Development of Colloid Gold-Based Lateral Flow Immunochromatographic Kits for Screening and Rapid Detection of Beta-Lactams Antibiotic Residues in Dairy Milk
Research Article
Development of Colloid Gold-Based Lateral Flow Immunochromatographic Kits for Screening and Rapid Detection of Beta-Lactams Antibiotic Residues in Dairy Milk
Sara Mohamed Hemeda1, R.H. Sayed2, Hani Hassan1, Sheima A.E.2, Hassan Aboul-Ella3, R. Soliman3*
1Institute for Animal Reproduction, Ministry of Agriculture, Egypt; 2Institute of animal vaccines and serum production, Ministry of agriculture, Abbasia, Egypt; 3Department of Microbiology, Faculty of Veterinary medicine, Cairo University, Egypt.
Abstract | An affordable, sensitive, specific, fast, and easy to be delivered to end-users immunochromatographic lateral flow kit (LFK) has been developed for the detection of β-lactams antibiotic residues in dairy milk. The developed LFK is based on a competitive immunochromatographic format using anti-antibiotic-specific polyclonal antibodies. Specific artificially induced active acquired polyclonal antibodies production were performed in female New Zealand rabbits against the following β-lactam antibiotics; Amoxicillin (AMX)- Keyhole Limpet Hemocyanin (KLH) and Penicillin-G (Pen G)-KLH conjugates. The prepared anti-β-lactam antibodies were conjugated with gold chloride nanoparticles and used in the development of competitive lateral flow kits for the detection of β-lactams antibiotic residues in dairy milk. The concentration of the gold chloride-antibody conjugate in the conjugation pad was adjusted to detect β-lactam antibiotics in milk samples that exceed the permissible limit. In the newly developed kits, each involved β-lactam antibiotic conjugated with KLH was placed in the test line to compete for the gold chloride anti-β-lactams antibodies conjugate with the free beta-lactam antibiotics supposedly exceeding the permissible limit and existing in the tested samples or vice versa. The control line of the developed lateral flow kits was coated with polyclonal unlabeled goat anti-rabbit antibodies. If the tested milk sample contains a high antibiotic level that exceeds the permissible limits it will be consumed by reaction with the gold anti-β-lactam antibody conjugate in the conjugation pad and the test line read negative with no color development, while if the tested samples contain antibiotics lower than the permissible limits it will not consume the whole conjugate that will, in turn, reacts with the β-lactam antibiotic in the test line producing a deep red color in the test line, so the presence of two deep ruby red lines indicates acceptable Beta-lactam antibiotic residues beneath the permissible limit and vice versa in case of one deep ruby red line. The results could be accomplished within 8 minutes without the need for any other equipment. The recorded lower detection limit of β-lactams by the developed kits was 10ppb in skimmed milk and 5ppb in phosphate buffer saline. 75 cattle milk samples were examined by the developed kits and by the commercially available new existing and commercially available SNAP β-Lactam test kit. The results were compared, and the determined specificity, sensitivity, and accuracy of the locally prepared kits were 93.7%, 95.3%, and 94.6%, respectively.
Keywords | Polyclonal antibodies (PAbs) production, Nano-gold applications, Lateral flow immunochromatographic assay (LFA), Antibiotic residues, Rapid detectors
Received |March 19, 2022; Accepted | June 06, 2022; Published | July 04, 2022
*Correspondence | R. Soliman, Department of Microbiology, Faculty of Veterinary medicine, Cairo University, Egypt; Email: [email protected]
Citation | Hemeda SM, Sayed RH, Hassan H, Sheima AE, Aboul-Ella H, Soliman R (2022). Development of colloid gold-based lateral flow immunochromatographic kits for screening and rapid detection of beta-lactams antibiotic residues in dairy milk. Adv. Anim. Vet. Sci. 10(7):1616-1622.
DOI | https://dx.doi.org/10.17582/journal.aavs/2022/10.7.1616.1622
ISSN (Online) | 2307-8316
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Beta-lactam antibiotics are among the most frequently used in the dairy animal production process, especially as an extensively used antimicrobial agent in the treatment of mastitis. The Beta-lactam group includes those members that shared the same building chemical structure bearing the Beta-lactam ring; Penicillin derivatives, Cephalosporins and Cephamycin (Cephems), Monobactams carbapenems, and Carbapenems (Karageorgou et al., 2012).
