Comparison of Heavy Metals Concentration in Different Tissues of Four Wild Bird Species
Research Article
Comparison of Heavy Metals Concentration in Different Tissues of Four Wild Bird Species
Noha M. El-Shabrawy1, Atef M. Kamel2, Aza S. Goda1, Gehad R. Donia1, Ahmed M. Salah-Eldein2*
1Department of Animal and Poultry Health, Animal and Poultry Production Division, Desert Research Center, Cairo, Egypt; 2Department of Wildlife and Zoo, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
Abstract | The extensive growth of human activity, urbanization, agricultural and industrial processes result in increasing the number of pollutants introduced into the environment. Continuous monitoring of such pollutants required an indicator sensitive to environmental toxins and represents its accumulative effect. The aim of the present study is to determine the preferable tissue and bird that can be used as a bio-indicator for heavy metals pollution in El-Salam Canal which is considered as an important source of water for land irrigation and drinking of livestock in north Sinai, Egypt. A total of 58 wild birds belonging to 4 different species; Egyptian barn swallow (Hirundo rustica), house sparrow (Passer domestics), great white egret (Ardea alba) and striated heron (Butorides striata) were hunted by a net trap to determine the concentration of lead (Pb), cadmium (Cd), copper (Cu), zinc (Zn) and manganese (Mn) in their muscle, liver, kidney and feather. Also, 16 water samples were collected to assess the level of heavy metals pollution in the study area. The result revealed that the kidney is the most tissue burdens the highest concentration of heaviest metals among the examined tissues. Egyptian barn swallow and house sparrow have a great tendency to accumulate heavy metals in their tissues among other examined birds. Levels of Pb in Egyptian barn swallow and house sparrow have exceeded the normal background level. Levels of Cu exceed the normal background level in all examined birds except great white egret. Levels of Pb and Cd concentrations in water exceed the permissible limits.
Keywords | Wild birds, Bio-indicator, Heavy metals, Pollution
Received | September 13, 2021; Accepted | December 02, 2021; Published | January 05, 2022
*Correspondence | Ahmed M. Salah-Eldein, Lecturer of Wildlife and Zoo, Faculty of Veterinary Medicine, Suez Canal University, 41522 Ismailia, Egypt; Email: [email protected]
Citation | El-Shabrawy NM, Kamel AM, Goda AS, Donia GR, Salah-Eldein AM (2022). Comparison of heavy metals concentration in different tissues of four wild bird species. Adv. Anim. Vet. Sci. 10(2): 307-315.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.2.307.315
ISSN (Online) | 2307-8316
Introduction
Nowadays, the degradation of ecosystems is increasing due to the extensive growth of human activity (Xu et al., 2013) and as a result of industrial processes and excessive use of fertilizers and other chemicals in agriculture, large quantities of pollutants were accumulated in the environment (Martinez-Lo´pez et al., 2005). Unlike other pollutants, heavy metals are non-biodegradable and persistent in nature for a long time causing biological magnification that may lead to severe health problems to human and wildlife (Malik and Zeb, 2009). Exceeding the permissible limits of heavy metals in the environment are considered to be toxic and interfere with essential metabolic and biochemical processes in a living organism by altering the activity of different enzymes, increasing free radicals and disrupting the antioxidant mechanism (Isaksson, 2010). Hence, the need for biological indicators for continuous monitoring of the level of heavy metals in the environment is significantly increased. Wild birds can be used as a bio-indicator for heavy metals pollution as it widely distributed, long lived, occupy several trophic levels according to their feeding habits and are sensitive to environmental pollutants (Kekkonen, 2011). Moreover, wild birds reflect the bio-accumulation of such meals in the environment and express their hazards to human rather than the physical environmental elements (Edison et al., 2007).
El-Salam Canal is one of the irrigation projects in Egypt that located at the northern Sinai aimed to reclaim an estimated 620,000 feddans of desert situated along the Mediterranean coast of Sinai by diverting considerable amounts of agricultural drainage water to newly reclaimed areas after blending with Nile water in a ratio about 1:1. The canal water is acceptable for irrigation, with much concern directed towards the chemical contents of the trace elements because serious pollution, in the long run, may be caused due to the over-usage of agricultural wastewater (Othman et al., 2012).
In the current study, levels of lead (Pb), cadmium (Cd), copper (Cu), zinc (Zn) and manganese (Mn) were determined in muscle, liver, kidney and feather of four wild birds; Egyptian barn swallow (Hirundo rustica), house sparrow (Passer domestics), great white egret (Ardea alba) and striated heron (Butorides striata) and also in water samples from the study area.
So, this work is aimed to determine the significance between metal concentrations in different tissues of examined birds (muscle, liver, kidney and feather) and to evaluate the species differences in metal accumulation.
Materials and Methods
Study area
El-Salam Canal consists of two main parts; the first part (El-Salam Canal) lies west of the Suez Canal with (89.750 km) long, while the second part (El-Sheikh Gaber Canal); the extension of El-Salam Canal, is located east of the Suez Canal with a total length of (163.000km). Both parts are connected through a (770m) long siphon, under the Suez Canal (Elkorashey, 2012). This study was carried out in village Baloza which lies between latitude (30° 58’ and 30° 59’ N) and longitude (32° 25’ and 32° 28’ E); the northwestern part of Sinai, Egypt; irrigated with water through El-Salam Canal.
