Copper Nanoparticles Embedded Rice Husk for the Removal of Bacterial Contaminants from Drinking Water
Copper Nanoparticles Embedded Rice Husk for the Removal of Bacterial Contaminants from Drinking Water
Farzana Bashir2, Aysha Saleem1, Khalid Iqbal Khichi2, Rubina Nelofer3*
and Rauf Ahmad Khan2
1Applied Environmental Sciences, Lahore College for Women University, Lahore
2Centre for Environmental Protection Studies, PCSIR Laboratories Complex, Lahore
3Food and Biotechnology Research Centre, PCSIR Laboratories Complex, Lahore
ABSTRACT
The objective of the study was to prepare copper nanoparticles (Cu-NP) embedded on rice husk and to use them as a cost effective approach for the removal of microbial contaminant from drinking water. The copper nanoparticles (Cu-NP) were prepared by using copper sulphate, sodium hydroxide and ascorbic acid and then embedded on rice husk for the removal of bacterial contaminants from drinking water. The Cu-NP embedded rice husk then characterized by SEM, EMAX and ICP-OES techniques. The antibacterial activity was determined by column studies using response surface methodology (RSM) based on three levels, three factorial Box Behnken Design (BBD). The significance of independent variables (flow rate, bacterial count and column height) and their interactions were tested by means the analysis of variance (ANOVA) with 95% confidence limits. The antibacterial susceptibility was optimized for flow rate, bacterial count and column height. High regression coefficient between the variable and response (R2=99.4%) showed good evaluation of experimental data by polynomial regression model. The optimum conditions suggested by the model for the variables such as flow rate, bacterial load and column height were 46 ml/min, 262 bacterial count/ml and 8 cm respectively with maximum actual removal of bacterial contaminants was of 95.5% and is very close to predicted value (94.15%). The prepared copper nanoparticles (Cu-NP) embedded on rice husk was found very effective for the removal of microbial contaminants (E. coli) from drinking water.
Article Information
Received 09 October 2018
Revised 12 May 2019
Accepted 20 November 2020
Available online 03 March 2022
(early access)
Published 27 October 2022
Authors’ Contribution
RAK designed the study. FB and AS prepared the copper nano particles embedded rice husk and conducted the experiments using that. Khalid carried out the characterization of the prepared Cu-NP. Rubina analyzed the effect of Cu-NP on the bacterial decontamination of water. .
Key words
Copper nanoparticles (Cu-NP), Response surface methodology, Column study, Bacterial load, Decontamination of water
DOI: https://dx.doi.org/10.17582/journal.pjz/20181009121054
* Corresponding author: [email protected]
0030-9923/2023/0001-251 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
In developing countries, the use of contaminated water for drinking purposes contributes many water-born diseases in poor community (Prüss et al., 2002). Worldwide, it is tried to solve the problems related to contaminated water by using different emerging strategies. One of the modern strategy is to use nanoparticles for treating drinking water and to make it fit for human consumption. Copper, having strong biocidal properties and have been used from a long time for antimicrobial purposes and can be used successfully for the removal of bacteria from drinking water (Shuster, 2010). Its germicidal properties are similar to that of silver (Santo et al., 2008) but it is has an extra advantage that it is much cheaper than silver.
Copper nanoparticles (Cu-NP) were found effective in killing a range of bacterial pathogens involved in hospital acquired infections. But a high concentration of Cu-NP is required to achieve a bactericidal effect (Mahmoodi et al., 2018; Islam et al., 2021).
Copper ions released by the nanoparticles may connect to the negatively charged bacterial cell wall and burst it that leads to protein de-naturation and cell death (Lok et al., 2006). Copper nanoparticles are copper based particles 1 to 100 nm in size. Like numerous different types of other nanoparticles, copper nanoparticles can be prepared by normal procedures or through synthesis. Cu-NP are quite cheap, high yields in mild reaction conditions and they have short reaction times as compared to traditional catalysts. There are different methods for the synthesis of Cu-NP like chemical, electrochemical, photochemical, sonochemical and thermal techniques (Kim et al., 2007; Phong et al., 2011).
