Determination of Maternal Rabies Virus Neutralizing Antibody in Young Puppies
Determination of Maternal Rabies Virus Neutralizing Antibody in Young Puppies
Uzma Farid Durrani1,*, Sagar Mal Goyal2, Asim Khalid Mahmood1, Cathleen Hanlon3, Susan Moore3, Muhammad Waqas1 and Raheela Akhtar4
1Pet Centre, Faculty of Veterinary Science, University of Veterinary and Animal Sciences, Lahore, Pakistan
2Veterinary Diagnostic Laboratory, College of Veterinary Medicine, University of Minnesota, Saint Paul, MN, USA
3Rabies Laboratory, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA
4Department of Pathology, Faculty of Veterinary Science, University of Veterinary and Animal Sciences, Lahore, Pakistan
ABSTRACT
A pre-vaccination serological study was conducted on 100 puppies (< 3 months age), in rabies endemic Lahore city of Pakistan, to evaluate their maternal rabies virus neutralizing antibody titer (mRVNA). All puppies were whelped by dams regularly vaccinated with high quality inactivated rabies vaccines. Rapid Fluorescence Focus Inhibition Technique (RFFIT) revealed mRVNA titer (>0.5 IU/mL) in 29 puppies while 48 puppies showed mRVNA titer less than minimum protective level (0.2-0.4 IU/mL). RFFIT did not reveal mRVNA in 23 puppies that were declared lacking maternal rabies immunity. One basis of findings of this study it was concluded that puppies population lacking maternal rabies immunity is one of the responsible factors for increasing incidence of rabies in humans and dogs living in Lahore, Pakistan.
Article Information
Received 04 November 2015
Revised 21 October 2016
Accepted 27 November 2016
Available online 26 May 2017
Authors’ Contributions
UFD conceived the idea, performed the experiment and wrote the article. SMG and AKM supervised the work. CH and SM acted as facilitators of RFFIT technique. MW helped in manuscript preparation. RA helped in statistical analysis.
Key words
Maternal rabies virus neutralizing antibody (mRVNA), Rapid fluorescent focus inhibition technique (REFFIT), Puppies.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.3.sc11
* Corresponding author: ufdurrani@uvas.edu.pk
0030-9923/2017/0003-1147 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Pakistan is a rabies endemic country where 99% cases of rabies are followed by the rabid dog exposure every year (Durrani et al., 2012). These prevailing circumstances have urged the people for rabies prophylaxis of their pet dogs. Both active and maternal (passive) immunities are important for protection against rabies. In puppies, whelped by rabies vaccinated dams; maternal rabies virus neutralizing antibodies (mRVNA) are transferred to puppies. Maternal immunity (RVNA) of puppies persists till 3 months age (Morters et al., 2015; Hogenesch et al., 2004).
In Pakistan currently there is no test available to determine the mRVNA and in routine clinical practice primary vaccination of puppies is started at 3 months age to avoid interference by mRVNA (Durrani et al., 2012; Kasempimolporn et al., 1996). This serological ignorance of potential cases of maternal immunity failure is posing a serious risk of rabies spread to humans and other animals ultimately leading to the hike in rabies toll.
Rapid fluorescence focus inhibition technique (RFFIT) is a gold standard technique for the determination of RVNA titers (Bahloul et al., 2002). The test has good specificity (100%) and reproducibility (P>0.05) and is highly reliable in detecting rabies immunity status of dogs (Yu et al., 2012).
The present study was conducted to determine the mRVNA titers of young puppies at 1-3 months age and compare the effect of age on mRVNA titer.
Materials and methods
This study was conducted on 1-3 months old 100 healthy puppies of either sex. Puppies belonged to different areas of Lahore, Pakistan that is a rabies endemic city. Selection of puppies was made on the basis of rabies vaccination history of their dams and consent of the owners. Data about age, health status and medication history of puppies was collected for each puppy. Puppies were divided into 3 age based groups i.e. groups of 1 month, 2 months and 3 months old puppies.
