, Fe 30 mg, Co 0.1 mg, Cu 5 mg and Se 1 mg.
Table 2: Zinc concentration in diets
| Diets | Calculated (mg/kg) | Analyzed (mg/kg) |
| Zn-40 | 40 | 39 |
| Zn-50 | 50 | 48 |
| Zn-60 | 60 | 59 |
| Zn-70 | 70 | 71 |
Statistical Analysis
Data collected for all treatments were analyzed through one-way analysis of variance using the general linear model procedure of statistical analysis system . To determine the difference between the mean values, Tukey’s test was performed (P<0.05). In addition, linear and quadratic trends were determine d through polynomial contrast analysis (Steel and Torrie, 1960).
RESULTS
During the starter phase (1-21 days), the lowest (P=0.006) FCR was observed in Zn-50, and other parameters were not affected during this phase. During the finisher phase (22-35 days), a significant effect of varying Zn levels was observed on body weight gain (P=0.013) and feed intake (P=0.007). During this phase, weight gain and feed intake of broilers in Zn-70 were significantly higher (P<0.05) than those of the control group (Zn-40). Overall data showed that the weight gain of broilers in all Zn-supplemented groups was higher (P=0.006) than the control group, but there was no statistical difference among them (Table 3).
Dressing percentage was increased (P<0.05) in birds by feeding higher dietary Zn levels than NRC recommendations. However, no significant effect (P>0.05) was observed on other parameters of carcass characteristics, but abdominal fat was reduced (p<0.05) as Zn supplementation was increased as shown in Table 4.
The relative weight of the thymus and bursa was significantly (P<0.05) affected by dietary treatments. However, the relative weight of the spleen and HI titer levels against Newcastle disease remained unaffected (Table 5). Broilers
Table 3: Effect of dietary Zn supplementation on growth performance in broilers
| Parameters | Treatments | SEM | ANOVA | Linear | Quadratic | |||
| Zn-40 | Zn-50 | Zn-60 | Zn-70 | |||||
| Starter phase (0-3 weeks) | ||||||||
| Feed intake (g) | 1,248.68 | 1,208.76 | 1,242.98 | 1,368.92 | 39.6 | 0.055 | 0.040 | 0.052 |
| Body weight (g) | 399.92 | 442.24 | 418.92 | 414.35 | 15.1 | 0.290 | 0.771 | 0.140 |
| Feed conversion ratio |
3.15ab |
2.74b |
2.97ab |
3.32a |
0.14 | 0.006 | 0.255 | 0.018 |
| Finisher phase (4-5 weeks) | ||||||||
| Feed intake (g) |
1565.72b |
1760.88ab |
1732.44ab |
1863.76a |
73.1 | 0.007 | 0.017 | 0.978 |
| Body weight (g) |
602.75b |
745.01ab |
798.16a |
805.55a |
42.3 | 0.013 | 0.003 | 0.131 |
| Feed conversion ratio | 2.70 | 2.37 | 2.19 | 2.34 | 0.17 | 0.210 | 0.106 | 0.164 |
| Overall period (0-5 weeks) | ||||||||
| Feed intake (g) | 2,748.92 | 2,911.85 | 2,975.42 | 3,062.32 | 0.08 | 0.087 | 0.013 | 0.643 |
| Body weight (g) |
1011.58b |
1187.25 a |
1216.10 a |
1228. 20a |
0.04 | 0.006 | 0.002 | 0.064 |
| Feed conversion ratio | 2.74 | 2.46 | 2.45 | 2.51 | 0.1 | 0.190 | 0.148 |
0.113 |
Zn-40, Zn-50, Zn-60, Zn-70 conatined 40, 50, 60 and 70 mg/kg Zn per kg diets, respectively. Different superscripts with means values showed significant difference (P<0.05).
Table 4: Effect of dietary Zn supplementation on carcass characteristics in broilers
| Parameters | Treatments | SEM | P-value | |||
| Zn-40 | Zn-50 | Zn-60 | Zn-70 | |||
| Carcass characteristics (%) | ||||||
| Dressing percentage |
55.96 b |
59.80 a |
59.66ab |
57.48ab |
0.93 | 0.027 |
|
Breast yield |
35.80 | 36.70 | 36.50 | 38.20 | 1.17 | 0.560 |
| Thigh yield | 17.60 | 16.50 | 16.8 | 18.20 | 0.56 | 0.170 |
| Wings | 10.60 | 9.71 | 9.81 | 9.36 | 0.44 | 0.270 |
| Heart | 1.30 | 0.93 | 0.98 | 1.05 | 0.08 | 0.059 |
| Liver | 2.95 | 2.72 | 2.89 | 2.85 | 0.17 | 0.210 |
|
Gizzard |
4.83 | 4.35 | 4.40 | 4.42 | 0.20 | 0.880 |
| Abdominal fat pad |
2.67a |
1.67b |
2.22ab |
2.23ab |
0.21 |
0.028 |
Zn-40, Zn-50, Zn-60, Zn-70 conatined 40, 50, 60 and 70 mg/kg Zn per kg diets, respectively. Different superscripts with means values showed significant difference (P<0.05).
