Compatibility of Chemical and Biological Control for the Management of Maize Stem Borer, Chilo Partellus, (Swinhoe) (Lepidoptera; Pyralidae)
Compatibility of Chemical and Biological Control for the Management of Maize Stem Borer, Chilo Partellus, (Swinhoe) (Lepidoptera; Pyralidae)
Inzimamul Haq1*, Shahid Sattar1, Bashir Ahmed1, Qamar Zeb2 and Amjad Usman3
1Department of Plant Protection, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Entomology, Agricultural Research Institute Tarnab, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Department of Entomology, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | With a view to use Trichogramma chilonis as parasitoid in the integrated pest management of Chilo partellus in maize field, basic studies on the efficacy of some insecticides against Chilo partellus in Jalal variety of maize and selectivity for the bio-control agent, T. chilonis were carried out under field conditions at Agricultural Research Institute, Tarnab Peshawar during 2016. Treatments viz Proclaim (Emamectin benzoate® 1.9 EC) + T. chilonis, Confidor® (Imidacloprid 200 SL) + T. chilonis, Chlorpyrifos® 40 EC + T. chilonis, Neem seed extract + T. chilonis and release of T. chilonis alone were applied to the Jalal variety. For C. partellus infestation, the data was recorded on the basis of leaf injury scale from 1 to 5 and percent dead hearts. Results showed that Imidacloprid + T. chilonis resulted in significantly lower leaf injury m-2 (1.32) and dead hearts (4.16 %) closely followed by the plots treated with emamectin benzoate + T. chilonis with leaf injury m2 (1.74) and dead hearts (7.50 %) while significantly higher leaf injury m2 (3.64) and dead hearts (20.0 %) were recorded in control plots. Yield data followed almost the same pattern as observed in case of leaf injury and dead hearts. Significantly lower yield was recorded in the control plots (3.54 ton-ha), while significantly higher yield was recorded in the plots treated with imidacloprid (6.61 ton-ha) followed by emamectin benzoate (6.10 ton-ha) treated plots. Results of the field test showed that significantly higher (88.0 %) parasitism of host eggs by T. chilonis was recorded in T. chilonis alone released plots followed by neem extract of (69.33 %) parasitism, imidacloprid with (65.33 %) parasitism, while significantly lower (30.66 %) parasitism was recorded in chlorpyrifos treated plots. We conclude from this experiment that for the management of C. partellus, Imidacloprid is recommended as it exhibited high level of selectivity for T. chilonis parasitization capacity. However, further investigation is needed to study the effect of these chemicals on the whole life cycle of T. chilonis.
Received | August 06, 2017; Accepted | October 22, 2018; Published | November 25, 2018
*Correspondence | Inzimamul Haq, The University of Agriculture, Peshawar, Pakistan; Email: [email protected]
Citation | Haq, I., S. Sattar, B. Ahmed, Q. Zeb and A. Usman. 2018. Compatibility of chemical and biological control for the management of maize stem borer, Chilo Partellus, (Swinhoe) (Lepidoptera; Pyralidae). Sarhad Journal of Agriculture, 34(4): 904-911.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.4.896.903
Keywords | Maize, Chilo partellus, Trichogramma chilonis, Imidacloprid, Emamectin benzoate, Neem seed extract, Chlorpyrifos
Introduction
Maize, stem, borer, Chilo partellus is one of the noticeable biotic obstacles in maize production around the world (Pingali, 2000), especially in Asia, and Africa (Arabjafari and Jalali, 2007). It came to knowledge that huge losses, in maize, crop occurred each year in Pakistan (Khan et al., 2015). Yield reduction of 25-76 percent has been noted, by the, infestation of this insect pest alone (Kumar, 1997). Farid et al. (2007) reported that 12–48 percent damage is caused by C. partellus in district Peshawar.
Different insecticides as granules and foliar formulations have been used against maize stem borer but the granular formulations are preferred and recommended against maize stem borer (Saleem et al., 2014). Granular formulations of chlorpyrifos and Carbofuron were reported effective against maize stem borer by Bhat and Baba (2007). Singh and Sharma (2009) determined that chlorpyrifos was ineffective as compared to Cypermethrin (foliar application). Foliar application of insecticides Spinosad (Tracer) (Ahmed et al., 2002), Confidor (Ali et al., 2014; Mashwani et al., 2011) are conventionally recommended for the management of maize stem borer.
