Comparative Methods for Controlling Root Knot Nematode, Meloidogyne javanica under Laboratory and Greenhouse Condition
Comparative Methods for Controlling Root Knot Nematode, Meloidogyne javanica under Laboratory and Greenhouse Conditions
Mohammed Abdel-Mageed Abdel-Aziz Abdel-Mageed1, Eman Alsayed Hammad2*, Nashaat Abdel-Aziz Mahmoud1 and Anas Farag El-Mesalamy1
1Agriculture Zoology and Nematology Department, Faculty of Agriculture, Al-Azhar University, Assiut, Egypt; 2Nematode Disease Research, Plant Pathology Research Institute, Agricultural Research Center (ARC) in Giza Egypt.
Abstract | Under laboratory conditions, aqueous, acetonic and methanolic extracts of Solanum nigrum, Atropa belladonna, Hyoscyamus muticus, Capsicum frutescens, Datura innoxia and Withania somnifera were tested against root-knot nematode, Meloidogyne javanica. All the tested materials affected the survival of the nematode juveniles and egg-masses hatched depending on materials property, concentrations and solvents used in extraction. The aqueous extract had the best percentage of mortality and inhibition of egg hatching compared to the acetonic and methanolic extracts. Whereas, aqueous extracts of deadly nightshade, winter cherry and chili pepper at concentration 500 ppm applied to the plants as foliar sprays alone, foliar sprays with chelates and soil drenches were tested against root-knot nematode M. javanica infecting tomato plants cv. Strain-B under greenhouse conditions. Almost tested materials have significantly reduced nematode parameters compared to Oxamyl 24% L. and the untreated plants (check). The degree of nematode reduction varied according to the method of applications and the type of materials. Adding the extract with the chelating nutrients improved the properties of the plant, but adding the extract as doses to the soil reduced the numbers of nematodes better than other methods of application.
Received | October 24, 2021; Accepted | December 03, 2021; Published | December 09, 2021
*Correspondence | Eman Alsayed Hammad, Nematode Disease Research, Plant Pathology Research Institute, Agricultural Research Center (ARC) in Giza Egypt; Email: mohammed.abdo9999@gmail.com, dreman.hammad@yahoo.com
Citation | Abdel-Mageed, M.A-M.A-A., Hammad, E.A., Mahmoud, N.A-A., and El-Mesalamy, A.F., 2021. Comparative methods for controlling root knot nematode, Meloidogyne javanica under laboratory and greenhouse conditions. Pakistan Journal of Nematology, 39(2): 88-94.
DOI | https://dx.doi.org/10.17582/journal.pjn/2021.39.2.88.94
Keywords | Root-knot nematode, Mortality, Hatching, Meloidogyne javanica
Introduction
Plant-parasitic nematodes have the greatest impact on crop productivity when they attack the roots of plants immediately after seed germination (Ploeg and Stapleton, 2001). Root-knot nematodes, Meloidogyne spp. are common pathogen that parasitizes vegetables and other crops and cause significant yield reductions worldwide (Sasser, 1980). Nematicides such as oxamyl, Thionazin, carbofuran are effective in controlling nematodes but are not ecofriendly for their serious threat to the ecological balance. The influenced of plant parts on controlling nematodes and consequently improving plant growth, voluminous studies had been done on several economic vegetable crops by many workers (Montasser et al., 1999; Olanigi et al., 2005; Sowley et al., 2014). Solanaceous plants have nematicidal activity for management nematode parasites (Hussain et al., 2011; Saeed et al., 2015) and the materials used in extractions varied depending to workers (Khan et al., 2008; Meena et al., 2010; Nandakumar et al., 2017a; Correia, 2014; Oplos et al., 2018). Youssef et al. (2016) used garlic clove and acetylsalicylic acid (ASA) aqueous extracts by soil drench and foliar spraying for controlling root-knot nematode, Meloidogyne incognita infecting sugar beet cv. Gazelle.
The work has summarized literatures on the use of plant extracts prepared as aqueous extracts or extracted with chemical solvents including methanol and acetone in the control of plant parasitic nematodes M. javanica in vitro. Whereas, the objectives of greenhouse study to determine the extracts against the infection of M. javanica and to compare between modes of applications, soil drenches, foliar spray alone and foliar spray with chelates.
Materials and Methods
Nematode inoculum
Pure culture of M. javanica was raised from single egg mass and maintained on tomato roots in greenhouse. Infected plants were uprooted from soil and the entire root system was dipped in water and washed gently to remove adhering soil. Egg-masses were picked with forceps and rinsed with sterile water then placed in 0.5% sodium hypochlorite (NaOCl) solution agitated for 4 minutes and rinsed with sterile water on a 26 µm sieve (Hussey and Barker, 1973). The eggs were incubated for 3-5 days using a modified Baermann funnel method (Southey, 1986) to obtain second stage juveniles (J2) for in vitro and pots experiments.