Table 5: Effect of varying dietary Zn levels on immune response in broilers
| Parameters (%) | Treatments | SEM | P-value | |||
| Zn-40 | Zn-50 | Zn-60 | Zn-70 | |||
| Immune organs indexes (%) | ||||||
| Spleen | 0.003 | 0.003 | 0.004 | 0.005 | 0.001 | 0.053 |
| Thymus |
0.014b |
0.014b |
0.014b |
0.023a |
0.002 | 0.010 |
| Bursa |
0.006b |
0.005b |
0.004b |
0.01a |
0.001 | 0.010 |
|
HI titer levels (log2) against Newcastle disease |
||||||
|
17th day |
7.1 | 7.1 | 6.7 | 7.6 | 0.334 | 0.330 |
|
35th day |
1.3 | 1.1 | 2.3 | 1.6 | 0.440 |
0.280 |
Zn-40, Zn-50, Zn-60, Zn-70 conatined 40, 50, 60 and 70 mg/kg Zn per kg diets, respectively. Different superscripts with means values showed significant difference (P<0.05).
in Zn-70 group had the highest relative weight of bursa (P=0.010) and thymus (P=0.010).
Results showed that Zn-intake, Zn-excretion, and Zn-retention were significantly affected by dietary treatments (Figure 1). Zn-intake was increased (P<0.001) by increasing the supplementation levels of Zn in diet, and the highest Zn intake was observed in Zn-70 group. Zn excretion in Zn-70 was higher (P<0.05) than in lower dietary Zn level groups (Zn-40 and Zn-50). Zn retention in Zn-60 and Zn-70 was higher (P=0.002) than in Zn-40.
DISCUSSION
Growth Performance
Zinc supplementation revealed variable effects on broiler growth performance in this study. In tropical countries, broiler production is usually suboptimal as shown by decreased weight gains and higher FCR. Apparently this is all because of high environmental temperature and humidity present in these areas (Hidayat et al., 2020). In current study, overall performance of birds was poor due to high ambient temperature of open house in which trial was conducted but it was similar for all the experimental units. Some studies have reported no effect of increasing Zn levels on feed intake, weight gain (Liu et al., 2011), and FCR (Ezzati et al., 2013). However, many studies have reported contradictory findings that feed intake (Ezzati et al., 2013), weight gain (Mohanna and Nys, 1999), and FCR (Bao et al., 2007) were improved in broilers in response to Zn supplementation. A previous experiment on broilers showed that different dietary Zn levels were similar regarding growth performance in broilers starter, finisher and overall phases under heat stress (Akhavan-Salamat and Ghasemi, 2019) . Variation in the results could be due to various factors, including dietary Zn levels, its source (organic, inorganic or nanoparticles), Zn level in basal diet (Hassan, 2024) , response criteria, and type of birds used in the experiment . In addition, NRC (1994) (Council, 1994) recommendation for dietary Zn were obtained by using purified or semi-purified diets (egg white, casein-dextrose and synthetic amino acids) such as used by (Hassan, 2016). It may not show the same results when the same Zn levels are used in corn-soybean diets because of the presence of different anti-nutritional factors. Differences in the type of birds (strains of broiler) and criteria chosen may offer possible explanations for variable findings observed across experiments.
Carcass Characters
Zinc supplementation could increase the percentage of eviscerated yield (Liu et al., 2011). Experiments on steers stated that increasing Zn concentration in diet could increase steers’ yield grade and quality grade (Spears and Kegley, 2002). Similarly, (Malcolm-Callis et al., 2000) stated that supplementation of Zn in diet could increase the yield grade of steers. An increase in dressing percentage by increasing dietary Zn levels could be due to higher body weight gain in broilers. As Zn is the component of insulin growth hormone, and supplementation of Zn could enhance the production or working of enzymes, enhancing the weight gain and dressing percentage. Organs and wing weights were not influenced by varying Zn concentrations that agreed with previous results (Mohammadi et al., 2015).