Cu-NPs can be incorporated onto different materials to carve their utilization according to requirement. Just little amounts of copper are required because these nano particles are very effective even at low concentration. The use of copper nanoparticles for water sterilization is an advantageous and reasonable strategy for managing water contamination. In this study, an easy and novel method for embedding nanoparticles on rice husk has been described which involved the synthesis of Cu-NP by using ascorbic acid as reducing agent followed by dipping the rice husk into it and allow to stand then filtered and dried. To study the bactericidal activity of the prepared material, the synthetic water contaminated with E. coli was passed through the column filled with Cu-NP embedded rice husk and then analyzed the effluent water for the removal of bacteria.
Response surface methodology (RSM) is a statistical technique that makes treatment process simple and efficient in light of time and resource utilization. RSM has been widely used for optimization of the process variables of adsorption studies (Singh et al., 2010; Ponnusamy and Subramaniam, 2013). In the present study RSM is used for the optimization of processes in which all factors are varied over a set of experimental runs (Myers and DCM, 2009). Usually, in most of the studies, the optimization process is generally conducted to investigate the effects of several process parameters such as initial pH, initial concentration, temperature, and adsorbent dose on the adsorption in batch process. To the best of our knowledge, little information about applying RSM to optimize the process parameters for in column studies with desired properties was reported.
Hence, in present investigation is to quantify the antibacterial activity of Cu-NP rice hush against E. coli as a cheaper process for drinking water purification. The optimization was carried out by the RSM in a mathematical model to describe the effects and relationships of the independent variables (column height, flow rate, bacterial count) to dependent variable i.e., percent decrease in bacterial count.
MATERIAL AND METHODS
Preparation of copper nanoparticles and adsorption on rice husk
Copper sulfate and sodium hydroxide were used for the preparation of Cu-NP (Dankovich and Smith, 2014). Alkaline solution of copper hydroxide is prepared by adding 1M sodium hydroxide to 0.32M copper sulfate to form gelatinous precipitates. To this solution 20 gm. of washed, dried rice husk was added and 10 M NaOH were added and allow it to stand for two days at room temperature (25±5 ̊C). Then rice hush filtered and thoroughly washed with distilled water to remove excess base. After washing the rice husk soaked in 10% ascorbic acid at 85 ̊C for 30 minutes and again washed with distilled water and then dried at 70 ̊C in hot air oven and kept in glass jar for further analysis.
Characterization
For the estimation of copper contents absorbed by the rice husk, it was digested in triplicate with nitric acid and hydrochloric acid (3:2) on a hot plate at 95±5 ̊C till clear and then diluted to 100ml with deionized water. The digested samples then analyzed for copper estimation by ICP-OES (Optima DV 5300, Perkin Elmer. The surface morphology was determined by SEM instrument. The elemental composition of synthesized NP,s was analyzed through EDAX. (S 3700 N, Hitachi Japan)
Column studies
Fixed bed column studies were conducted in glass column of 3.5 cm diameter and bed height of 15.0 cm. The experimental setup consisted of three parallel glass columns set. The glass column was filled with Cu-NP adsorbed rice husk on gauze and cotton wool support. One set was used as reference, second set for blank, and third set for experimental study. The effect of column height was investigated by varying amount of rice husk from 4 to 12 cm at room temperature (25±5°C) with varying height 4.0, 8.0 and 12.0 cm. E. coli (ATTCC No. 8739) was selected as indicator organism for synthetic water preparation used in all antimicrobial susceptibility studies. The E. coli concentration was set at 50, 250 and 500 bacterial count/ml. The synthetic water was introduced into the column and allowed to flow by gravity at flow rate of 15, 50 and 80 mL/min which was maintained and controlled at the bottom neck with the use of stopper. For column study, the treated sample was collected manually and bacterial count was measured by pore plate method (Federation and Association, 2005).