Sampling
A total of 58 resident wild birds was trapped using nets in the non-migrating season (March, April, May and June) and they were identified into four species; Egyptian barn swallow (Hirundo rustica) (n=27), house sparrow (Passer domestics) (n=16), great white egret (Ardea alba) (n=9) and Striated heron (Butorides striata) (n=6).
Breast feathers were collected and stored in clean labelled plastic bags. Pectoral muscles, livers and kidneys were dissected and stored separately in polyethylene bags at −20oC till preparation and digestion.
A total of 16 water samples (one liter for each) was collected from the study area in clean, dry and screw-capped plastic bottles. The water samples were then preserved via adding 5 ml concentrated nitric acid at collection to reduce the pH below 2 to prevent the microbial reactions (Hegazy et al., 2016).
Preparation of samples and analysis
Feathers samples were digested according to Adout et al. (2007) by adding 2 ml of high purity concentrated nitric acid and 1 ml of 30% hydrogen peroxide in test tube. Test tubes with loosely screwed caps were then placed in a hot water bath, at a close to boiling. In most cases, the sample was completely digested after about 60 minutes. Another portion of the digesting solution was added for a second round if a visible precipitate remained.
Muscle, kidney and liver samples were digested according to Al Ghais (1995) by adding a mixture of 10 ml nitric and perchloric acid (4:1) to the tissue samples. The Initial digestion was carried out for 3-4 hours at room temperature, followed by one hour of careful heating at 40-45 oC to prevent frothing. The temperature was raised to 70-80 oC with gentle shaking until the digestion was complete.
The residue was allowed to cool to room temperature before being diluted to 20 ml with deionized water and filtered by Whatman filter paper (0.45μm).
Water samples were prepared according to Jan and Young (1978) in a separation funnel by adding 5ml of 1% ammonium pyrolidine dithiocarbamate (APDC) and 10 ml of methyl isobutyl ketone (MIBK) to the water sample. The mixture was shaken for 2 minutes and left for 30 minutes to allow the layers to separate. The MIBK layer was used for analysis.
The samples were analysed using inductively coupled plasma (ICAP- 6500 Due) Thermo Scientific, England in Desert Research Center. To check for contamination, blank samples were prepared and analysed using the same procedure. All heavy metals concentration (µg/g) in tissues was estimated on a dry weight basis.
The bioconcentration factor (BCF) was calculated according to Walker et al. (1996) to compare values of heavy metals in different tissue of examined birds to its values in water.
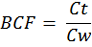
Where Ct is the concentration of heavy metals in the tissue of the bird and Cw is the concentration of the heavy metals in water.
The statistical analysis of data was carried out using SPSS 17.0 version (Chicago, USA) program. One-way ANOVA with Bonferroni test at a significance difference (P<0.05) was used according to (Snedecor and Cochran, 1989) to compare the level of heavy metals between different tissues and birds.
This study was approved by the Scientific Research Ethics Committee at the Faculty of Veterinary Medicine, Suez Canal University (Ismailia, Egypt) (code # 2016004).
Results and Discussion
Comparison of heavy metals concentration within different tissues of each bird
Concentrations of Pb, Cd, Cu, Zn and Mn in muscle, liver, kidney and feather of the different examined birds were shown in (Table 1). The kidney of Egyptian barn swallow, house sparrow and striated herons appeared the highest accumulation of all metals with a significant difference with the other examined tissues (p< 0.05) except for Zn in striated heron where the feather showed the highest accumulation among the examined tissues (p< 0.05).
Regarding the great white egret, the kidney showed the highest accumulation for Pb and Mn with a significant difference with the other examined tissues (p< 0.05) while there is no significant difference between the kidney and liver of great white egret in the concentration of Cu and Zn (p>0.05) but both of them has a significant difference with the other examined tissues (p< 0.05). Also, its feather showed the highest accumulation of Cd with a significant difference with the other the examined tissues (p< 0.05).
This result revealed that the kidney has the highest concentration of heaviest metals among the examined tissues. Feather of striated heron and great white egret showed promising results as indicators for heavy metals pollution with Zn and Cd, respectively.
Comparison of heavy metal concentrations within tissues of different birds
Concentrations of Pb, Cd, Cu, Zn and Mn in different tissues of Egyptian barn swallow, house sparrow, great white egret and striated herons were shown in (Table 2).
The muscle of the Egyptian barn swallow showed the highest level of Pb and Cu (Figures 1, 3) with a significant difference with the muscle of the other examined birds while the muscle of house sparrow showed the highest accumulation of Cd (Figure 2) among the muscles of the examined birds (P<0.05).
There is no significant difference between the accumulation of Mn in the muscle of Egyptian barn swallow and house sparrow but both of them showed a significant difference with the muscle of the other examined birds (Figure 5). Also, there is no significant difference in the concentration of the Zn in the muscle of all examined birds (P>0.05) (Figure 4).