One of the most incriminated causes of the presence of antibiotic residues in dairy milk and its long-run adverse impact on antimicrobial resistance and its public health concerns is the uncontrolled use of antibiotics and the inconsideration of the withdrawal time of the used antibiotics (Kebede et al., 2014).
The antibiotic residues and especially β-lactam antibiotic residues have serious health hazardous effects on the end consumers including allergic reactions in susceptible persons and subsequent antibiotic resistance, especially to Penicillin and other ß-lactam antibiotics such as cephalosporins and carbapenems. These antibiotic residues could cause allergies if high levels if it exists in milk consumed by penicillin-allergic persons (Conzuelo et al., 2013). Maximum residue limits (MRLs) of antibiotics in milk have been set by the European Commission to protect the health of consumers (European Commission, 2010). For example, the MRL for ampicillin, penicillin G, and Amoxicillin is 4μg/kg, the limit above which the milk is shouldn’t be consumed.
The already established methods which are used for the detection of β-lactams antibiotic residues in milk are divided into two major groups; the first group consists of screening methods including the microbial inhibition assay (MIA) and the enzyme-linked receptor binding assay. These methods, however, are characterized by low sensitivity, low specificity, and they are time-consuming methods (Wang, 2010).
Beta-lactams residue in dairy milk can also be detected by Bacterial Growth Inhibition methods such as Milch test (Juščáková and Kožárová, 2017), Delvotest SP (Sierra et al., 2009), Inhibition MRL test (Nihad et al., 2014), Rosa Charm β-Lactam test (Bion et al., 2016) and competitive binding methods such as the SNAP β-lactam test (Nihad Fejzić et al., 2014).
The Milch test is one of the most recent bacterial growth inhibition methods developed for the determination of antibiotic residues in raw, thermally treated, and dried milk. This test combines the principle of agar diffusion tests with indicator color changes due to test strain Bacillus stearothremophillus var. calidolactis active metabolism at the absence of inhibitor (Juščáková and Kožárová, 2017). The Rose Charm β-lactam test is qualitative microbial receptor assay in which β-lactam residues have a specific, irreversible propensity for enzyme sites on the cell wall of microorganisms (Bion et al., 2016).
The second group of tests comprises confirmatory methods, instrumental and immunoassay. Instrumental assays such as gas chromatography and liquid/gas chromatography coupled with mass spectrometry (Mahony et al., 2013) and immunoassays such as (Enzyme immunoassay) (Chen et al., 2016), gold immunochromatographic assay (GICA), or lateral flow immunoassay (Wang et al., 2017). The application of the instrumental analysis was limited in well-equipped laboratories, and it requires expensive equipment, professional personnel, and preparation of samples before analysis. Therefore, they aren’t suitable as field tests due to time and cost consumption (Grebe and Singh., 2011).
GICA is a particularly good method as a field test due to its time efficiency, cost-effectiveness, and simple preparation compared with the complex steps required for ELISA (Xing et al., 2015).
The present work aimed to develop rapid, simple, and cost-effective local lateral flow kits for the detection of β-lactam antibiotics residues in milk. This work includes the production of antibodies that are class-specific to the selected antibiotics and utilizing the prepared anti-antibiotic-specific antibodies for the development of antibiotic residues detection Lateral flow kit (LFK). The sensitivity, specificity, and accuracy of the developed LFK were determined by comparing it with the commercially available SNAP β-Lactam test kit and showed very promising competitive results.
Materials and Methods
Preparation of immunogen from the β-lactam antibiotics, namely Penicillin G (Pen G) and Amoxicillin (AMX)
These antibiotics to be immunogenic were conjugated with the keyhole Limpet Hemocyanin (KLH) using the glutaraldehyde method according to (Bremus et al., 2012).