Trichogramma chilonis is the typical gregarious egg parasitoid widely distributed in the world (Haffmans, 2008). It has been recorded that T. chilonis parasitized the eggs of Chilo partellus, Helicoverpa armigera, Agrotis ipsilon, Spodoptera litura (Hassan et al., 1998). Jalali and Singh, 2003 evaluated potential of Trichogramma spp as a biological control agent and found appreciable results of T. chilonis parasitizing 77.98 % eggs of Chilo partellus when released at 0.1 million/ha. Ahmed et al., 2003 found T. chilonis little effective in controlling C. partellus infestation from maize plots.
The idea of unified insect pest control first appears to be assumed by Hoskin et al. (1993) when they mentioned that “biological control agents and chemical pesticides for the control of insect pest are considered as supplementing one another. Nature’s own balance provides the major part of the protection hence insecticides should be used as little as possible so as to least interfere with natural control of pests (Sattar et al., 2011). The trends to integrate the chemical and biological control in integrated pest management were successfully studied by (Brunner, 1998; Saljoqi and Walayati, 2013) using selective insecticides and bio-control agents.
Dependence on the chemical, pesticides, to avoid the infestation caused by the insect pests is a common practice in; Pakistan. The use of chemical pesticides for insect pest management has both useful and harmful effects. The useful effects include convenience, simplicity, flexibility and cost effectiveness. The usage of pesticide has created many problems, such as harmful effect on non-target organism (wildlife and humans), resistance adaptation by insect pest, resurgence and secondary pest’s outbreak (Badshah et al., 2015b). Certain insecticides have been noted dangerous showing different degrees of toxicity to the parasitoid Trichogramma spp (Preetha et al., 2010). Adult emergence and parasitism of Trichogramma spp was greatly lowered by the application of deltamethrin, cypermethrin, endosulfan, carbosulfan and profenofos (Bastos et al., 2006). Hussain et al. (2010) indicated that emamectin benzoate, lufenuron, triflumuron and imidacloprid have least effect on pre-imaginal development, so these insecticides could be used in conjunction with T. chilonis to control lepidopteron pests.
Keeping in view the value of maize crop in Khyber Pakhtunkhwa, the economic and social losses caused by the C. partellus, the present research was planned to screen out efficacy of insecticides along with T. chilonis for C. partellus in maize crop and determine its effect on maize yield. During the study these objectives were focused,
1). To determine the infestation level of maize stem borer in local Jalal variety of maize; 2). To find out compatibility of the insecticides with T. chilonis under field conditions.
Materials and Methods
Layout of experiment
The trial was carried out in ARI Tarnab, Peshawar. The experiment was laid out in Randomized Complete Block Design (RCBD). The experiment comprised six treatments replicated three times. The field was divided into subplots and each subplot size was (5×4 m2). Row to row and plant to plant distance were 58 cm and 16 cm respectively. A buffer zone of 1-meter width was kept in between subplots to avoid insecticide drift effect (Ahmed et al., 2003). Maize variety Jalal was sown in lines on a well-prepared seedbed in summer 2016. Standard agronomic practices were applied equally to all subplots. Following treatments were applied to the subplots.
T1= Emamectin benzoate + Trichogramma chilonis
T2= Imidacloprid + Trichogramma chilonis
T3= Neem seed extract + Trichogramma chilonis
T4= Chlorpyrifos + Trichogramma chilonis
T5= Trichogramma chilonis
T6= Control (water only)
Treatment description
Trichogramma chilonis: Trichogramma chilonis is an important egg parasitoid of C. partellus and found effective in parasitizing the eggs of C. partellus by several researchers like Muresan et al. (1989), Rawat et al. (1994), Cheng et al. (1995), Bonhof et al. (1997), Ahmad et al. (2003).
Insecticides: The insecticides Proclaim®-Emamectin benzoate-1.9 EC @ 500 ml ha-1 (Syngenta), Confidor®-Imidacloprid-200 SL @ 150 ml ha-1 (Buyer Crop Sciences), Chlorpyrifos®-40 EC @ 100 ml ha-1 (Arysta Life Science) and neem seed extract-10 % @ 500 ml ha-1 (Khyber Bazar, Peshawar) were used. The insecticides were diluted into application volumes according to their respective recommended concentrations. First spray of insecticides was done at 15 days after the emergence of maize plant in the field (Ali et al., 2014). Care was taken to avoid the drift effect of insecticides to other treatments. Second spray was done after two weeks interval.
Emamectin benzoate: Emamectin benzoate belongs to avermectins which is derived from Gram-positive soil bacterium Streptomyces avermitilis (Burg). Emamectin benzoate attacks its target both, through contact and stomach. Rameash et al., 2012 found Emamectin benzoate (0.002 %) to provide satisfactory control of C. partellus in maize.