Table 1: Comparison of heavy metals concentration (µg/g) within different tissues of each birds.
| Muscle | Liver | Kidney | Feather | P Value | ||
| Egyptian barn swallow | Pb |
46.09a±1.9 |
56.61a±2.9 |
244.86b±11.5 |
47.76a±3.7 |
0.000 |
| Cd |
2.18a±0.2 |
3.16a±0.2 |
20.62b±0.9 |
3.52a±0.2 |
0.000 | |
| Cu |
42.51a±3.2 |
35.71a±1.9 |
267.65b±18.4 |
31.44a±1.7 |
0.000 | |
| Zn |
44.25a±3.9 |
61.1a±1.7 |
221.56b±13.8 |
144.74c±3.4 |
0.001 | |
| Mn |
77.76a±4.05 |
62.65a±1.4 |
888.18b±27.2 |
101.88a±6.9 |
0.000 | |
| House sparrow | Pb |
37.37ac±3.1 |
124.69a±6.4 |
872.54b±52.9 |
22.52c±1.2 |
0.039 |
| Cd |
3.24a±0.2 |
18.65b±1.7 |
25.00c±1.1 |
6.4a±0.3 |
0.000 | |
| Cu |
31.17a±1.03 |
47.56b±1.7 |
252.33c±17.9 |
29.11a±2.6 |
0.032 | |
| Zn |
40.46a±3.6 |
74.89b±5.2 |
193.58c±14.3 |
106.00b±8.4 |
0.048 | |
| Mn |
68.53a±5.06 |
121.41a±4.2 |
936.39b±28.3 |
116.37a±7.2 |
0.001 | |
| Great white egret | Pb |
5.54a±0.4 |
5.73a±0.2 |
13.08b±0.6 |
3.26c±0.2 |
0.002 |
| Cd |
0.12a±0.009 |
0.23a±0.01 |
0.31a±0.02 |
1.06b±0.09 |
0.011 | |
| Cu |
11.08a±0.9 |
17.04b±1.5 |
19.19b±1.1 |
8.74a±0.5 |
0.044 | |
| Zn |
32.47a±2.06 |
61.36b±2.2 |
62.26b±1.4 |
33.86a±2.2 |
0.000 | |
| Mn |
16.27a±0.71 |
18.69a±0.9 |
57.7b±2.02 |
4.81c±0.39 |
0.001 | |
| Striated heron | Pb |
4.08a±0.3 |
2.37a±0.7 |
29.46b±1.9 |
9.95c±0.31 |
0.018 |
| Cd |
0.81a±0.04 |
0.11b±0.00 |
3.32c±0.2 |
0.41ab±0.00 |
0.032 | |
| Cu |
23.27a±0.6 |
26.17a±1.7 |
52.73b±4.5 |
33.42a±2.9 |
0.000 | |
| Zn |
28.95a±0.8 |
92.06b±5.08 |
88.7b±5.6 |
107.09c±0.3 |
0.047 | |
| Mn |
9.28a±0.6 |
15.6a±0.9 |
108.49b±6.8 |
44.59c±0.8 |
0.000 | |
Data are presented as (Mean±SE). Within the same row, means carrying different superscript are significant at (P<0.05).
Table 2: Comparison of heavy metal concentration (µg/g) within each tissue of the different birds.
| Egyptian barn swallow | House sparrow | Great white egret | Striated heron | P value | ||
| Muscle | Pb |
46.09a±1.9 |
37.37b±3.1 |
5.54c±0.4 |
4.08c±0.3 |
0.017 |
| Cd |
2.18a±0.2 |
3.24b±0.2 |
0.12c±0.009 |
0.81ac±0.04 |
0.039 | |
| Cu |
42.51a±3.2 |
31.17b±1.03 |
11.08c±0.9 |
23.27b±0.6 |
0.039 | |
| Zn |
44.25a±3.9 |
40.46a±3.6 |
32.47a±2.06 |
28.95a±0.8 |
0.081 | |
| Mn |
77.76a±4.05 |
68.53a±5.06 |
16.27b±0.71 |
9.28b±0.6 |
0.040 | |
| Liver | Pb |
56.61a±2.9 |
124.69b±6.4 |
5.73c±0.2 |
2.37c±0.7 |
0.035 |
| Cd |
3.16a±0.2 |
18.65b±1.7 |
0.23a±0.01 |
0.11a±0.00 |
0.33 | |
| Cu |
35.71a±1.9 |
47.56b±1.7 |
17.04c±1.5 |
26.17ac±1.7 |
0.040 | |
| Zn |
61.1a±1.7 |
74.89a±5.2 |
61.36a±2.2 |
92.06b±5.08 |
0.049 | |
| Mn |
62.65a±1.4 |
121.41b±4.2 |
18.69c±0.9 |
15.6c±0.9 |
0.001 | |
| Kidney | Pb |
244.86a±11.5 |
872.54b±52.9 |
13.08c±0.6 |
29.46c±1.9 |
0.000 |
| Cd |
20.62a±0.9 |
25.00b±1.1 |
0.31c±0.02 |
3.32c±0.2 |
0.038 | |
| Cu |
267.65a±18.4 |
252.33a±17.9 |
19.19b±1.1 |
52.73b±4.5 |
0.018 | |
| Zn |
221.56a±13.8 |
193.58a±14.3 |
62.26b±1.4 |
88.7b±5.6 |
0.043 | |
| Mn |
888.18a±27.2 |
936.39a±28.3 |
57.7b±2.02 |
108.49b±6.8 |
0.000 | |
| Feather | Pb |
47.76a±3.7 |
22.52b±1.2 |
3.26c±0.2 |
9.95bc±0.31 |
0.044 |
| Cd |
3.52a±0.2 |
6.4b±0.3 |
1.06a±0.09 |
0.41a±0.00 |
0.009 | |
| Cu |
31.44a±1.7 |
29.11a±2.6 |
8.74b±0.5 |
33.42a±2.9 |
0.000 | |
| Zn |
144.74a±3.4 |
106.00b±8.4 |
33.86c±2.2 |
107.09b±0.3 |
0.001 | |
| Mn |
101.88a±6.9 |
116.37a±7.2 |
4.81b±0.39 |
44.59c±0.8 |
0.000 | |
Data are presented as (Mean±SE). Within the same row, means carrying different superscript are significant at (P<0.05).