Immune Response
A previous study reported the increased weight of the thymus and bursa by dietary supplementation (90 mg/kg) of Zn (Feng et al., 2010). However, another study reported that the weights of immune organs in broilers was not affected by feeding higher dietary Zn concentration (Mohammadi et al., 2015); results might be inconsistent due to high environmental temperature (35 °C) in the present study. The HI titer levels against Newcastle disease were not affected by dietary Zn levels as reported in the previous experiment (Bao et al., 2007), which might be due to low dietary Zn concentration (30-60 mg/kg) in the previous and present studies (35-70 mg/kg), as Zn consumed by the birds are preferentially used to support the essential metabolic functions and growth performance and limited is available for the immune system. Zn is critical for the immune system as its deficiency could reduce the development of immune organs or the population of mature T lymphocytes in blood (Cui et al., 2004). In broilers, lymphoid organs are the immune system’s structural and functional components, which protect the body from invading microorganisms. The Zn is required for structural development and proper functionality of immune organs in broilers (Kidd et al., 1996; Hidayat et al., 2020). It might be the reason of increase in the weight of lymphoid organs in groups fed higher amounts of Zn. Similarly, another study reported thymic atrophy, decrease innate and adaptive immune and antibody secretion of B and T cells at lower dietary Zn levels (Hidayat et al., 2020).
Zinc Retention
Present results of Zn retention are supported by previous findings that extra supplementation of Zn increases its excretion and retention (Kidd et al., 1996; Burrell et al., 2004; Zhang et al., 2018). Previous experiment stated that 80 ppm was the threshold level for Zn deposition in broilers; exceeding dietary Zn levels beyond this value might increase excessive excretion of Zn and reduce its deposition (Zhang et al., 2018). In the present study, dietary Zn levels were within the threshold level, which might be the reason for significantly increased Zn retention with dietary supplementation of Zn in broilers.
CONCLUSION
Supplementation of dietary zinc (70 mg/kg) could enhance the body weight gain, dressing percentage, and immune organ weights in broilers under higher environmental temperature (34±1.3 °C), possibly due to enhanced zinc retention, without adversely affecting carcass characteristics. Zinc deposition in bones and different other organs should also be studied in future research.
Acknowledgments
Provision of Zinc free vitamin-mineral pre-mix by Mukhtar Feeds (Pvt.) Ltd., Samundri is thankfully acknowledged.
Conflict of Interest
No conflicts of interest.
novelty statement
This article covers the estimation of Zinc requirements for Hubbard Broilers strain rearing under (local) hot climate along with their immune parameters.
Authors’ Contribution
SH, SAB and FA planned and designed the experiments. SH conducted the feeding trial. SAB supervised the experiments. SAB and AUH and MUY performed the statistical analysis. MA, MN and AM did proof reading and editing. MS did correspondence.
References
Ahmadi F, Ebrahimnezhad Y, Maheri Sis N, Ghalehkandi JG (2013). The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid, concentrations in broiler chickens during starter period. Int. J. Biosci. 3:23-29. http://dx.doi.org/10.12692/ijb/3.7.2329
Akhavan-Salamata H, Ghasemib HA (2019). Effect of different sources and contents of zinc on growth performance, carcass characteristics, humoral immunity and antioxidant status of broiler chickens exposed to high environmental temperatures. Livest. Sci. 223: 76-83. https://doi.org/10.1016/j.livsci.2019.03.008
Allan WH, Gough RE (1974). A standard Haemagglutination Inhibition test for Newcastle disease. A comparison of macro and micro methods. Vet. Rec. 95:120-123.
Bao Y, Choct M, Iji P, Bruerton K (2007). Effect of organically complexed copper, iron, manganese, and zinc on broiler performance, mineral excretion, and accumulation in tissues. J. Appl. Poult. Res. 16: 448-455. https://doi.org/10.1093/japr/16.3.448
Burrell A, Dozier W, Davis A, Compton M, Freeman M, Vendrell P, Ward T (2004). Responses of broilers to dietary zinc concentrations and sources in relation to environmental implications. Brit. Poult. Sci. 45: 225-263. https://doi.org/10.1080/00071660410001715867
Council NR (1994). Nutrient requirements of poultry: National Academies Press USA.