Imidacloprid: Imidacloprid is a systemic insecticide that acts as an insect neurotoxin and belongs to a class of chemicals called the neonicotinoids which act on the central nervous system of insects, with much lower toxicity to mammals. Mashwani et al., 2011 and Arunkumara et al., 2017 recorded highest mean grain yield of maize in Imidacloprid treated plots.
Neem seed extract: Neem or Azadirachta indica A. Juss (Meliaceae) is a native tree of the Indo-Pak subcontinent having several beneficial compounds, some of which act as insecticides. It may act as a feeding and ovipositional deterrent, growth inhibitor or reduce the fecundity of insect (Sattar et al., 2011). With foliar application of Neem seed products, appreciable results were found in suppressing the densities of C. partellus (Tekie et al., 2016)
Chlorpyrifos: Chlorpyrifos is an organophosphate insecticide. Chlorpyrifos acts on pests primarily as a contact poison, with some action as stomach poison. Adamu et al., 2015 study revealed that foliar application of Chlorpyrifos was effective against the maize stem borer.
Release of Trichogramma chilonis: Culture of Sitotroga cerellela and T. chilonis were collected from rearing laboratory of Agriculture Research Institute Tarnab, Peshawar. The Trichocards were made by using 100 eggs of Angoumois grain moth, S. cerellela parasitized by T. chilonis. Saljoqi and Yu-rong (2004b) reported method was used for the preparation of Trichocards. Before first insecticide application these Trichocards impregnated with pupal stage of T. chilonis from which adults were ready to emerge within 24 hours were stapled on the lower side of leaves in the fields @ 1 card/subplot.
Placing Sitotroga cerellela eggs cards: Eggs of S. cerellela was artificially released in maize variety to evaluate the parasitism rate of T. chilonis (Cheema et al., 2004). Counted number of less than 24 hours old S. cerellela eggs glued to small paper cards of 2×5 cm size (50 eggs/card) was consistently assigned to all the treatments such that within the field one card received by each subplot, hung to one of the terminal shoot of a plant. The S. cerellela eggs cards were collected after two days from each treatment and were placed in petri dishes bearing their respective treatment number in manipulated environment section (25±20C temperature, 60±5 % R.H and 14:10 L: D period) (Jalali and Singh, 2003).
Preparation of neem seed extract: The neem seed powder was used as an aqueous suspension at the rate of 10% (Tekie et al., 2016). Neem seed was crushed using blender and the powder was sieved through mesh. For preparing a stock solution of 20 % aqueous neem seed extract, 1 kg of neem seed extract was mixed with 5 liters of water for 24 hours along with 10 g surf (detergent) and was stirred at interval of about 4 hours (Badshah et al., 2015b). After 24 hours, the mixture was filtered through muslin cloth to obtain an aqueous neem seed extract. Further concentration of 10 % (v/v) was prepared from it by using following formula (Khan et al., 2010).
V1 C1= V2 C2
V1 = Required volume; V2 = Given volume; C1 = Given concentration; C2 = Required concentration
Maize stem borer, Chilo partellus infestation
Leaf injury: Leaf injury were recorded at intervals of 3, 7 and 14 days after insecticide application on scale of 1 to 5, from ten selected plants of maize in each subplot using the standardized C. partellus leaf damage scoring system (Dissemond and Weltzein, 1986). The scale was.
Scale: Damage Symptoms: 1). Plant exhibiting slight damage pinholes on 1-2 leaves; 2). Plant showing slight damage on 3-4 leaves; 3). Plant showing injury pin opening, shot hole slit in about 1/3 total leaves, 4). Plant showing 50 percent of leaf damage; 5). Plant showing 2/3 total leaf injuries.
Dead hearts (%): The observation for dead hearts by maize stem borer was recorded two times at 10 day’s intervals after insecticides application (Ali et al., 2014). Twenty plants were randomly chosen at set intervals along parallel transect through the crops while avoiding the border rows. The selected plants were used for closer observation for the attack of maize stem borer. Number of dead hearts was then converted into total dead hearts (%). The dead hearts (%) was calculated by using the following formula; (Muresan et al., 1989).
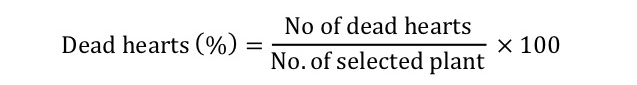
Grain yield (tons ha-1): Grain yield was calculated for each plot by the following formula, (Ali et al., 2014).

Field Parasitism (%) for Trichogramma chilonis: Percent parasitization of S. cerellela eggs by T. chilonis was computed after the emergence of T. chilonis adult from the parasitized eggs. The percent parasitism was calculated by the following formula; (Ahmed et al., 2003).