The liver of the House sparrow showed the highest concentration of Pb, Cd, Cu and Mn (Figures 1, 2, 3 and 5) with a significant difference with the liver of other examined birds (p<0.05) while the liver of striated heron showed the highest concentration of Zn (Figure 4) with a significant difference with the liver of the other examined birds (p<0.05).
Table 3: Heavy metal concentration (mg/l) in water samples from El-Salam Canal (Mean±SE).
| Heavy metals | Mean±SE | Permissible limits |
| Pb | 0.070±0.00 | 0.01* |
| Cd | 0.064±0.00 | 0.003* |
| Cu | 0.043±0.00 | 2* |
| Zn | 0.049±0.00 | 5** |
| Mn | 0.128±0.0045 | 0.2** |
* WHO 2011; **WHO 2008.
The kidney of the house sparrow showed the highest accumulation of Pb and Cd (Figures 1 and 2) with a significant difference with kidneys of the other examined birds (p<0.05) while there is no a significant difference between the kidney of the house sparrow and Egyptian barn swallow in the concentration of Cu, Zn and Mn (Figures 3, 4 and 5) but both of them showed a significant difference with the kidneys of the other birds (P< 0.05).
The feather of the Egyptian barn swallow showed the highest concentration of Pb, Cd and Zn (Figures 1, 2 and 4) with a significant difference with the feather of the other examined birds (p<0.05) while there is no significant difference between feathers of Egyptian barn swallow, house sparrow and striated heron in the concentration of Cu (Figure 3) (p<0.05) but all of them show a significant difference with the feather of great white egret (P>0.05).
Regarding Mn (Figure 5), there is no significant difference in its concentration in the feather of Egyptian barn swallow and house sparrow (p>0.05) but both of them showed a significant difference with the feather of the other examined birds (p<0.05).
Heavy metals concentration in water samples
The levels of heavy metals in water of El-Salam Canal were summarized in table 3 and were compared with the permissible limits of heavy metals in water according to (WHO, 2008, 2011). Levels of Pb and Cd were above the permissible limits while levels of Cu, Zn and Mn were within the permissible limits.
Bioconcentration factor in the different tissue of examined birds
As shown in Table 4, the muscle and feathers of Egyptian barn swallow showed the highest bioconcentration of Pb levels between muscles and feathers of the other examined birds respectively, while the liver and the kidney of house sparrow showed the highest bioconcentration of Pb levels between livers and kidneys of the other examined birds.
Regarding Cd levels, muscle, liver, kidney and feathers of house sparrow showed the highest bioconcentration levels among the same tissues of the other examined birds.
Regarding Cu levels, the muscle and kidney of Egyptian barn swallow showed the highest bioconcentration levels between muscles and kidneys of the other examined birds respectively, while the liver of house sparrow showed the highest bioconcentration of Cu levels among the livers of the other examined birds. Meanwhile, the feather of striated heron showed the highest bioconcentration of Cu levels between the feathers of the other examined birds.
Regarding Zn levels, muscle, kidney and feather of Egyptian barn swallow showed the highest bioconcentration levels among the same tissue the other examined birds, while the liver of striated heron showed the highest bioconcentration of Zn levels between livers of the other examined birds.
Regarding Mn levels, the muscle of Egyptian barn swallow showed the highest bioconcentration levels between muscles of the other examined birds while liver, kidney and feathers of house sparrow showed the highest bio-concentration of Mn levels among the same tissues of the other examined birds.