Extraction
Fifty grams of each plants powder were extracted with 500 ml of distilled water for 48 hrs. The suspension was filtered with Whatman No. 1 filter paper, and a concentration of “S” was prepared for each plant extract and was considered as standard. In addition, fifty grams of the powder of each plant was suspended in 500 ml of methanol or Acetone in a 1-L flask for 48 hours in the dark on an orbital incubator shaker at 200 rpm. The suspension was filtered under vacuum with Whatman No. 1 filter paper, then evaporated to dryness in a rotatory evaporator at 45 °C, and the resulting extract (5g) was stored at 5 °C until use. (Nandakumar et al., 2017b).
In vitro experiment
The evaluation was carried out in 5 cm clean Petri dishes in 3 replications for each treatment. The Petri dishes with distilled water were taken as control. One hundred second stage (J2) of M. javanica were suspended in 10 ml of different extracts with concentrations 250, 500 and 1000 ppm in acetone and methanol extracts. However, other dilutions of aqueous extract S/16, S/8 and S /4 were prepared by adding 16, 8 and 4 ml of distilled water to 1 ml of stander concentration “S” for studying the juveniles mortality. After 72 hours incubation, all dead and alive J2 were counted and the percentages of J2 mortality were calculated.
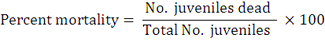
Five egg-masses medium size handpicked from the galls of eggplant were placed in each of Petri dishes containing 5 ml of extracts with concentrations 250 and 500 ppm in acetone and methanol extracts, other dilutions of aqueous extract S/8 and S /4 were prepared by adding requisite amount of distilled water to determine the effect on egg-masses hatching. Egg-masses kept in distilled water served as control. Each treatment were replicated 3 times. After 7 days exposure, the number of juveniles hatched were counted and the percentage of juveniles hatched and inhibition were calculated.


Greenhouse experiment
Two grams powder of Atropa belladonna, Withania somnifera leafs and Capsicum frutescens fruits were macerated in 100 ml of distilled water for 48 hrs. at 28o C (2% w/v) (Ardakani, 2012). The suspensions were filtered and used as standard concentrations (2%) for each plant and diluted with the appropriate amount of distilled water to obtain 500 ppm concentration. Also, the nematicide (Oxamyl) was applied according to the recommended dose with the same methods of applications. On the other hand, seeds of tomato var. Strain-B were cultured in seed boxes at the greenhouse and water daily for one month, then the slips were transferred gently at the pots filled with a 1:1 mixture of loamy sand soil. Two weeks after transplant, the tomato plants were inoculated with 2000 freshly newly hatched juveniles obtained from pure culture of Meloidogyne javanica.
Materials applied to plants as soil drenches, sprayed onto leaf surface alone or sprayed onto leaf surface with chelates1 g/L (Verdimix form EDTA) two days after inoculation (El-Eslamboly et al., 2019). All treatments including the check (untreated, nematode only, nematode and chelates and chelates only) were replicated four times. Forty-five days after inculcation the plants removed carefully from the soil, length and fresh weights of both shoots and roots were estimated. Also, the number of galls, egg-masses per root and eggs per egg-mass were counted. Nematode final population and rate of nematode reproduction were calculated. Data were analyzed according to Duncan’s Multiple Range Tests (1955).
Results and Discussion
Aqueous extracts of solanaceous plants were found to be highly effective having nematicidal potential against J2 of M. javanica in in vitro experiment, (Table 2). The nematode mortality was in the range of 54.33 to 100.0 % in concentrations S/16, S/8 and S /4 % compared to distilled water (1.33 %). Deadly nightshade and chili pepper extracts were more effective against second stage juveniles at concentration of S/4 followed by winter cherry, thorn apple, black nightshade and Egyptian henbane and had shown 100, 100, 99, 98.33, 93 and 75.67 %, respectively. Whereas, acetone extracts of deadly nightshade, Egyptian henbane, thorn apple and winter cherry were found to be highly toxic to juveniles of M. javanica and killed more than 75 % of the second stage juveniles. Methanolic extracts of deadly nightshade, Egyptian henbane and winter cherry with concentrations 250, 500 and 1000 ppm exhibited (82.00, 82.33 and 92.33%), (84.67, 92.00 and 95.67%) and (81.00, 83.33 and 99.00%) of larval mortality percentage after 72 hrs, respectively.