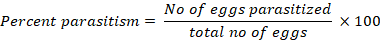
Statistical analysis: Data was statistically analyzed using M-STATC statistical package. F-test value was calculated at probability of 0.05%. LSD was used for means separation (Gomez and Gomez, 1980).
Results and Discussion
Effect of different treatments on stem borer damage and maize grain yield
Among all treatments (Table 1 and 2), Imidacloprid + T. chilonis has been observed to be efficacious in terms of lower mean leaf injury (1.32), mean percent dead hearts (4.16) caused by C. partellus and significantly higher yield (6.61-ton ha-1) in our findings.
Table 1: Effect of Trichogramma chilonis (Ishii) alone and in combination with different insecticides on leaf injury caused by Chilo partellus.
| Leaf Injury | ||||
| Treatments | 3-DAT | 7-DAT | 14-DAT | Means |
|
Emamectin benzoate + T. |
||||
| chilonis | 1.90 d | 1.60 d | 1.73 d | 1.74 d |
|
Imidacloprid + T. |
||||
| chilonis | 1.56 d | 1.26 d | 1.13 e | 1.32 e |
|
Neem seed extract + T. |
||||
| chilonis | 2.36 c | 2.20 c | 2.06 cd | 2.21 c |
|
Chloropyrifos + T. |
||||
| chilonis | 2.76 bc | 2.53 bc | 2.63 b | 2.64 b |
| Trichogramma chilonis | 2.86 b | 2.76 b | 2.30 bc | 2.64 b |
| Control | 3.53 a | 3.63 a | 3.76 a | 3.64 a |
| LSD (0.05) | 0.41 | 0.43 | 0.41 | 0.32 |
*Means sharing different letter in columns are different statistically by Least Significant Difference (LSD) test at 5 % level of probability; -DAT= Days after treatments.
Table 2: Effects of Trichogramma chilonis (Ishii) alone and in combination with different insecticides on dead hearts caused by Chilo partellus and grain yield.
| Treatments | 10-DAT | 25-DAT | Means | Yield (ton ha-1) |
|
Emamectin Benzoate + T. |
||||
| chilonis | 6.66 c | 8.33 cd | 7.50 c | 6.10 a |
|
Imidacloprid + T. |
||||
| chilonis | 5.0 c | 3.33 d | 4.16 c | 6.61 a |
|
Neem seed extract + T. |
||||
| chilonis | 1.66 b | 13.33 bc | 12.50 b | 5.27 b |
|
Chloropyrifos + T. |
||||
| chilonis | 15.0 ab | 16.66 ab | 15.83 b | 4.60 b |
| Trichogramma chilonis | 13.33 b | 15.0 b | 14.16 b | 4.94 b |
| Control | 18.33 a | 21.66 a | 20.0 a | 3.54 c |
| LSD (0.05) | 3.71 | 6.57 | 3.58 | 0.67 |
*Means sharing different letter in columns are different statistically by Least Significant Difference (LSD) test at 5 % level of probability; -DAT= Days after treatments.
The second best treatment was found emamectin benzoate + T. chilonis with mean leaf injury (1.74), mean percent dead hearts (7.50) and yield (6.10 ton ha-1). The highest mean leaf injury (3.64), mean percent dead hearts (20.0) and lower grain yield (3.54 ton ha-1) was obtained from the control plots. The results obtained by Ali et al. (2014) support the present findings as they reported that the application of imidacloprid caused minimum leaf injury of 1.15 (25 DAS) and 1.30 (40 DAS) by C. partellus in maize field. Imidacloprid was recorded effective against C. partellus by recorded minimum number of its infestation (Radha et al., 2006; Mashwani et al., 2011; Arunkumara et al., 2017). Emamectin benzoate has been reported to be effective against C. partellus by several researchers as Shahzad et al. (2010) found it superior compared to other tested chemicals in reducing the damage caused by C. partellus. Similarly, Ramesh et al. (2012) compared the efficacy of different insecticides against C. partellus in maize and recorded emamectin benzoate as the most better in lowering the damage caused by C. partellus. Neem seed extract + T. chilonis was found least effective against Chilo partellus compared to control by recording the mean leaf injury (2.21), mean percent dead hearts (12.50) and grain yield (5.27 ton ha-1). Our findings are in agreement with Ahmed et al. (2002), Tekie et al. (2016) who found neem derivative effective against Chilo partellus in maize crop. Treatment chlorpyrifos + T. chilonis was found ineffective compared to other treatments with mean leaf injury (2.64), mean percent dead hearts (15.83) and yield (4.60 ton ha-1). Our results are in line with Saleem et al. (2014) who recorded chlorpyrifos not effective against C. partellus. Our results are in contrast with Bhat and Baba (2007) who evaluated the efficacy of different insecticides against C. partellus and recorded chlorpyrifos effective in reducing the damages caused by C. partellus. The reason behind this might be difference in insecticides formulation and application method as we use chlorpyrifos as a foliar application and they did whorl applications of granular insecticide chlorpyrifos 10G @ 0.75 kg a.i/ha. Also, Bhat and Baba (2007) observed that granular formulation of insecticides showed superior efficacy than foliar application.