Table 4: Bio-concentration factor of heavy metals in the different tissue of examined birds.
| Egyptian barn swallow | House sparrow | Great white egret | Striated heron | ||
| Pb | Muscle | 658.4 | 533.8 | 79.1 | 58.2 |
| Liver | 808.7 | 1781.2 | 81.8 | 58.2 | |
| Kidney | 3498 | 12464.8 | 186.8 | 420.8 | |
| Feather | 682.2 | 321.7 | 46.5 | 142.1 | |
| Cd | Muscle | 34.0 | 50.6 | 1.8 | 12.6 |
| Liver | 49.3 | 291.4 | 3.5 | 1.7 | |
| Kidney | 322.1 | 390.6 | 4.8 | 51.8 | |
| Feather | 55 | 100 | 16.5 | 6.4 | |
| Cu | Muscle | 988.6 | 724.8 | 257.6 | 541.1 |
| Liver | 830.4 | 1106.0 | 396.2 | 608.6 | |
| Kidney | 6224.4 | 5868.1 | 446.2 | 1226.2 | |
| Feather | 731.1 | 676.9 | 203.2 | 777.2 | |
| Zn | Muscle | 903.0 | 825.7 | 662.6 | 590.8 |
| Liver | 1246.9 | 1528.3 | 1252.2 | 1878.7 | |
| Kidney | 4521.6 | 3950.6 | 1270.6 | 1810.2 | |
| Feather | 2953.8 | 2163.2 | 691.0 | 2185.5 | |
| Mn | Muscle | 607.5 | 535.39 | 127.1 | 72.5 |
| Liver | 489.4 | 948.5 | 146.0 | 121.8 | |
| Kidney | 6938.9 | 7315.5 | 450.7 | 847.5 | |
| Feather | 795.9 | 909.1 | 37.5 | 348.3 | |
According to our knowledge and the available literatures, this study is considered to be the first one dealing with resident wild birds as a bio-indicator for some heavy metal pollution at El-Salam Canal.
In the current study, the kidney of all the examined birds showed the highest concentration of all metals except Zn in striated heron and Cd in great white egret where the feather showed the highest accumulation among the other examined tissues. This result disagreed with the result of Mansouri and Majnoni (2014) who recorded that the liver is accumulated the highest level of metals in both coot and mallard; that may due to the difference in the food items between birds in the two studies. Salah-Eldein et al. (2012) recorded that the feather of little tern showed the highest level of Zn and Cd among the examined tissues which also has the same food preference of great white egret and striated heron in our study; all of them feed on fish and crustacean. Our result indicated that the level of both Pb and Cd in the kidney and liver of Egyptian barn swallow and house sparrow were higher than the other examined tissue, this result agreed with Mansouri and Majnoni (2014). Liver and kidney are the most organs responsible for the detoxification in the body and this explains their high metal content. Also, the accumulation of toxic heavy metals in the kidney may indicate chronic exposure (Janaydeh et al., 2017).
Hepatic Pb level in waterfowl was suggested by Pain (1996), (7.5 µg/g d.w.) as background levels, (7.5– 23 µg/g d.w.) for subclinical poisoning, (23–57 µg/g d.w.) for clinical poisoning and (57 µg/g d.w.) for severe clinical poisoning. House sparrow in the current study recorded hepatic Pb concentration exceeded the level of severe clinical poisoning and Egyptian barn swallow recorded hepatic Pb concentration close to the level of clinical poisoning. Pb concentrations of 4 µg/g in feathers are known to be potentially toxic (Burger and Gochfeld, 2008). All the examined birds in the current study showed higher concentrations of Pb than 4 µg/g in their feathers except Great white egret.
Normal and toxic range of hepatic Cd for bird species (0.02– 1.5μg/g) and (70– 140 μg/g) respectively were described by Kitowski et al. (2017). None of the examined birds in the present study showed a toxic range of hepatic Cd. Kidney damage occurred in many species of birds when the Cd levels approached 20µg/g (Toman et al., 2005). The present study showed Cd level exceeded 20µg/g in the kidneys of Egyptian barn swallow and house sparrow. The kidney of the house sparrow showed the highest accumulation of both Pb and Cd.
Cu and Zn and Mn are essential elements responsible for various physiological functions in the body. Excessive Cu intake may cause ocular damage, respiratory malfunctions and certain disorders in gastrointestinal, hepatic, reproductive, hematological and endocrine systems (Stern, 2010). The background level of Cu in the liver of shorebirds reported by Kim et al. (2010) ranged from 10.6 to 25.9 µg/g. All of the examined birds except the great white egret exceed this level in the present study. Hepatic Zn concentration level of 525mg/kg d.w. can be regarded as indicative of poisoning (Taggart et al., 2009). None of the examined birds in the current study recorded that level. Mn is involved in several biochemical reactions in the living organism and acts as an essential micronutrient. Excessive intake of Mn causes neurobehavioral defects (Burger and Gochfeld, 2000) but the levels of Mn in tissues associated with these defects are often not determined (Burger, 2002). The kidney of the Egyptian barn swallow showed the highest concentration of Cu and Zn while the kidney of the house sparrow showed the highest concentration of Mn among the examined birds. The elevated concentration of Cu, Zn and Mn may be due to the extensive use of bactericides, fungicides and fertilizer in agriculture (Canova et al., 2020; Binkowski et al., 2013; Abdullah et al., 2015).