Blood samples were collected from cephalic vein. Sera were separated, stored at -80°C before shipping these to K- State Rabies Laboratory at Kansas State University, Manhattan, K.S., USA for mRVNA screening by rapid fluorescent focus inhibition technique (RFFIT). For this analysis 11 µl of serum samples were placed in a water bath at 56°C for 1 h to inactivate complement (Kramer et al., 2009). Three serial fivefold dilutions of test Sera and a 2 IU/mL stock solution of standard serum prepared in RFFIT media were transferred to 8-well Lab Tek chamber slides followed by addition of 100µL of pre-diluted CVS-11 (CCID50). The mixture was incubated at 37°C for 90 min. followed by the addition of 200 µL of baby hamster kidney cells suspension (5×105 /mL). After 24 h of incubation at 37°C, the chamber slides were fixed in 80% cold acetone followed by a rinse in phosphate buffer saline (PBS) for 3 min. After drying in a bio safety cabinet for 10 min, 156 µL of fluorescein conjugated rabies antibody (Kramer et al., 2009) was added to all wells and incubated at 37°C for 45 min. followed by PBS washing and then dried. Under a fluorescent microscope, 20 microscopic fields were examined in each well of the slide at 200X magnification. Number of fields containing at least one fluorescent cell was counted starting at the same corner of the slide and taking four rows of the five fields. Control slides were read first and the number of positive fields in each serum dilution was recorded.
| Age (months) |
mRVNA titer (IU/mL) |
||
|
≥ 0.5 |
0.2 - 0.3 |
≤ 0.2 |
|
| 1 |
14 |
20 |
13 |
| 2 |
11 |
16 |
5 |
| 3 |
4 |
12 |
5 |
| Total |
29 |
48 |
23 |
There were two serum samples per slide and end point titer of the test serum samples was calculated on the basis of number of positive fields in corresponding dilution (1:5) using Reed and Muench calculation chart (Reed and Muench, 1938), according to which dilution field with viruses 1:5, 0/20, 1/20 and > 2/20 has titer of 0.1, 0.1 and < 0.1, respectively.
mRVNA concentration (IU/mL) for test serum samples was calculated by using the following formula:
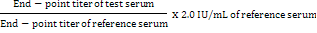
Data were statistically analyzed using Student’s t-test and One Way Analysis of Variance (ANOVA) and multiple comparisons were carried out by using Least Significant Difference test (Snedecor and Cohran, 1967). Confidence level was 95% and the P value was considered significant at < 0.05.
Results and discussion
The screening of mRVNA titer is an effective tool to determine the rabies protective immunity in animals and humans. RFFIT is a WHO recommended gold standard serological assay used for RVNA determination (Durrani et al., 2012).
The under discussion study was conducted on 100 puppies (1-3 months old) whelped by regularly rabies vaccinated dams. RFFIT revealed a variation of mRVNA titers among different age groups of puppies (Table I). Statistical analysis proved a significant difference (P<0.05) among mRVNA titers of different age groups. According to the documented findings of previous studies it is considered that there are different factors that can affect mRVNA titers and age may be one of these factors (Durrani et al., 2012; Kennedy et al., 2007; Muirhead et al., 2008; Mansfield et al., 2004). In the present study RFFIT results revealed that out of 100 puppies only 29 puppies (1 month old) exhibited protective mRVNA titers ≥ 0.5 IU/mL (Fig. 1), while 48 puppies (2 months old) exhibited mRVNA below minimum protective level i.e. 0.1-0.4 IU/mL, however, there was no detectable mRVNA titer in 23 puppies (3 months old). mRVNA results presented mRVNA declining trend from 1 month age to 3 months age (Fig. 1). According to Muller et al. (2002) and Vos et al. (2001) disappearance rate of maternal antibody is independent of dam’s RVNA titer and decreases exponentially with the age but level of maternal antibodies is directly proportional to the level of antibody titers in the dam. Earlier another group of scientists performed a study on the maternal immunity of Thai puppies whelped by rabies vaccinated dams and the researchers reported that in puppies; from rabies immunized dams, maternal immunity exists till the age of three months and sometimes slightly beyond. They also added that rabies vaccine failure in dams can be one of the several reasons for maternal immunity failure in puppies (Kasempimolporn et al., 1996). The same observation was recorded in the present study as a result of an age based comparison of mRVNA that revealed a declining trend of mRVNA titers from 1-3 months age (Fig. 1). These findings are also in line with the findings of (Morters et al., 2015) who reported that maternal antibodies are maintained at the levels >0.5 IU/mL till 36 days after birth and then this level starts declining to <0.5 IU/mL. On the basis of these observations they suggested that in rabies endemic areas all dogs, including those less than 3 months age, must be vaccinated with high quality inactivated rabies vaccine to secure the animal and human population against rabies. Puppies from non-vaccinated dams respond well to vaccination after the 4-10 weeks of age with a progressive increase in RVNA titer. On the contrary, puppies from vaccinated dams respond only at 10 weeks of age, although detectable mRVNA titer decreases by 6 weeks (Moore and Hanlon, 2010; Rashid et al., 2009; Winters, 1981). It is also reported earlier that a continuous use of corticosteroids, immune-suppressive agents and some health disorders can interfere the immunity level (Rashid et al., 2009).