Experimental design and data analysis
RSM is a statistical technique, used for the optimization process in order to evaluate the significance of variables in the presence of interaction. In this study, the three most important input variables; column bed height, flow rate and bacterial concentration were optimized. Their ranges and levels were shown in Table I. The actual experimental design matrix which is a total16 experiments in duplicate has been designed by Box Behnkin Design (BBD) as shown in Table II.
All the experiments described in Table II were performed in duplicate and results were analyzed by response plots and analysis of variance. For RSM, the most commonly used second order polynomial equation developed to fit the results of experimental data and determine the relevant model terms can be written as:
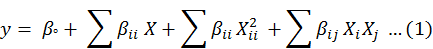
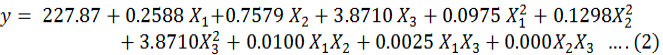
Where y represents a predicted response, i.e. the percentage removal of bacteria, βo is the constant coefficient, βi is the linear coefficient of input factor X1, βii is the coefficient of quadratic effect, and βij is the coefficient of interaction effect of X1 and Xj.
Table I. Experimental factors and levels of BBD.
|
Sr. No. |
Factors |
Levels |
||
|
+ 1 |
0 |
-1 |
||
|
1. |
Column height (cm) |
4 |
8 |
12 |
|
2. |
Flow rate (ml/min) |
15 |
50 |
80 |
|
3. |
Bacterial count (ml) |
100 |
250 |
500 |
Table II. BBD for bacterial removal by using Cu-NP rice husk.
|
Exp. No. |
Column height (cm) |
Flow rate (ml/min) |
Bacterial count (ml) |
Actual bacterial removal (%) |
Predicted bacterial removal (%) |
|
1. |
12 |
50 |
50 |
78.5 |
78.66 |
|
2. |
12 |
50 |
500 |
75.9 |
76.64 |
|
3. |
12 |
80 |
250 |
68.6 |
69.02 |
|
4. |
12 |
15 |
250 |
95.5 |
94.19 |
|
5. |
4 |
50 |
50 |
65.6 |
64.66 |
|
6. |
4 |
50 |
500 |
55.9 |
55.84 |
|
7. |
4 |
80 |
250 |
61.2 |
62.58 |
|
8. |
4 |
15 |
250 |
65.3 |
64.82 |
|
9. |
8 |
15 |
50 |
90.5 |
91.81 |
|
10. |
8 |
15 |
500 |
78.1 |
78.59 |
|
11. |
8 |
80 |
50 |
72.3 |
71.67 |
|
12. |
8 |
80 |
500 |
74.1 |
72.94 |
|
13. |
8 |
50 |
250 |
77.5 |
77.50 |
|
14. |
8 |
50 |
250 |
77.5 |
77.50 |
|
15. |
8 |
50 |
250 |
77.5 |
77.50 |
|
16. |
8 |
50 |
250 |
77.5 |
77.50 |
Statistical analysis
The statistical design of experiments, data analysis and plots was performed using STATISTICA software version 7. The regression coefficient ‘P’ ‘F’ and ‘t’ values for all linear, quadratic and interaction effects of the parameters were determined in order to determine the significance of model. The coefficient of correlation determination (R2) was calculated to evaluate the performance of the regression equation. The optimum levels of the three variables were obtained from the desirability charts. The competence of RSM was justified through the analysis of variance (ANOVA) Table III. A variable was considered statistically significant if the value of ‘P’ (probability) with 95% confidence level was smaller than the level of significance (0.05).
RESULTS AND DISCUSSION
The use of nanotechnology is vast in many fields such as nano-medicines, solar cells, sensor development and most importantly in the control of water pollution (Hameed et al., 2013). The basic reason of their effectiveness is their high reactivity due to the large surface to volume ratio.