The findings of this study had shown that; Egyptian barn swallow and house sparrow have a great tendency to accumulate heavy metals in their tissues among other birds; this finding is in agreement with (Millaku et al., 2014). They are intermediate level consumers whose diet consists of invertebrates such as insects in case of Egyptian barn swallow, and seeds and soil invertebrates as in the case of house sparrow which may contribute to high Cd hepatic and renal concentrations (Carpene et al., 2006) and also the soil pollutants may easily get into the bird´s body (Sundaramahalingam et al., 2016). Birds living in terrestrial ecosystems contained more heavy metals than aquatic birds because of the high metabolic rate of the small birds. This may mean that smaller size species are more susceptible than the larger ones (Lebedeva, 1999). So the results of the current study suggest that the Egyptian barn swallow and house sparrow are suitable species to provide information on metal contamination, and this finding is in agreement with that stated for house sparrow by Baker et al. (2017) and Millaku et al. (2015). Also, the kidney considers the most tissue burdens the highest concentration of most of the examined heavy metals.
In the present study, the concentration of heavy metals in water samples collected from El-Salam Canal was in the following order: Mn>Pb>Cd>Zn>Cu respectively. The concentration of Pb and Cd is above the permissible limits according to (WHO, 2008, 2011). A higher concentration of heavy metals in water samples from El-Salam Canal was recorded by Saber et al. (2016); Donia et al. (2017) except for Pb and Cd, Abu Hashim and Negm (2018) and Ramadan et al. (2018). Meanwhile, lower concentrations of heavy metals in El-Salam Canal water than that detected in the current study were recorded by Geriesh et al. (2015), Abdel-Hamid et al. (2017) except for Pb and Zn Abdel-Mottaleb et al. (2017). These variations can be attributed to the differences between the localities, the amount of pollution from one area to another and the season in which studies were carried out. The increase of heavy metals in El-Salam Canal water may be attributed to the discharging of wastewater from El-Serw and Hadous drains; which are agricultural drains; constitute higher concentration values because of excess usage of agricultural fertilizers and agricultural pesticides (Geriesh et al., 2015; Abdel-Hamid et al., 2017).
Bioconcentrtion factor reflects the case when the level of toxin in an organism exceeds the level of that toxin in the surrounding environment. This factor is defined by the ratio of the heavy metal concentration of the organism to that of water (Kennish, 1992). The higher ratio, the more intense the bioconcentration of toxins (Sauliut et al., 2017). In the current study, the results of bioconcentration factor in the examined tissues of different birds confirmed our previous results that the kidney is the most organ accumulating the heavy metals and the most preferable tissue can be used as bioindicator for heavy metals pollution, where kidney of house sparrow showed the highest bioconcentration of Pb and Cd among the other examined tissues and the kidney of Egyptian barn swallow showed the highest bioconcentration of Cu, Zn and Mn among the other examined tissues.
Conclusions and recommendations
The kidney is the most tissue burdens the highest concentration of most of the heavy metals in the examined birds. Egyptian barn swallow and house sparrow have a great tendency to accumulate heavy metals in their tissues among other examined birds and can be considered as the most preferable birds to use as a bio-indicator for heavy metals pollution. Levels of Pb in Egyptian barn swallow and house sparrow have exceeded the normal background level. Levels of Cu exceed the normal background level in all examined birds except great white egret. Levels of Pb and Cd concentrations in water exceed the permissible limits.
Novelty Statement
According to our knowledge and the available literatures, this study is considered to be the first one dealing with resident wild birds as a bio-indicator for some heavy metal pollution at El-Salam Canal.
Author’s Contribution
Noha M. El-Shabrawy was responsible for the laboratory work and writing original draft and resources of manuscript.
Atef M. Kamel was participated in the design of the study.
Aza S. Goda was participated in the design of the study and drafting and revising the work. Gehad R. Donia was participated in the design of the study and drafting and revising the work. Ahmed M. Salah-Eldein was participated in data analysis, curation and investigation, manuscript writing, and final approval of the submitted version.
Conflicts of interest
The authors have declared no conflict of interest.
References
Abdel-Hamid MI, El-Amier YA, Abdel-Aal EI, El-Far GM (2017). Water quality assessment of El-Salam Canal (Egypt) based on physico-chemical characteristics in addition to hydrophytes and their epiphytic algae. Int. J. Ecol. Dev. Res., 3(1): 28-43.
Abdel-Mottaleb O, Ibrahim S, Moured S (2017). Ecological Assessment of some Trace Elements Status in North Sinai. Alex. Sci. Exch. J., 38(4): 856-871. https://doi.org/10.21608/asejaiqjsae.2017.4770
Abdullah M, Fasola M, Muhammad A, Eqani S (2015). Avian feathers as a non-destructive bio-monitoring tool of trace metals signatures. A case study from severely contaminated areas. Chemosphere, 119: 553-561. https://doi.org/10.1016/j.chemosphere.2014.06.068
Abu Hashim M, Negm A (2018). Sustainability of agricultural environment in Egypt. In: The Handbook of environmental chemistry, pp. 41-46.