Preparation of amoxicillin–KLH conjugate
In a sterile beaker, a separate antibiotic suspension was performed as follows; 40mg Amoxicillin/4ml of 0.01M phosphate-buffered saline (PBS), pH 7 was prepared and mixed with an equal volume of a solution composed of 6.68mg KLH/4ml PBS. In a dark place, 160µl of 25% glutaraldehyde solution was added dropwise to the above mixture over 10 minutes with continuous stirring using a magnetic stirrer at room temperature (25°C). The mixture was stirred at the same temperature for 4hrs. The formed AMX-KLH was then dialyzed for 72 hours using 3 successive changes of PBS at 4ºC and stored at -70ᴏC until it was used for immunization. The successful conjugation was confirmed by comparing the UV spectra of the AMX-KLH product with that of the AMX and KLH alone.
Preparation of Penicillin G KLH conjugate using glutaraldehyde reaction (Bremus et al., 2012)
In a sterile beaker, 40 mg Pen G /4 ml of 0.01M PBS, pH 7 was prepared and mixed with an equal volume of a solution composed of 8mg KLH/4ml PBS. Further steps were carried out exactly as in the case of Amoxicillin conjugate preparation.
Immunization of rabbits by the previously prepared Amoxicillin-KLH and Penicillin G-KLH
Four adult heifer female white New Zealand rabbits weighing 2 Kg each were divided into two groups each consisting of 2 members. A separate two members group was used to be immunized with each antibiotic-KLH Conjugate (Two rabbits for each immunogen). The process of immunization was carried out according to (Yeh et al., 2008). One volume containing 1mg of the AMX-KLH immunogen was mixed with an equal volume of complete Freund’s adjuvant and 1mg of the Pen G-KLH immunogen was also mixed with an equal volume of complete Freund’s adjuvant separately. Pen G-KLH conjugate immunization emulsion and AMX–KLH conjugate immunization emulsion were injected intradermally (I/D) along both sides of the neck of the involved laboratory animal (New Zealand rabbits). Booster doses were prepared by mixing one volume of AMX-KLH immunogen (1mg) with an equal volume of incomplete Freund’s adjuvant and penicillin-G-KLH immunogen (1mg) with an equal volume of incomplete Freund’s adjuvant. Boosters were injected I/D with 2 weeks intervals for two months and a half at the following days 15, 30, 45, 60, and finally 75 after the priming dose. Blood samples were collected from the auricular vein 1 week after the final booster injection and serum was separated. The anti-β-lactam antibiotics specific IgG was separated from the collected and separated rabbit serum using the caprylic acid method acid according to (Elke et al., 2008), which is described briefly as follows; 50 ml of 0.06M sodium acetate buffer, pH 4.6 were mixed with 25 ml of each serum sample in a beaker. After that, centrifugation of the expanded and diluted serum at 10000×g for 30 minutes was performed, and the obtained pellets were discarded while the supernatants were collected for further step processing. At room temperature magnetic stirring, 2.02 ml caprylic acid was added slowly dropwise for 30 minutes on the prepared serum, followed by centrifugation at 10000×g for 20 minutes. The outcome supernatants were retained, and the pellets were discarded. Finally, the supernatants were dialyzed against PBS buffer at 4oC overnight with three successive buffer changes, and the concentration of purified IgG was measured spectrophotometrically to ensure at least 1 mg/ml concentration.
Preparation of colloidal gold nanoparticles (GNP) 40 nm
The gold chloride (GC) nanoparticles were prepared according to (Herizch et al., 2014) as follows; one ml of 1% HAuCl4 was added rapidly to 50 ml of purified water containing 0.01% (w/v) sodium citrate. The mixture then was boiled and stirred vigorously till the color of the solution changed to a stable red (about 2 minutes) indicating the formation and proper reduction of GC nanoparticles, then the solution was left at boiling temperature for another 10 minutes. Sodium azide 0.02% (w/v) was added and the content was cooled at room temperature under constant stirring and the GNP was collected in a clean glass bottle. The diameter of the prepared nanoparticles was checked by scanning within the range of 400-600 nm using a spectrophotometer against purified water as blank.