Concentration (S/8) of aqueous extracts gave the highest percentage hatch inhibition in winter cherry (97.63%) followed by Egyptian henbane, deadly nightshade, black nightshade, thorn apple and chili pepper with percentage inhibition of egg hatching (95.42, 94.60, 92.87, 79.61 and 67.97%), respectively. While, acetonic extracts showed inhibition in hatching at both lower and higher concentrations with winter cherry treatment as the best (93.45 and 98.88%) followed by black nightshade (87.56 and 90.66%) followed by thorn apple (78.72 and 83.36%).
Methanolic extracts of winter cherry and black nightshade were found to be most effective in reducing percentage of egg-masses hatching in concentrations 250 and 500 ppm with percentage inhibition of egg hatching (93.45and98.88%) and (87.65and 90.66%), respectively. Methanolic extract of chili pepper proved to be less effective against hatchability of egg-masses of M. javanica (Table 2).
Data presented in Table 3 showed that all applications suppressed populations of nematodes and fewer root galls per plant were formed of applications. Therefore, all applications of the tested extracts significantly reduced the values of root galls per root, nematode reproduction on roots of tomato plants cv. Strain-B when compared with untreated plants (check). On the other hand, aqueous extracts of deadly nightshade (foliar sprays) and chili pepper (foliar sprays and soil drenches) treatments caused the lowest gall numbers and rate of nematode reproductions (38 and 0.73 and 25 and 0.35) respectively, compared to the untreated plants and plants treated with chemical control (Oxamyl). While, good nematode control was also achieved with aqueous extracts of deadly nightshade (soil drenches) and winter cherry (foliar sprays and soil drenches) with number of galls and rate of nematode reproductions (58, 1.88, 56, 1.99, 41 and 1.49), respectively.
Plant growths were inversely related to the level of nematode population resulting from the different treatments. No significant differences in tomato cv. Strain-B shoots and roots weights were observed among methods of application with all tested materials. Highly significant differences were observed in shoots and roots lengths. The greatest increase of plant length and weight (75.40-19.89 %) was observed in tomato treated with chili pepper applied as foliar spray with chelates followed by treatments with aqueous extract of winter cherry applied foliar spray with chelates (65.39 – 66.59%), respectively.
The potential of using plant extracts in controlling plant parasitic nematodes has been shown by several authors (Adegbite and Adesiyan, 2005; Orisajo et al., 2007; Abbasi et al., 2008) who reported that, aqueous extracts of tested plant leaves showed nematicidal effect against Meloidogyne ssp., Root-knot nematode reduced hatching of egg-masses, increased mortality of juveniles with an increase in exposure of the time. Phytochemical analysis revealed that plants are rich in alkaloids: atropine, meteloidine, nicotine, scopolamine, hyoscyamine, terpenoids and flavonoids, which have high rate of nematicidal activity (Shahwar et al., 1995; Pavela, 2004). The alkaloids killed 90 to 100% of Hoplolaimus indicus, Helicotylenchus multicinctus, and M. incognita (Qamar et al., 1995).
Table 1: Solanaceous plants used in the experiment.
|
Common name |
Scientific name |
Parts used |
Collected from |
|
|
1 |
Black nightshade |
Solanum nigrum |
leaves |
Univ. farms and gardens |
|
2 |
Deadly nightshade |
Atropa belladonna |
leaves |
Univ. farms and gardens |
|
3 |
Egyptian henbane |
Hyoscyamus muticus |
leaves |
New Valley governorate |
|
4 |
Thorn apple |
Datura innoxia |
leaves |
Univ. farms and gardens |
|
5 |
Winter cherry |
Withania somnifera |
leaves |
Univ. farms and gardens |
|
6 |
Chili pepper |
Capsicum frutescens |
fruits |
New Valley governorate |
Plant parts were washed thoroughly under running tap water, cut into small pieces, shade dried and used for extraction. Dried plant materials homogenized to a fine powder then stored in airtight bottles.