Adout A, Hawlena D, Mamana R, Paz-tal O, Karpas Z (2007). Determination of trace elements in pigeon and raven feathers by ICPMS. Int. J. Mass. Spectrom., 267: 109-116. https://doi.org/10.1016/j.ijms.2007.02.022
Al-Ghais S (1995). Heavy metals concentration in the tissue Sparus sebra from the United Arab Emirates. Bull. Environ. Contam. Toxicol., 55: 581. https://doi.org/10.1007/BF00196039
Baker N, Dahms S, Gerber R, Greenfield R (2017). Metal accumulation in house sparrow (Passer domesticus) from Thohoyandou, Limpopo Province, South Africa. Afri. Zool., 52(1): 43-53. https://doi.org/10.1080/15627020.2017.1293491
Binkowski J, Stawarz R, Zakrzewski M (2013). Concentrations of cadmium, copper and zinc in tissues of mallard and coot from southern Poland. J. Environ. Sci. Health B., 48(5): 410-415. https://doi.org/10.1080/03601234.2013.742725
Burger J (2002). Food chain differences affect heavy metals in bird eggs in Barnegat Bay, New Jersey. Environ. Res. A, 90: 33-39. https://doi.org/10.1006/enrs.2002.4381
Burger J, Gochfeld M (2000). Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci. Total. Environ., 257: 37-52. https://doi.org/10.1016/S0048-9697(00)00496-4
Burger J, Gochfeld M (2008). Mercury and other metals in feathers of common eider (Somateria mollissima) and tufted pun (Fratercula cirrhata) from the Aleutian chain of Alaska. Arch. Environ. Contam. Toxicol., 56: 596-606. https://doi.org/10.1007/s00244-008-9207-5
Canova L, Sturini M, Maraschi F (2020). Evidence of low-habitat contamination using feathers of three heron species as a biomonitor of inorganic elemental pollution. Int. J. Environ. Res. Publ. Health, 17(21): 7776. https://doi.org/10.3390/ijerph17217776
Carpene E, Andreani G, Monari M, Castellani G, Isani G (2006). Distribution of Cd, Zn, Cu and Fe among selected tissues of the earthworm (Allolobophora caliginosa) and Eurasian woodcock (Scolopax rusticola). Sci. Total Environ., 363: 126-135. https://doi.org/10.1016/j.scitotenv.2005.06.023
Donia G, Hafez A, Wassif I (2017). Studies on some heavy metals and bacterial pollutants in tilapia fish of El Salam Canal, Northern Sinai, Egypt. Egypt. J. Aquat. Biol. Fish., 21: 67-84. https://doi.org/10.21608/ejabf.2017.7178
Edison B, Carlos AB, Elisangela DP, Kennedy AS, Jose AH (2007). Heavy metal concentration in tissues of Puffinus gravis sampled on the Brazilian coast. Rev. Brasil. Ornitol., 15: 69-72.
Elkorashey RM (2012). Investigating the water quality of El-Salam Canal to reconnoiter the possibility of implementing it for irrigation purposes. Nat. Sci., 10(11): 109-205.
Geriesh M, El-Rayes A, Gom’aa R, Kaiser M, Mohamed M (2015). Geo-environmental Impact Assessment of El-Salam Canal on the Surrounding Soil and Groundwater Flow Regime, North Western Sinai, Egypt. Catrina. 11(1): 103-115.
Hegazy W, Hamed M, Toufeek M, Mabrouk B (2016). Determination of Some Heavy Metals in water of the Southern Region of Lake Manzala, Egypt. Egypt. J. Aquat. Biol. Fish., 20(4): 69-81. https://doi.org/10.21608/ejabf.2016.11179
Isaksson C (2010). Pollution and its impact on wild animals: A meta-analysis on oxidative stress. EcoHealth, 7: 342-350. https://doi.org/10.1007/s10393-010-0345-7
Jan TK, Young DR (1978). Determination of microgram amounts of some transition metals in seawater by methyl isobutyl ketone-nitric acid successive extraction and flameless atomic absorption spectrophotometry. Anal. Chem., 50: 1250-1253. https://doi.org/10.1021/ac50031a014
Janaydeh M, Ismail A, Omar H, Zulkifli S, Bejo M, Abdel-Aziz N (2017). Relationship between Pb and Cd accumulations in house crow, their habitat, and food content from Klang area, Peninsular Malaysia. Environ. Monit. Assess., 190: 47. https://doi.org/10.1007/s10661-017-6416-2
Kekkonen J (2011). Evolutionary and conservation biology of the Finnish house sparrow. Academic Dissertation, Department of Biosciences, University of Helsinki, Finland.
Kennish MJ (1992). Ecology of estuaries: Anthropogenic effects. Institute of Marine and Costal Science Fisheries and Aquaculture, Rutgers University New Brunswick, New Jersey, pp. 1-6.
Kim J, Lee D, Koo T (2010). Effects of age on heavy metal concentrations of black-crowned night herons Nycticorax nycticorax from Korea. J. Environ. Monit., 12: 600-607. https://doi.org/10.1039/B911598F
Kitowski I, Wiacek D, Sujak A, Komosa A, Świetlicki M (2017). Factors affecting trace element accumulation in livers of avian species from East Poland. Turk. J. Zool., 41: 901-913. https://doi.org/10.3906/zoo-1606-43
Lebedeva NV (1999). Ecotoxicology and biogeochemistry of geographic populations of birds, Nauka, Moscow, pp. 99.