Table 3: Effect of different treatments on parasitization potential of Trichogramma chilonis.
| Treatments | % Parasitism |
| Emamectin Benzoate + T. chilonis | 32.66 c |
| Imidacloprid + T. chilonis | 65.33 b |
| Neem seed extract + T. chilonis | 69.33 b |
| Chloropyrifos + T. chilonis | 30.66 c |
| Trichogramma chilonis | 88.0 a |
| Control | - |
| LSD (0.05) | 18.20 |
*Means in the same column followed by different letters are significantly different at 5 % level of probability.
Compatibility of insecticides with Trichogramma chilonis
The data (Table 3) showed that in the absence of insecticides, T. chilonis parasitized the host eggs in significantly higher numbers (88.0 %). Neem seed extract was recorded harmless compared to the candidate insecticides regarding T. chilonis ovipositional preference and recorded (65.66 %) parasitism. This was followed by imidacloprid in which (69.33 %) of host eggs were parasitized by T. chilonis. Highly toxic effect was exerted on the parasitism rate of the T. chilonis by emamectin benzoate and chlorpyrifos which recorded (32.66, 30.66 %) parasitization, respectively. Results regarding imidacloprid selectivity for T. chilonis are consistent with those of Preetha et al. (2010) who reported that imidacloprid caused minimum effects on parasitism rate of T. chilonis. Similar results was found by Hussain et al. (2010) who reported that imidacloprid exhibited selectivity to T. chilonis having minimum effects on the emergence and parasitism capacity of T. chilonis. Neem seed extract was recorded safe among the tested insecticides regarding T. chilonis parasitization rate and adult emergence (Badshah et al., 2017). Our results are same with Reddy and Manjunatha (2000) who found neem seed extract safe to immature and adult stage of T. chilonis. Gandhi et al. (2005) also reported slightly harmful effects of toxicity of the botanical extract to T. chilonis and determined 64.2 % eggs hatchability, 57.0 % adult emergence and 60.4 % parasitization with the application of neem product. Other investigation has reported the harmful impacts of the avermectins in reducing parasitism rate of T. chilonis (Shipp et al., 2000). Correspondingly, Moura et al. (2004) found abamectin unsafe to the developmental stages and adult’s survival of T. pretiosum based on residual test. Hassan et al. (1998) also published the harmful effects of avermectins to parasitoid such as T. cacoeciae. Negative impact of the avermectins on the survival and parasitism rates of wasp were also reported by Kapuge et al. (2003) and Vianna et al. (2009). Our results in terms of the mild effect of emamectin benzoate on T. chilonis parasitization ability are differ with Kanna and Chandraskaran (2006) who found emamectin benzoate harmless for parasitism of host eggs by T. chilonis. This is due to the fact that emamectin benzoate is safe for immature and adult stages of T. chilonis at concentration of less than or equal to 1 ml/liter of water (Giraddi and Gundannavar, 2006) and the field recommended concentration @ 2.02 ml/liter against the C. partellus proved harmful to T. chilonis immature stages and adult survival under field conditions in our findings. We recorded chlorpyrifos unsafe to T. chilonis regarding development and parasitization capability. Our results are in line with Hussain et al. (2010) who tested toxicity of different insecticides to T. chilonis under semi field and laboratory conditions and recorded chlorpyrifos toxic to T. chilonis in terms of adult emergence and reduction in parasitism of host eggs by T. chilonis in the presence of chlorpyrifos. Similarly, Saber et al. (2004) also reported chlorpyrifos toxic to T. chilonis.
Conclusion and Recommendation
From the results, it is concluded that imidacloprid, emamectin benzoate and to certain degree neem seed extract were effective against Chilo partellus. Imidacloprid in combination with the release of T. chilonis for C. partellus management is strongly recommended.
Author’s Contributions
Inzimamul Haq: Major reseracher of the study.