Characterization of Cu-NP adsorbed rice husk
Cu-NP were prepared and then embedded on rice husk and characterized by Electron microscope SEM (Fig. 1) EXAD (Fig. 2) technique and also for copper contents determination by ICP. The SEM analysis of Cu-NP adsorbed rice husk is shown in Figure 1. The surface of rice husk covered with smaller and larger nanoparticles. The larger nanoparticles appeared to be aggregated smaller particles. The aggregation of particles may be due to the absence of any stabilizing polymer in this system.
Table III. Analysis of variance for efficiency of bacterial removal using Box-Behnken model.
|
Variables |
Analysis of variance |
Parameter estimates |
|||||
|
SS |
Deg. of freedom |
MS |
F |
Co-efficient |
T values |
P values |
|
|
Intercept |
170.39 |
1 |
170.39 |
113.20 |
39.843 |
10.64 |
0.000041* |
|
Column height(cm) |
399.87 |
1 |
399.87 |
265.67 |
11.2013 |
16.30 |
0.000003* |
|
Flow rate (ml/min) |
7.52 |
1 |
7.52 |
5.00 |
0.159 |
-2.23 |
0.066774 |
|
Bacterial count (ml) |
27.11 |
1 |
27.11 |
18.01 |
-0.0419 |
-4.24 |
0.005417* |
|
Column height x column height |
215.53 |
1 |
215.53 |
143.19 |
-0.459 |
-11.97 |
0.00021* |
|
Flow rate× Flow rate |
15.38 |
1 |
15.38 |
10.22 |
0.0018 |
3.19 |
0.018692* |
|
Bacterial count x Bacterial count |
3.28 |
1 |
3.28 |
2.18 |
-0.00002 |
-1.48 |
0.190346 |
|
Column height x flow rate |
131.93 |
1 |
131.93 |
87.65 |
-0.0044 |
-9.36 |
0.00084* |
|
Column height x bacterial count |
11.63 |
1 |
11.63 |
7.73 |
0.002 |
2.78 |
0.031995* |
|
Flow rate x bacterial count |
53.01 |
1 |
53.01 |
35.22 |
0.0005 |
5.93 |
0.001022* |
|
Error |
9.03 |
6 |
9.03 |
||||
The peaks of EMAX pattern confirm the formation of Cu-NP. The Cu peaks are present along with other peaks of C, O, Si and Al (Table IV). Some small peaks of Cu are also observed, which indicated some minor oxide formation on the surface due to air. Copper oxide is not unexpected, some other researchers also have the same findings (Vainio et al., 2007; Cady et al., 2011). The concentration of copper estimated by digesting the Cu-NP adsorbed rice husk and then analyzing by ICP-OES was found to be 2962 mg/Kg.
Table IV. Percent of peaks of different elements in EMAX pattern.
|
Elements |
Weight (%) |
Atomic (%) |
|
C |
41.10 |
54.98 |
|
O |
37.89 |
38.06 |
|
Al |
0.45 |
0.27 |
|
Si |
4.66 |
2.67 |
|
Cu |
15.90 |
4.02 |
|
Total |
100.00 |
100.00 |
The uptake of copper by from cuprate solutions is time dependent. The sodium hydroxide swelling of rice husk cellulose fibers occurs within a few minutes and then it shrinks. However, to achieve high levels of copper uptake, the material requires enough time of soaking in cuprate solutions. In alkaline solutions, copper ions penetrate slowly into the rice husk, and have been suggested to change the crystalline structure of cellulosic material (Ogawa et al., 2014). In basic solution, these cellulose copper complexes are stable, but in solutions of ascorbate, copper leaches out of the cellulosic material and is reduced to nanoparticles on the surface. The copper contents were found to be ranged 3mg Cu per g to 5 mg per g rice husk and the soak time was 24 h to 48 h. Literature has the findings that 10-65 Cu per g by paper (Dankovich and Smith, 2014) and 225 mg of Cu per g of cotton cellulose (Davidson and Spedding, 1958). The SEM scan (Fig. 1) showed that CU-NP are present on the surface and not embedded inside. This indicates that the Copper uptake occurs completely at the outer surface and not penetrates inside the rice husk.