Malik R, Zeb N (2009). Assessment of environmental contamination using feathers of Bubulcus ibis L., as a biomonitor of heavy metal pollution, Pakistan. Ecotoxicology, 18(5): 522-536. https://doi.org/10.1007/s10646-009-0310-9
Manjula M, Mohanraj R, Devi M (2015). Biomonitoring of heavy metals in feathers of eleven common bird species in urban and rural environments of Tiruchirappalli, India. Environ. Monit. Assess., 187(5): 267. https://doi.org/10.1007/s10661-015-4502-x
Mansouri B, Majnoni F (2014). Comparison of the metal concentrations in organs of two bird species from western of Iran. Bull. Environ. Contam. Toxicol., 92(4): 433-439. https://doi.org/10.1007/s00128-014-1238-1
Martı´nez-Lo´pez E, Marı´a-Mojica P, Martı´nez J, Calvo J, Romero D, Garcı´a-Ferna´ndez A (2005). Cadmium in feathers of adults and blood of nestlings of three raptor species from a non-polluted mediterranean forest, South-Eastern Spain. Bull. Environ. Contam. Toxicol., 74: 477-484. https://doi.org/10.1007/s00128-005-0610-6
Millaku L, Imeri R, Trebicka A (2014). House sparrow (Passer domesticus) as bioindicator of heavy metals pollution. Eur. J. Exper. Biol., 4(6): 77-80.
Millaku L, Imeri R, Trebicka A (2015). Bioaccumulation of heavy metals in tissues of house sparrow (Passer domesticus). Res. J. Environ. Toxicol., 9(2): 107-112. https://doi.org/10.3923/rjet.2015.107.112
Othman A, Rabeh S, Fayez M, Monib M, Hegazi N (2012). El-Salam Canal is a potential project reusing the Nile Delta drainage water for Sinai desert agriculture: Microbial and chemical water quality. J. Adv. Res., 3(2): 99-108. https://doi.org/10.1016/j.jare.2011.04.003
Pain D (1996). Lead in waterfowl. In: Environmental contaminants in wildlife. Interpreting tissue concentrations. Lewis, Boca Raton, pp. 251-264.
Ramadan A, Abu-Zeid H, Talaat S, Abd El-Maksoud T, Sayed H, El-Hanbaly A (2018). Evaluation of Natural Radioactivity and Physico-Chemical Characteristics along El-Salam Canal, Egypt. Egypt. Int. J. Eng. Sci. Invent., 7(4): 51-63.
Saber M, Abu-Sedera S, Matter I, Zaghloul A (2016). Adverse impacts of El-Salam canal irrigation water on chemical characterizations of Sinai soils. Int. J. Pharm. Tech. Res., 9(9): 138-149.
Salah-Eldein AM, Gamal-Eldein MA, Lamiaa IM (2012). Resident wild birds as bio-indicator for some heavy metals pollution in Lake Manzala. Suez Canal Vet. Med. J., 17(1): 109-121.
Sauliut, G, Stankeviciut M, Svecevicius G, Baršien J, Valskien R (2017). Assessment of heavy metals bioconcentration factor (BCF) and genotoxicity response induced by metal mixture in Salmo salar tissues. Environmental Engineering. 10th International Conference. https://doi.org/10.3846/enviro.2017.043
Snedecor G, Cochran W (1989). Staistical Methods 8th edition. The Iowa State University Press.
Stern BR (2010). Essentiality and toxicity in copper health risk assessment. Overview, update and regulatory considerations. J. Toxicol. Environ. Health A, 73: 114-127. https://doi.org/10.1080/15287390903337100
Sundaramahalingam B, Somasundaram B, Jeyaraj P (2016). An opportunistic evaluation of heavy metal accumulation in house sparrow (Passer domesticus). Res. J. Biol., 4(1): 38-41.
Taggart M, Green A, Mateo R, Svanberg F, Hillstro L, Meharg A (2009). Metal levels in the bones and livers of globally threatened marbled teal and white-headed duck from El-Hondo, Spain. Ecotox. Environ. Saf., 72: 1-9. https://doi.org/10.1016/j.ecoenv.2008.07.015
Toman R, Massanyi R, Lukae N, Ducsay L, Golian J (2005). Fedility and content of cadmium in pheasant (Phasianus colchicus) following cadmium intake in drinking water. Ecotox. Environ. Saf., 62: 112-117. https://doi.org/10.1016/j.ecoenv.2005.02.008
Walker CH, Hpkin SP, Silby RM, Peakall DB (1996). Principals of ecotoxicology. UK Taylar and Francis Ltd, pp. 321.
WHO (2008). Guidelines for drinking water quality, Geneva.
WHO (2011). Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva.
Xu L, Wang T, Ni K, Liu S, Wang P, Xie S, Meng J, Zheng X, Lu Y (2013). Metals contamination along the watershed and estuarine areas of southern Bohai Sea, China. Mar. Pollut. Bull., 74: 453-463. https://doi.org/10.1016/j.marpolbul.2013.06.010
To share on other social networks, click on any share button. What are these?