Shahid Sattar: Major supervisor
Bashir Ahmed: Prepared field layout
Qamar Zeb: Co-supervised the study
Amjad Usman: Helped in data analysis statistically.
References
Adamu, R.S., M.S. Usman and R. Isah. 2015. Evaluation of four insecticides foliar spray for the management of maize stem borer, Busseola fusca (F.) on maize irrigated using furrow and basin irrigation methods at Kadawa, Kano state Nigeria. FUTA J. Res. Sci. (1): 7-14.
Ahmed, S., M.A. Saleem and I. Rauf. 2002. Field efficacy of some Bio-insecticides against maize and Jowar Stem Borer, Chilo partellus (Lepidoptera:Pyralidae). Int. J. Agric. Biol. 4(3): 332-334.
Ahmed, S., R.R. Khan and M. Khan. 2003. Some studies of Varietal Resistance in Spring Maize against Chilo partellus (Swinhoe) with and without release of Trichogramma chilonis. Int. J. Agric. Biol. 5(4): 552-554.
Ali, N., G. Singh, S.P. Singh, S.S. Dhaka, M. Ram and K.B. Tawar. 2014. Efficacy of different management practices against Chilo partellus (Swinhoe) in Kharif maize in Western Uttar Pradesh. Int. J. Adv. Res. 2(11): 952-956.
Arabjafari, K.H. and S.K. Jalali. 2007. Identification and analysis of host plant resistance in leading maize genotypes against spotted stem borer, Chilo partellus (Swinhoe) (Lepidoptera:Pyralidae). Pak. J. Biol. Sci. 10(11): 1885-1895. https://doi.org/10.3923/pjbs.2007.1885.1895
Arunkumara, C.G., M. Bheemanna and H.M. Shaila. 2017. Field evaluation of insecticides for management of spotted stem borer, Chilo partellus (Swinhoe) (Lepidoptera:Crambidae) on maize. J. Entomol. Zool. Std. 5(5): 1719-1723.
Badshah, H., F. Ullah, A. Farid, P.A. Calatayud and N. Crickmore. 2015b. Toxicity of neem seed Azadirachta indica Juss (Meliaceae) different solvents extracts against cotton Mealybug, Phenacoccus solenopsis Tinsley (Sternorrhyncha:Pseudococcidae) under Laboratory conditions. J. Entomol. Zool. Std. 3(4): 45-49.
Badshah, H., F. Ullah, P.A. Calatayud, H. Ullah and B. Ahmed. 2017. Can toxicants used against cotton mealybug Phenacoccus solenopsis be compatible with encyrtid parasitoid Aenasius bambawalei under laboratory conditions. Environ. Sci. Pollut. Res. 24(6): 5857–5867. https://doi.org/10.1007/s11356-016-8293-6
Basto, C.S., R.P. Almeida and F.A. Suinaga. 2006. Selectivity of pesticides used on Cotton, Gossypium hirustum to Trichogramma pretiosum reared on two laboratory hosts. J. Pest. Mgt. Sci. 62: 91-98. https://doi.org/10.1002/ps.1140
Bhat, Z.H. and Z.A. Baba. 2007. Efficacy of different insecticides against maize stem borer, Chilo Partellus (Swinhoe) and maize Aphid, Rhopalosiphum maids (Fit.) infesting maize. Pak. Ento. 29(2): 73-76.
Bonhof, M. J., W.A. Overholt, A. Huis, A.V. Polaszek and H.A. Van. 1997. Natural enemies of cereal stem borers in East Africa, a review. Insect. Sci. Appl. 17: 19-35.
Brunner, J.F. 1998. 41st Annual IDFTA Conference. Volume XXI: p. 199. Pest management-Novel chemical and Biological Control. Feb. Pasco, Washington, USA.
Cheng, W.Y., S.M. Chen, and Z.T. Wang. 1995. Differences in occurrence of Trichogramma chilonis and T. ostrinia between spring cane and sweet corn fields. Rept. Taiwan Sugar Res. Instt. 150: 23-41.
Cheema, G.M., A. Nasreen and M. Iqbal. 2004. Effect of different cotton genotypes on parasitism rate of Trichogramma chilonis (Ishii). Pak. J. Biol. Sci. 7(1): 87-89. https://doi.org/10.3923/pjbs.2004.87.89
Chaudhry, N.A. and M.A. Ansari. 1988. Insect pests of sugarcane in Pakistan. Programmed Farm. 3: 15-20.
Dissemond, A and H.C. Weltzein. 1986. Influence of sorghum and cowpea intercropping on plant pests in a semi-arid area of Kenya. Pak. Entomol. 51(3): 1147-1155.