Cui H, Xi P, Junliang D, Debing L, Guang Y (2004). Pathology of lymphoid organs in chickens fed a diet deficient in zinc. Avian Pathol. 33: 519-524. https://doi.org/10.1080/03079450400003528
Edwards H, Baker D (2000). Zinc bioavailability in soybean meal. J. anim. Sci. 78: 1017-1021. https://doi.org/10.2527/2000.7841017x
Feng J, Ma W, Niu H, Wu X, Wang Y, Feng J (2010). Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace Elem. Res. 133: 203-211. https://doi.org/10.1007/s12011-009-8431-9
Hassan S (2024). Effect of zinc nanoparticles on egg production, egg quality, bone mineralization and antioxidant capacity in caged layers. PhD dissertation, University of Agriculture, Faisalabad, Pakistan.
Hassan S, Hassan F, Rehman MS (2020). Nano-particles of trace minerals in poultry nutrition: potential applications and future prospects. Biol. Trace Elem. Res. 195:591–612. https://doi.org/10.1007/s12011-019-01862-9
Hidayat C, Jayanegara A, Wina E (2020). Effect of zinc on the immune response and production performance of broilers: a meta-analysis. Asian-Australas. J. Anim. Sci. 33(3): 465-479. https://doi.org/10.5713/ajas.19.0146
Kidd M, Ferket P, Qureshi M (1996). Zinc metabolism with special reference to its role in immunity. World’s Poult. Sci. J. 52: 309-324. https://doi.org/10.1079/WPS19960022
Liu Z, Lu L, Li S, Zhang L, Xi L, Zhang K, Luo X (2011). Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult. Sci. 90: 1782-1790. https://doi.org/10.3382/ps.2010-01215
Malcolm-Callis K, Duff G, Gunter S, Kegley E, Vermeire D (2000). Effects of supplemental zinc concentration and source on performance, carcass characteristics, and serum values in finishing beef steers. J. Anim. Sci. 78: 2801-2808. https://doi.org/10.2527/2000.78112801x
Mohammadi F, Ahmadi F, Andi MA (2015). Effect of zinc oxide nanoparticles on carcass parameters, relative weight of digestive and lymphoid organs of broiler fed wet diet during the starter period. Intl. J. Biosci. 6: 389-394. https://doi.org/10.12692/ijb/6.2.389-394
Mohanna C, Nys Y (1999). Effect of dietary zinc content and sources on the growth, body zinc deposition and retention, zinc excretion and immune response in chickens. Brit. Poult. Sci. 40: 108-114. https://doi.org/10.1080/00071669987926
Nielsen F (2012). History of zinc in agriculture. Adv. Nutr. 3: 783–789. https://doi.org/10.3945/an.112.002881
Perkin E (1982). Analytical methods for atomic absorption spectrophotometry. Perkin Elmer Corporation, Norwalk, Connecticut, USA.
Roberson RH, Schaible PJ (1958). Zinc requirement of the chick. Science. 127: 875-876. https://doi.org/10.1126/science.127.3303.875
Saenmahayak B (2007). Complexed trace mineral supplementation of broiler diets, PhD dissertation.
Sajadifar S, Miranzadeh H, Moazeni M (2011). Immune responses of broiler chicks supplemented with high levels of zinc. J. Anim. Feed Res. 6: 493-496.
Soni N, Mishra SK, Swain R, Das A, Chichilichi B, Sethy K (2013). Bioavailability and immunity response in broiler breeders on organically complexed zinc supplementation. Food Nutr. Sci. 4: 1293. https://doi.org/10.4236/fns.2013.412166
Spears J, Kegley E (2002). Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80: 2747-2752. https://doi.org/10.2527/2002.80102747x
Tomaszewska E, Muszyński S, Dobrowolski P, Kwiecień M, Winiarska-Mieczan A, Świetlicka I, Wawrzyniak A (2017). Effect of zinc level and source (zinc oxide vs. zinc glycine) on bone mechanical and geometric parameters, and histomorphology in male Ross 308 broiler chicken. Braz. J. Poult. Sci. 19: 159-170. https://doi.org/10.1590/1806-9061-2016-0285
Wedekind K, Lewis A, Giesemann M, Miller P (1994). Bioavailability of zinc from inorganic and organic sources for pigs fed corn-soybean meal diets. J. Anim. Sci. 72: 2681-2689. https://doi.org/10.2527/1994.72102681x
Zhang T, Liu J, Zhang J, Zhang N, Yang X, Qu H, Xi L, Han J (2018). Effects of dietary zinc levels on the growth performance, organ zinc content, and zinc retention in broiler chickens. Braz. J. Poult. Sci. 20: 127-132. https://doi.org/10.1590/1806-9061-2017-0604
To share on other social networks, click on any share button. What are these?