Conclusion
It is concluded that there is a high risk of rabies maternal immunity failure under field conditions and a significant relation exists between mRVNA titers and age of the young puppies. In this scenario it is recommended that for puppies under 3 months age sero-screening must be practiced to confirm the mRVNA titer prior to especially in rabies endemic areas. RFFIT is a gold standard diagnostic assay for mRVNA determination however, currently this diagnostic technique is not available in Pakistan and here rabies antibody titer is diagnosed on the basis of ELISA.
Acknowledgement
We are highly grateful to Prof. Dr. Gary Anderson, Director Veterinary Diagnostic Laboratory, Kansas State University, USA for accommodating this project at their laboratories as well as for their cooperation during this study. We also acknowledge the financial assistance by Higher Education Commission of Pakistan under International Research Support Initiative Program to perform RFFIT in the U.S.A.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Bahloul, C., Taieb, D., Kaabi, B., Diouani, M.F., Hadjahmed, S.B., Chtourou, Y., B’chir, B.I. and Burr, K., 2002. Res. Vet. Sci., 72: 25. https://doi.org/10.1016/S0034-5288(02)90071-8
Durrani, U.F., Khan, M.S., Khan, M.A., Hanlon, C. and Moore, S., 2012. Pakistan J. Zool., 44: 291-296.
HogenEsch, H.S., Thompson, A.D., Ceddia, M. and Hayek, M., 2004. Vet. Immunol. Immunop., 97: 77-85. https://doi.org/10.1016/j.vetimm.2003.08.010
Kennedy, L.J., Lunt, M., Barnes, A., McElhinney, L., Fooks, A.R., Baxter, D.N. and, W.E.R., 2007. Vaccine, 25: 8500-8507. https://doi.org/10.1016/j.vaccine.2007.10.015
Kramer, B., Schildger, N., Nicol, H.P., Hanschmann, A.B. and Duchow, K.M., 2009. Biologicals, 37: 119-126. https://doi.org/10.1016/j.biologicals.2009.01.001
Mansfield, K.L., Burr, P.D., Snodgrass, D.R., Sayers, R. and Fooks, A.R., 2004. Vet. Rec., 154: 423-426. https://doi.org/10.1136/vr.154.14.423
Moore, S.M. and Hanlon, C., 2010. PLoS Negl. Trop. Dis., 4: 595. https://doi.org/10.1371/journal.pntd.0000595
Morters, M.K., McNabb, S., Horton, D.L., Fooks, A.R., Schoeman, J.P., Whay, H.R. and Wood, J.L.N., Cleaveland, S., 2015. Vet. Rec., 177: 150. https://doi.org/10.1136/vr.102975
Muller, T., Selhorst, T., Schuster, P., Vos, A., Wenzel, U. and Neubert, A., 2002. BMC Infect. Dis., 2: 10. https://doi.org/10.1186/1471-2334-2-10
Rashid, A., Rasheed, K. and Akhtar, M., 2009. J. Anim. Pl. Sci., 19: 22-25.
Reed, L.J. and Muench, H., 1938. Am. J. Hyg., 27: 493-497.
Snedecor, G.W. and Cohran, W.G., 1967. Statistical methods. 6th ed., Lowa State University Press, Ames, Lowa, USA.
Vos, A., Muller, T., Schuster, P., Selhorst, T. and Wenzel, U., 2001. Acta Vet. Hung., 49: 291-294. https://doi.org/10.1556/004.49.2001.3.5
Winters, WD., 1981. Vet. Rec., 108: 295-299. https://doi.org/10.1136/vr.108.14.295
Yu, J., Li, H., Tang, Q., Rayner, S., Han, N., Guo, Z., Liu, H., Adams, J., Fang, W., Tao, X., Wang, S. and Liang, G., 2012. PLoS. Negl. Trop. Dis., 6: e1640. https://doi.org/10.1371/journal.pntd.0001640
To share on other social networks, click on any share button. What are these?