Farid, A., M.I.N. Khan, A. Khan, S.U.K. Khattak and A. Sattar. 2007. Study on maize stem borer, Chilo partellus (Swinhoe) in Peshawar valley: Pak. J. Zool. 9(2): 127-131.
Gandhi, P.I., K. Gunasekaran, A. Poonguzhali, S. Anandham, R. Kim, G.H. Chung and S.A. Taere. 2005. Laboratory evaluation of relative toxicities of some insecticides against Trichogramma chilonis (Hymenoptera: Trichogrammatidae) and Chrysoperla carnea (Neuroptera; Chrysopidae). J. Asia-Pacific. Entomol. 8(4): 381-386. https://doi.org/10.1016/S1226-8615(08)60267-8
Giraddi, R.S. and K.P. Gundannavar. 2006. Safety of Emamectin benzoate, an Avermectin Derivative to the egg parasitoid, Trichogramma spp. Karnataka. J. Agric. Sci. 19(2): 417-418.
Gomez, K.A. and A.A. Gomez. 1980. Statistical procedures for Agriculture Research. p. 139-240 Wiley. Int. Sci. Pub. John Wiley and Sons, New York, Brisbane, Singapore.
Haffmans, S. 2008. Phasing in alternatives to Endosulfan, Joint paper of the PAN (Pesticide Action Network). Volume XII: International working group Alternatives to Synthetic Pesticides, Germany.
Halimie, M.A., M.S. Mughal, S.A. Mehdi and Z.A. Rana. 1989. Response of different timings of carbofuran (Furadan 3G) on the maize stem borer incidence and yield of the crop. J. Agric. Res. Lahore. 27(4): 337-40.
Hassan, S.A., B. Hafes, P.E. Degrande and K. Herai. 1998. The side effects of pesticides on the egg parasitoid, Trichogramma cacoeciae (Mar.), acute dose response and persistence tests. J. Appl. Entomol. 122: 569-573. https://doi.org/10.1111/j.1439-0418.1998.tb01547.x
Hoskin, W.M., A.D. Borden and A.E. Michellbachar. 1993. Recommendations for a more discriminating use of insecticides. Annu. Rev. Entomol. 37(5): 273-302.
Hussain, D., M. Ikram, Z. Iqbal, A. Ali and M. Saleem. 2010. Effect of insecticides on Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) on immature and adult survival. J. Agric. Res. 48(4): 531-537. Jalali, S.K. and S.P. Singh. 2003. Effect of neem product and bio-pesticides on egg parasitoid, Trichogramma chilonis, Ishii. J. Appl. Zool. Res. 14(2): 125-128.
Kapuge, S.H., S. Mcdougall and A.A. Hoffmann. 2003. Effects of methoxyfenozide, indoxacarb and other insecticides on the beneficial egg parasitoid Trichogramma chilonis (Hymenoptera: Trichogrammatidae) under laboratory and field conditions. J. Econ. Entomol. 96: 1083-1090. https://doi.org/10.1093/jee/96.4.1083
Kanna, S. S. and S. Chandrasekaran. 2006. Safety of emamectin 5 SG on Trichogramma chilonis and Chrysoperla carnea under laboratory conditions. J. Ecotoxicol. Environ. 16(6): 509-514.
Khan, I.A., M.N. Khan, R. Akbar, M. Saeed, A. Farid, I. Ali, M. Alam, K. Habib, W. Fayaz, S. Hussain, B. Shah and S.R.A. Shah. 2015. Assessment of different control methods for the control of maize stem borer, Chilo partellus in maize crop at Nowshera-Pakistan. J. Entomol. Zool. Stu. 3(4): 327-330.
Khan, M.H., M. Sarwar, A. Farid and F. Syed. 2010. Compatibility of Pyrethroid and different concentrations of Neem seed extract on parasitoid Trichogramma chilonis (Hymenoptera: Trichogrammatidae) under laboratory conditions. The Nucleus. 47(4): 327-331.
Kumar, H. 1997. Resistance in maize to Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae): role of stalk damage parameters and biological control. Crop. Protect. Sci. 16: 375-381. https://doi.org/10.1016/S0261-2194(96)00107-X
Mashwani, M.A., F. Ullah, S. Sattar, S. Ahmed and M.A. Khan. 2011. Efficacy of different insecticides against maize stem borer, Chilo partellus (Swinhoe) (Lepidoptera:Pyralidae) at Peshawar and Swat valleys of Khyber Pakhtunkhwa, Pakistan. S. J. Agric. 27(3): 459- 465.