Anciently silver has been used for drinking water treatment due to its effective purification properties (Campbell, 2005; Prema, 2011; Chan et al., 2015). However, Cu-NP is proved as effective to control and suppress bacterial escalation. Number of applications has been developed to check the efficacy of Cu-NP against bacterial growth (Dey, 2012). Anciently silver has been used for drinking water treatment due to its effective purification properties (Campbell, 2005; Prema, 2011; Chan et al., 2015).
Copper in an essential trace element required for human health. According to WHO guidelines and PAK PSQCA (Pakistan Standard Quality Control Association) standard, the permissible limits for copper in drinking water are 2.0 and 1.0 ppm respectively. Ingesting levels higher than 3ppm in drinking water for two weeks can cause gastrointestinal irritation (2004). The average copper concentration in the filter effluent was from filtering synthetic water through Cu-NP adsorbed rice husk was the 0.86 ppm, which is below the limits.
This low copper release per liter suggests the CuNP paper could be a long-lasting water purifier. Literature the findings are available that Cu-NP also efficient against Staphylococcus, Aureus, Bacillus subtilis, Pseudomonas, Aeruginosa and Escherichia coli (Azam et al., 2012).
RSM for bactericidal activity of Cu-NP adsorbed rice husk
The 16 experiments of BBD in duplicate were performed in a glass column to study the bactericidal activity of Cu-NP adsorbed rice husk column height, flow rate and bacterial load. Different combination of variables along with observed and predicted values of responses are presented in Table II. The calculated values of coefficient of determination (R2) and adjusted R2 were 0.994 and 0.985 respectively. This indicates that 99.4 % of experimental data was compatible with the predicted data as observed by applying the model. The R2 value is always between 0 and 1, and a value > 0.75 indicates aptness of the model. For a good statistical model, the value of coefficient R2 should be close to 1.0.
The significance of each coefficient was determined by Student’s t-test and p-values, and are listed in Table IV. The larger the magnitude of the t-values and smaller the p-value, the more significant is the corresponding coefficient. The values of “Prob > F” which are less than 0.05 indicate that the model terms are significant. In this study, the linear effect of flow rate and square effect of bacterial count are non-significant, while all other linear, square and interactive effects of column height, flow rate and bacterial count are significant because their p-values are greater than 0.05.
Optimization by response surface modeling
From the desirability charts, the optimum conditions for variables were 8 cm, 45.9 ml/min and 262.2 per ml for column height, flow rate and bacterial count respectively for the percentage removal of 94.15 %. These optimized conditions of all the variables were verified by conducting experiments. The experimental value was 95.5 %, very close to predicted values in model.
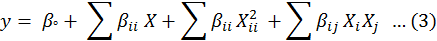
Effect of column height
Effect of column height on the percentage removal of bacteria was studied for 4.0, 8.0 and 12.0 cm. Table II shows that at 12 cm column height, 15ml/min flow rate and 250/ml bacterial load. The bacterial removal was 95.5% and when column height decreased to 4 cm, the bacterial removal was decreased to 65.3 %. As packed column height increased, bacterial count decreased because the contact time increased and bacterial removal efficiency increases.
Effect of flow rate
Effect of flow rate on the percentage removal of bacterial contents was studied for 15, 5, and 80 mL/min. Table II shows that at 12 cm column bed height, 250 bacterial count/mL and 15mL/min flow rate, the percentage removal of bacteria was 95.5%. When the flow rate was increased to 80 mL/min at same values of other variables, the bacterial removal was decreased to 68.6 %.
As the results show, when the flow rate is high, the breakpoint occurs faster as it is obvious that that an increase in flow rate decreased the percentage removal of bacteria. At high flow rate the contact time between nanoparticles and bacteria is minimized which leads to early breakthrough. Effect of flow rate is critical for large scale fixed bed treatment systems.