Preparation of gold chloride conjugated β-lactam antibiotic-specific antibodies
Conjugation of the β-lactam antibiotic-specific rabbit IgG with the colloidal gold (CG) was performed according to (Kong et al., 2017) as follows; The CG was firstly adjusted to pH 8.5 using 0.02M K2CO3 and 0.3 ml of IgG antibodies at a concentration of 0.2 mg/ml was added to 30 ml of adjusted CG solution with gentle stirring for 10 minutes. The resulting solution was mixed over a rotating wheel at room temperature and incubated for 30 minutes. Polyethylene glycol (PEG) (20000 1% w/v final concentration) was added to block unreacted sites of GNPs followed by further incubation for 15 minutes at room temperature. The mixture was centrifuged at 10000×g for 30 minutes and the supernatants were removed while the pellets containing the antibody–gold conjugate were resuspended in 1ml diluted buffer (20 mM Tris containing 3% w/v sucrose, 1% w/v BSA, and 0.02 % w/v sodium azide, and stored at 4˚C.
Assembly of the lateral flow kits according to (Lata et al., 2013), (Figure 1)
Sample pad: A glass fiber pad, pretreated with sample pad solution, pH 8.5 that is composed of purified water containing 1% (w/v) polyvinyl pyrrolidone (PVP), 3.81% (w/v) Borax, 0.1% (w/v) casein sodium salt, 0.5% (w/v) sodium chloride 2% (w/v), Triton X 100, 0.02% (w/v) sodium azide and 0.15% (w/v) SDS then dried at 37oC.
Conjugation pad: A glass fiber, pretreated with conjugation treatment 20mM PBS solution, pH 7.4 containing 2% (w/v) BSA, 0.3% (w/v) PVP, 1% (w/v) Triton X 100, 2.5% (w/v) sucrose and 0.02% (w/v) sodium azide, then dried at 37oC and kept in dry condition. Finally, the conjugation pad was saturated with 10µl CG-conjugated β-lactam specific rabbit IgG, dried at 37oC for 1hr, and kept dry.
Nitrocellulose membrane: The dispenser (Iso flow) was used to dispense two lines on the nitrocellulose (NC) membrane (25mm X 300mm). 1mg/ml of Penicillin G-KLH conjugate and Amoxicillin-KLH conjugate were dispensed across the NC membrane at the test line (2 µL/1mm line) whiles 0.5 mg/ml of the polyclonal goat anti-rabbit antibodies (MILLIPORE Cat. No. AP132) were dispensed at the upper position at the control line (1 µl/1mm line). The distance between the two lines was 5mm. The loaded NC membrane was dried at 37oC for 2hrs and kept in dry condition. The treated sample pad, treated conjugation pad, loaded NC membrane and absorption pad were stuck down in the PVC card. After that, the collected PVC card was cut into 4 mm width test strips by using an automated cutter machine.
Determination of the sensitivity of the prepared kit (Kiran Lata et al., 2016)
Two-fold serial dilution was made from the two selected antibiotic stock solutions starting from 40ng/0.1ml to 2.5ng/0.1ml in skimmed milk and saline. The different concentrations of antibiotics were dispensed on the sample pads of the prepared lateral flow strips and the results at the control and test line were recorded.
Determination of specificity of the prepared kits (Kiran Lata et al., 2016)
The specificity of test strips was tested in a cross-reactivity study with other groups of antibiotics including Chloramphenicol, sulphonamides, and tetracycline that were spiked in skimmed milk at 50 and 100 ppb/ml and tested with the prepared kits.
Determination of the sensitivity and specificity of the prepared kits in the detection of β-lactam antibiotic residues in dairy milk samples as compared with the new, existing, and commercially available SNAP β-Lactam test kit
Seventy-five collected milk samples were tested with the prepared kit and the new SNAP Beta-Lactam test kits, and the results were recorded and compared.
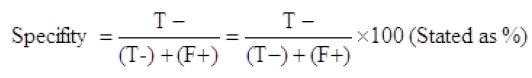
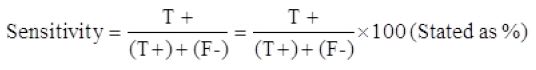
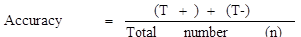
Results and Discussion
Preparation of AMX immunogen (AMX–KLH) and penicillin G immunogen (Pen G–KLH)
AMX has an absorbance peak at 256nm and KLH has a peak at 278nm, while after conjugation, there was a shift in peak at 270 nm for AMX–KLH. Penicillin G has an absorbance peak at 260nm and KLH has a peak at 278 nm, while after conjugation, there was a shift in peak at 272nm for Pen G–KLH.