Moura, A.P., G.A.A.E. Carvalho, G.A. Pereira and L.C.D. Rocha. 2004. Selectivity evaluation of insecticides used to control tomato pests to Trichogramma pretiosum. Biol. Control. 51: 769-778.
Muresan, N., S. Mushtaq and R. Ahmed. 1989. Relative toxicity of neem products and insecticides against maize stem borer and maize aphid. Pak. J. Zool. 31(5): 237-40.
Pingali, P.I. CIMMYT. 2000. World Maize Facts and Trends. Meeting World Maize Need: Technological Opportunities and Priorities for the Public Sector. CIMMYT, Mexico. D.F. 57p.
Preetha, G., T. Manoharan, J. Stanley, S. Suresh and S. Kuttalam. 2010. Impact of Imidacloprid on egg, egg-larval and larval parasitoid under laboratory conditions. J. Plant Protect. Res. 50(4): 535-540.
Rawat, U.S., A.D. Pawar and V. Joshi. 1994. Impact of inundative releases of Trichogramma chilonis for the control of maize stem borer, Chilo partellus in Himachal Pradesh. Plant Protec. Bull. 46: 28-30.
Rameash, K., A. Kumar and H. Kalita. 2012. Biorational Management of Stem borer, Chilo partellus in Maize. Ind. J. Plant Protect. 40(3):208-2013.
Radha, I.T.S., T. Madhumathi and V.S. Rao. 2006. Studies on management of major insect pests on maize with different groups of insecticides. Ind. J. Plant Protect. 34(2): 252-255.
Rameash, K., A. Kumar and H. Kalita. 2012. Biorational management of stem borer, Chilo partellus in maize. India. J. Plant Protec. 40(3): 208-213.
Reddy, G.V.P and M. Manjunath. 2000. Laboratory and field studies on the integrated pest management of Helicoverpa armigera in cotton, based on performance trap catch threshold level. J. Appl. Entomol. 125(5): 212-216.
Saleem, Z., J. Iqbal, S.G. Khattak, M. Khan, N. Muhammad, Z. Iqbal, F.U. Khan and H. Fayyaz. 2014. Effect of different insecticides against maize stem borer infestation at Barani Agricultural Research Station, Kohat, KPK, Pakistan During Kharif. Int. J. Life. Sci. Res. 2(1): 23-26.
Saljoqi, A.U.R. and W.K. Walayati. 2013. Management of sugarcane stem borer Chilo infuscatellus (Snellen) (Lepidoptera: Pyralidae) through Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae) and selective use of insecticides. Pak. J. Zool. 45(6): 1481-1487.
Saljoqi, A.U.R. and H.E. Yu-Rong. 2004b. Effect of host and parasite density on Trichogramma ostrinae. J. South. China. Agric. Univ. 25(3): 120 – 122.
Sattar, S., F. Ullah, A.U.R. Saljoqi, M. Arif, H. Sattar and J.I. Qazi. 2011. Toxicity of some new insecticides against Trichogramma chilonis under laboratory and extended laboratory condition. Pak. J. Zool. 43(6): 1117-1125.
Saber, M., M.J. Hejazi and S.A. Hassan. 2004. Effects of Azadirachta/neemazal on different stages and adult life table parameters of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Appl. Entomol. 97(3): 905-910.
Shipp, J.L., K. Wang and G. Ferguson. 2000. Residual toxicity of avermectins b1 and pyridaben to eight commercially produced beneficial arthropod species used for control of greenhouse pests. Biol. Control. 17: 522-131. https://doi.org/10.1006/bcon.1999.0784
Shehzad, M.A., Z.A. Rana, I.U. Haq and H. Tariq. 2010. Screening of different insecticides against maize shoofly, Atherigona soccata (Rond.) and maize borer, Chilo partellus (Swin.). Sci. Int. 22(7): 293-295.
Singh, P. and R.K. Sharma. 2009. Effect of insecticides for the control of maize stem borer, Chilo partellus (Swinhoe). Mysore. J. Agric. Sci. 43(3): 577-78.
Tekie, H., E. Soeyoum and R.C. Saxena. 2016. Potential of neem, Azadirachta indica in the management of Chilo partellus on maize in Kenya. African. Entomol. 14(2): 373-379.
Vianna, U.R., D. Pratissoli, J.C. Zanuncio, E.R. Lima, J. Brunner, F.F. Pereira and J.E. Serrao. 2009. Insecticide toxicity to Trichogramma pretiosum (Trichogrammatidae:Hymenoptera) females and effect on descendant generation. Ecotoxicol. 18: 180-186. https://doi.org/10.1007/s10646-008-0270-5
To share on other social networks, click on any share button. What are these?