Effect of bacterial concentration
The initial concentration of bacterial count in synthetic water is also important and limiting factor, since a given mass of adsorbent can only adsorb a fixed number of bacteria and also the rate of adsorption decreased with the increase in bacterial concentration. At higher initial concentration, binding sites were quickly filled, resulting a decrease in equilibrium time. It has also been observed (Table II) bacterial concentration increased from 50 to 500 while keeping other variables constant the percentage removal of bacteria decreased ~ 3-10 %. The percentage of bacterial removal decreased with increase in initial concentration. Other researchers have examined the specific mechanism of copper inactivation of bacteria and suggest that the copper ions cause irreversible damage to bacterial membranes by increasing membrane permeability and destabilizing the cells (Santo et al., 2011).
CONCLUSION
Cu-NP were prepared and embedded on rice husk from copper sulphate solution using ascorbic acid as reducing agent. The prepared Cu-NP rice was husk characterized by SEM, EMAX and ICP-OES. The effect of independent variables of column bed height, flow rate and bacterial count on bacterial removal was monitored. The RSM model chosen for present column study shows correlation coefficient (0.9). The F-test with a very low probability value (p), also describes a very high significance for the second-order study. The optimum treatment conditions for column bed height, flow rate and bacterial count/mL were determined as respectively, and the predicted removal of bacterial count was 94.15%. The actual experimental value was 95.5%, both values are very close to each other. This material prepared by the Cu-NP embedded on rice husk has the potential to become a low cost way for drinking water purification.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Azam, A., Ahmed, A.S., Oves, M., Khan, M. and Memic, A., 2012. Size-dependent antimicrobial properties of cuo nanoparticles against gram-positive and-negative bacterial strains. Int. J. Nanomed., 7: 3527. https://doi.org/10.2147/IJN.S29020
Cady, N.C., Behnke, J.L. and Strickland, A.D., 2011. Copper-based nanostructured coatings on natural cellulose: Nanocomposites exhibiting rapid and efficient inhibition of a multi-drug resistant wound pathogen, a. Baumannii, and mammalian cell biocompatibility in vitro. Adv. Funct. Mater., 21: 2506-2514. https://doi.org/10.1002/adfm.201100123
Campbell, E., 2005. Study on life span of ceramic filter colloidal silver pot shaped (csp) model. http://potterswithoutborders.com/wp-content/uploads/2011/06/filter-longevity-study.pdf
Chan, C.C., Neufeld, K., Cusworth, D., Gavrilovic, S. and Ngai, T., 2015. Investigation of the effect of grain size, flow rate and diffuser design on the cawst biosand filter performance. Int. J. Serv. Learn. Eng. Humanit. Eng. Soc. Entrep., 10: 1-23. https://doi.org/10.24908/ijsle.v10i1.5705
Dankovich, T.A. and Smith, J.A., 2014. Incorporation of copper nanoparticles into paper for point-of-use water purification. Water Res., 6: 245-251. https://doi.org/10.1016/j.watres.2014.06.022
Davidson, G. and Spedding, H., 1958. The absorption of copper by cotton cellulose from sodium cuprate solutions. The normann compound. J. Text. Inst. Trans., 49: 621-626. https://doi.org/10.1080/19447025808662470
Dey, T., 2012. Magnetic nanoparticles and cellulosic nanofibers to remove arsenic and other heavy metals from water. In: Nanotechnology for water purification (ed. T. Dey), Ind. Edn. Universal-Publishers, Florida, pp. 1-28.
Hunt, M.E. and Rice, E.W., 2005. Microbial examination. In: Standard methods for the examination of water and wastewater (eds. A.D. Eaton, L.S. Clesceri, E.W. Rice and A.E. Greenberg), 21st Edn. American Public Health Association (APHA): Washington, DC, USA. pp 1-169.
Hameed, M., El-Aassar, M., Said, A. and Shawky, H., 2013. Using silver nanoparticles coated on activated carbon granules in columns for microbiological pollutants water disinfection in abu rawash area, great Cairo, Egypt. Aust. J. Basic appl. Sci., 7: 422-432.