Sensitivity of the prepared kit
The two-fold serial dilution of two antibiotic stock solutions started from 40ng/0.1ml to 2.5ng/0.1ml were tested in skimmed milk and PBS. The limit of detection of the prepared kit was 10ng/0.1ml in skimmed milk and 5ng/0.1ml in PBS, Figure 2.
Specificity of prepared kits
The prepared kit gives negative results for chloramphenicol, sulphonamides, and tetracycline, Figure 3.
Accuracy of prepared kits
As shown in Table 1, seventy-five collected raw milk samples were tested with the prepared kit and also with the new SNAP β-Lactam test kit, and the obtained results were compared. The true positive, false positive, true negative, and false negative were 30, 2, 41, and 2, respectively. The sensitivity, specificity, and accuracy of the developed kits were 93.7%, 95.3%, and 94.6%, respectively.
Table 1: Result of detection of β-lactams antibiotic residues in 75 raw milk samples by the prepared kit and the new, existing, and commercially available SNAP β-Lactam test kit.
|
New, existing, and commercially available SNAP Beta-Lactam test kit |
The newly developed LFA kit |
|||
|
Positive |
Negative |
Total |
||
|
Positive |
30 |
2 |
32 |
|
|
Negative |
2 |
41 |
43 |
|
|
Total |
32 |
43 |
75 |
|
AMX and Pen G are small molecular weight molecules (MW < 1,000 Da) and they may diffuse out from the site of injection so alone they would not be able to stimulate immune responses and antibody formation. To render these antibiotics immunogenic, they should be conjugated with carrier molecules. AMX and Pen G are usually conjugated with various types of carrier proteins e.g., KLH or BSA, or OVA. The greater number of functional groups in these carrier molecules facilitate their conjugation with the antibiotics. The KLH carrier molecules were selected in the current work because it stimulates a stronger immune response than BSA or OVA and also contains lysine, which helps in reaction initiation (Kiran et al., 2016). In the present work, AMX and Pen G were converted to strong immunogen by conjugation with KLH carrier molecules.
Carbodiimides were used to mediate the formation of amide linkages between carboxylates and amines and EDC with NHS are widely used for conjugation of antibiotics with protein (Bremus et al., 2012). while AMX-BSA coupled with the glutaraldehyde method (GA) method was considered more effective in producing antibodies than was the carbodiimide method (Yeh et al., 2008) and confirmation of conjugation was done with spectroscopic observations for successful immunization and conjugation and the shift in the peak of the complex Cefalexin-KLH indicates the successful conjugation (Kiran et al., 2016). The spectrophotometer technique has also been used successfully to ensure the conjugation of tetracycline to KLH (Siti and Nur, 2011).
In the present study, the glutaraldehyde method (GA) was used to conjugate AMX to KLH and Pen G to KLH protein, and spectroscopic observation was used to confirm the conjugation. AMX and Pen G have an absorbance peak at 256, and 260nm, respectively, and KLH has a peak at 278nm, while after conjugation, there is a shift in peak at 270nm for AMX–KLH and 272nm for pen G–KLH. The shift in the peak of the complex indicates successful conjugation between these antibiotics and carrier protein.
In the present study 10μl of gold-labeled beta-lactams, antibody conjugate was used and was the same in all strips and the test line became colorless at a concentration of 5 ppb in phosphate buffer saline and 10ppb in case of skimmed milk (limit of detection). Time taken for analysis was 7 or 8 minutes. These results are similar to those recorded by (Laxmana et al., 2014; Kiran et al., 2016).
The sensitivity, specificity, and accuracy of the developed kit are 93.7%, 95.3%, and 94.6% respectively. These results indicated that the locally prepared LFK is suitable for beta-lactam antibiotics residue detection in dairy milk.
Conclusions and Recommendations
This work was designed to develop accurate, affordable, sensitive, specific, user-friendly, rapid, robust, deliverable to end-users, devices less, bedside, handheld, field, and cost-effective for development and production, lateral flow immunochromatographic kit for detection of Beta-lactams antibiotic residues in dairy milk. The detection limit of β-lactams in milk using the developed LFA was 5 ppb in phosphate buffer saline and 10ppb in skimmed milk samples and the time taken for analysis was 7-8 min without the need for any types of equipment. Also, the sensitivity, specificity, and accuracy of this test were 93.7%, 95.3%, and 94.6%, respectively, as compared with the new, existing, and commercially available SNAP Beta-Lactam test kit. The developed assay is specific and easy to use, and the accuracy and precision of this assay also met the requirements for qualitative analysis. The locally developed lateral flow assay could be an effective tool for the direct and routine screening of β-lactams antibiotic residues in dairy milk samples.
Acknowledgment
We want to acknowledge all dairy animal veterinarians in the Greater Cairo Area (GCA), Egypt for their help during the dairy milk samples collection stage to be tested for Beta-lactams antibiotic residues by the newly developed kit.
Ethical statement
The guidelines of the institutional animal care and use committee (IACUC) were completely followed during any procedures involving animal use through the current conducted study. And the IACUC protocol approval code for the current study is VETCU23052022455.
Funding
This study was funded by the science and technology development fund (STDF) project Grant (No. 27811).
Novelty Statement
The misuse of antibiotics in the dairy animal has adverse effects on public health through the impermissible limit of antibiotic residues in dairy animal products mainly milk, finding an easy to be used technique to act as a rapid and accurate detector of the antibiotic residues in milk would be a great advancement in this field. Through the current conducted study a newly developed immunochromatographic kit has been introduced and compared with other already existing methods and showed very promising competitive results.
Author’s Contribution
All authors are contributed equally through the different stages of the current conducted study.
Conflict of interest
The authors have declared no conflict of interest.
References
Bion C, Beck Henzelin A, Qu Y, Pizzocri G, Bolzoni G, Buffoli E (2016). Analysis of 27 antibiotic residues in raw cow’s milk and milk-based products validation of Delvotest®T. Food Addit. Contam. A Chem. Anal. Contr. Expo Risk Assess., 33(1): 54-59. https://doi.org/10.1080/19440049.2015.1104731
Bremus A, Dietrich R, Dettmar L, Usleber E, Märtlbauer E (2012). A broadly applicable approach to prepare monoclonal anti-cephalosporin antibodies for immunochemical residue determination in milk. Anal. Bioanal. Chem., 403: 503–515. https://doi.org/10.1007/s00216-012-5750-z
Chen Y, Kong D, Liu L, Song S, Kuang H, Xu C (2016). Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Anal. Methods, 9(4): 905–914. https://doi.org/10.1007/s12161-015-0262-z
Conzuelo F, Campuzano S, Gamella M, Pinacho DG, Reviejo AJ, Marco MP, Pingarrón JM (2013). Integrated disposable electrochemical immunosensors for the simultaneous determination of sulfonamide and tetracycline antibiotics residues in milk. Biosens. Bioelectron., 50: 100–105. https://doi.org/10.1016/j.bios.2013.06.019
Elke S, Bergmann-Leitner, Ryan M, Mease, Elizabeth H, Duncan, Farhat K, John W, Evelina A (2008). Evaluation of immunoglobulin purification method and their impact on quality and yield of antigen-specific antibodies. Malar. J., 7: 129. https://doi.org/10.1186/1475-2875-7-129
European Commission, Council Regulation (2010). No. 37/2010 of 22 December 2009 on pharmacologically residue limits in foodstuffs of animal origin. Off. J. Eur. Union. L15/1-72.
Grebe SK, Singh RJ (2011). LC-MS/MS in the Clinical laboratory Where to from here? Clin. Biochem. Rev., 32: 5.
Herizchi R, Elham A, Morteza M, Abolfazl A (2014). Current methods for synthesis of gold nanoparticles. Artif. Cells, Nanomed. Biotechnol., 44(2): 596-602. https://doi.org/10.3109/21691401.2014.971807
Juščáková D, Kožárová (2017). Determination of antibiotic residues in milk by microbial inhibitory tests I. Folia Vet., 61(3): 57-64. https://doi.org/10.1515/fv-2017-0028
Karageorgou EG, Samanidou VF, Papadoyannis IN (2012). Ultrasound-assisted matrix solid-phase dispersive extraction for the simultaneous analysis of β-lactams (four penicillins and eight cephalosporins) in milk by high-performance liquid chromatography with photodiode array detection. J. Sep. Sci., 35: 2599–2607. https://doi.org/10.1002/jssc.201200514
Kebede G, Zenebe T, Disassa H, Tolosa T (2014). Review on detection of antimicrobial residues in raw bulk milk in dairy farms. Afr. J. Basic Appl. Sci., 6(4): 87–97.