Kim, J.S., Kuk, E., Yu, K.N., Kim, J.H., Park, S.J. and Lee, H.J., 2007. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med., 3: 95-101.
Islam, N., Ahmed, K., Nafees, M.A. Khalil, M., Hussain, I., Ali, M. and Imran, R., 2021. Physico-chemical and bacteriological analysis of drinking water of springs of Sherqilla, District Ghizer, Gilgit-Baltistan, Pakistan. Pakistan J. Zool., 53: 713-720. https://dx.doi.org/10.17582/journal.pjz/20160717150758
Lok, C.-N., Ho, C.-M., Chen, R., He, Q.-Y., Yu, W.-Y., Sun, H., Tam, P.K.-H., Chiu, J.-F. and Che, C.-M., 2006. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res., 5: 916-924. https://doi.org/10.1021/pr0504079
Mahmoodi, S., Elmi, A. and Nezhadi, S.H., 2018. Copper nanoparticles as antibacterial agents. J. Mol. Pharm. Org. Process. Res., 6: 140. https://doi.org/10.4172/2329-9053.1000140
Myers, R.H. and Dcm, C.M., 2009. Response surface methodology. In: Process and product optimization using designed experiments. 4th Edn. John Wiley and Sons New York, NY USA.
Phong, N.T.P., Khuong, V.Q., Tho, T.D., Van, Du, C., Minh, N.H., 2011. Green synthesis of copper nanoparticles colloidal solutions and used as pink disease treatment drug for rubber tree. Proc. Int. Workshop Nanotechnol. Appl. (IWNA), 2011: 10-12.
Ponnusamy, S.K. and Subramaniam, R., 2013. Process optimization studies of congo red dye adsorption onto cashew nut shell using response surface methodology. Int. J. Ind. Chem., 4: 17. https://doi.org/10.1186/2228-5547-4-17
Prema, P., 2011. Chemical mediated synthesis of silver nanoparticles and its potential antibacterial application. In: Progress in molecular and environmental bioengineering-from analysis and modeling to technology applications (ed. A. Capri). In Tech. https://doi.org/10.5772/22114
Prüss, A., Kay, D., Fewtrell, L. and Bartram, J., 2002. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ. Hlth. Perspect., 110: 537. https://doi.org/10.1289/ehp.110-1240845
Santo, C.E., Lam, E.W., Elowsky, C.G., Quaranta, D., Domaille, D.W., Chang, C.J. and Grass, G., 2011. Bacterial killing by dry metallic copper surfaces. Appl. environ. Microbial., 77: 794-802. https://doi.org/10.1128/AEM.01599-10
Santo, C.E., Taudte, N., Nies, D.H. and Grass, G., 2008. Contribution of copper ion resistance to survival of escherichia coli on metallic copper surfaces. Appl. environ. Microbial., 74: 977-986. https://doi.org/10.1128/AEM.01938-07
Shuster, N.A., 2010. Antibacterial effects of solid copper on waterborne bacteria. A Project of California State Science Fair. http://csef.usc.edu/History/2010/Projects/J1126.pdf
Singh, R., Chadetrik, R., Kumar, R., Bishnoi, K., Bhatia, D., Kumar, A., Bishnoi, N.R. and Singh, N., 2010. Biosorption optimization of lead (ii), cadmium (ii) and copper (ii) using response surface methodology and applicability in isotherms and thermodynamics modeling. J. Hazard. Mater., 174: 623-634. https://doi.org/10.1016/j.jhazmat.2009.09.097
Vainio, U., Pirkkalainen, K., Kisko, K., Goerigk, G., Kotelnikova, N. and Serimaa, R., 2007. Copper and copper oxide nanoparticles in a cellulose support studied using anomalous small-angle x-ray scattering. Clusters Nanostruct., 42: 93-101. https://doi.org/10.1140/epjd/e2007-00015-y
To share on other social networks, click on any share button. What are these?