Kiran L, Rajan S, Laxmana N, Yudhishthir SR, Bimlesh M (2016). Lateral flow assay based rapid detection of cephalexin in milk. J. Food Qual., ISSN 1745-4557.
Kong MM, Yang B, Gong CJ, Wang H, Li X, Zhao K, Li JJ, Wu F, Liu X, Hu Z (2017). Development of immunochromatographic colloidal gold test strip for rapid detection of Haemophilus influenzae in clinical specimens. J. Appl. Microbiol., ISSN 1364-5072. https://doi.org/10.1111/jam.13489
Lata K, Naik L, Sharma R, Mann B, Rajput YS (2013). Lateral flow assay concept and its applications in food analysis. Indian Food Ind., 32(5): 22-32.
Laxmana N, Kiran L, Rajan S, Bimlesh M, Rajput YS (2014). Production of polyclonal antibody for oxytetracycline and their use in lateral flow assay. J. Microbiol. Immunol. Biotechnol., 1: 08-17.
Mahony JO, Clarke L, Whelan M, Kennedy RO, Lehotay SJ, Danaher M (2013). The use of ultra-high-pressure liquid chromatography with tandem mass spectrometric detection in the analysis of agrochemical residues and mycotoxins in food – Challenges and applications. J. Chromatogr. A, 1292: 83–95. https://doi.org/10.1016/j.chroma.2013.01.007
Nihad F, Muris B, Sabina Š-H, Muhamed S (2014). Beta-lactam antibiotics residues in cow’s milk: Comparison of the efficacy of three screening tests used in Bosnia and Herzegovina. J. Assoc. Basal Med. Sci., 14(3): 155–159. https://doi.org/10.17305/bjbms.2014.3.109
Sierra D, Sánchez A,LuengoC,Corrales JC, Morales CT,dela C, Guirao FI, Gonzalo C (2009). Detection limits of four antimicrobial residue screening tests for β-lactams in goat’s milk, 92(8): 3585-3591. https://doi.org/10.3168/jds.2008-1981
Siti B, Nur AMS (2011). Production of polyclonal antibody against tetracycline using KLH as a carrier protein. J. Anim. Sci., 14: 61-66.
Virolainen NE, Pikkemaat MG, Elferink JWA, Karp MT (2008). Rapid detection of tetracyclines and their 4-epimer derivatives from poultry meat with bioluminescent biosensor bacteria. J. Agric. Food Chem., 56(23): 11065–11070. https://doi.org/10.1021/jf801797z
Wang C, Li X, Peng T, Wang Z, Wen K, Jiang H (2017). Latex bead and colloidal gold applied in a multiplex immunochromatographic assay for high-throughput detection of three classes of antibiotic residues in milk. Food Contr., 77: 1–7. https://doi.org/10.1016/j.foodcont.2017.01.016
Wang J (2010). Analysis of antibiotics in milk and its products. In: Handbook of dairy foods analysis; (eds. Nollet, L.M.L., Toldrá, F.,); CRC Press: Boca Raton, FL, USA, pp. 801–820. ISBN 978-1-4200-4631-1. https://doi.org/10.1201/EBK1439848173-39
Xing C, Liu L, Song S, Feng M, Kuang H, and Xu C (2015). Ultrasensitive immunochromatographic assay for the simultaneous detection of five chemicals in drinking water. Biosens. Bioelectron., 66: 445-453. https://doi.org/10.1016/j.bios.2014.12.004
Yeh LC, Lee WM, Koh BW, Chan JP, Liu CH, Kao JP, Chou CC (2008). Development of amoxicillin enzyme-linked immunosorbent assay and measurements of tissue amoxicillin concentrations in a pigeon microdialysis mode. Poult. Sci., 87: 577–587. https://doi.org/10.3382/ps.2007-00167
To share on other social networks, click on any share button. What are these?