Table 2: Evaluation of aqueous, acetonic and methanolic extracts of some solanaceous plants on larval mortality and egg masses hatching of M. javanica at different concentrations in vitro.
|
Materials |
Aqueous extract |
Chemical extracts |
||||||
|
Conc. |
No. larval mortality after 72 hrs. |
Inhibition of egg hatched % |
Conc. ppm |
Acetone |
Methanol |
|||
|
No. larval mortality after 72 hrs. |
Inhibition of egg hatched % |
No. larval mortality after 72 hrs. |
Inhibition of egg hatched % |
|||||
|
Black nightshade (Solanum nigrum) |
S/16 |
65.33 cd |
78.64 |
250 |
29.00 e |
87.56 |
27.67 e |
70.87 |
|
S/8 |
75.00 bc |
92.87 |
500 |
73.67 d |
90.66 |
34.67 e |
73.41 |
|
|
S/4 |
93.00 a |
------ |
1000 |
83.67abcd |
------ |
56.67 d |
------ |
|
|
Deadly nightshade (Atropa belladonna) |
S/16 |
97.33 a |
92.06 |
250 |
83.67abcd |
59.49 |
82.00 ab |
44.65 |
|
S/8 |
98.33 a |
94.60 |
500 |
95.67 ab |
86.82 |
82.33 ab |
51.33 |
|
|
S/4 |
100.00 a |
------ |
1000 |
100.00 a |
------- |
92.33 a |
------- |
|
|
Egyptian henbane (Hyoscyamus muticus) |
S/16 |
54.33 d |
84.42 |
250 |
75.33 cd |
62.46 |
84.67 ab |
31.48 |
|
S/8 |
66.67 cd |
95.42 |
500 |
80.67 bcd |
85.96 |
92.00 ab |
59.43 |
|
|
S/4 |
75.67 bc |
------- |
1000 |
91.00 abc |
------- |
95.67 a |
------ |
|
|
Chili pepper (Capsicum frutescens) |
S/16 |
89.00 ab |
69.22 |
250 |
4.67f |
5.17 |
3.33 f |
57.86 |
|
S/8 |
99.00 a |
67.97 |
500 |
6.33f |
60.01 |
11.00 f |
34.20 |
|
|
S/4 |
100.00 a |
------- |
1000 |
10.33f |
------- |
5.00 f |
------- |
|
|
Thorn apple (Datura innoxia) |
S/16 |
94.33 a |
72.23 |
250 |
80.67 bcd |
78.72 |
61.67 cd |
17.87 |
|
S/8 |
96.00 a |
79.61 |
500 |
79.67 bcd |
83.36 |
74.33 bc |
71.43 |
|
|
S/4 |
98.33 a |
------- |
1000 |
84.67abcd |
88.67 ab |
------- |
||
|
Winter cherry (Withania somnifera) |
S/16 |
88.00 ab |
88.21 |
250 |
91.00 abc |
93.45 |
81.00 ab |
54.79 |
|
S/8 |
93.33 a |
97.63 |
500 |
99.00 a |
98.88 |
83.33 ab |
64.63 |
|
|
S/4 |
99.00 a |
------- |
1000 |
100.00 a |
------- |
99.00 a |
------- |
|
|
Distilled water (Control) |
1.33 e |
0.00 |
1.33 g |
00 |
1.33 f |
0.00 |
||
|
Mean |
88.31 a |
84.41 a |
83.18 b |
74.38b |
75.73 c |
52.66 c |
||
Means in each column followed by the same letters are not significantly different by (P=0.05) according to Duncan’s multiple range test.
The study relates to control plant parasitic nematodes using systemic nematicides by applying to plants a non-toxic nematicides effective amount of organic plants extracts. It has been recognized that an ideal solution might reside in the use of materials that could be applied to the foliage or stems of growing plants in such conventional forms as sprays, dusts, pastes. In accordance with this work, we have found to treat plants infected by nematodes with that organic plants extract which were used alone, or with chelating agents. Chelates are a class of compounds which when applied to the foliage and stems of growing plants are absorbed into said plants. Thus, it is possible to carry the used materials and systemically translocate to areas where nematodes choose to attack, primarily the roots, and thereby repel and/or eventually kill nematodes attacking plants. Data in the present study showed that, almost the plants sprayed by chelates were significantly the best in improving plant growth. Absence of zinc increased nematode density in soil and
Table 3: Effect of some materials applied as soil drenches and foliar surface against root-knot nematode, M. javanica infecting tomato cv. Strain-B under greenhouse condition.
|
Materials |
Nematode parameters |
Plant growth |
|||||
|
Applications |
No. Galls |
(Pf) |
(Pf/Pi) |
Redaction % |
Increase in plant length % |
Increase in plant weight % |
|
|
Deadly nightshade (Atropa belladonna) |
Foliar |
32g |
1632 |
0.82 |
88.60 |
24.39 |
13.28 |
|
Foliar +Chelates |
66de |
7974 |
3.99 |
44.51 |
44.4 |
22.2 |
|
|
Soil drenches |
58def |
3766 |
1.88 |
73.85 |
53.07 |
6.52 |
|
|
Chili pepper (Capsicum frutescens) |
Foliar |
38g |
1470 |
0.73 |
89.85 |
40.78 |
14.64 |
|
Foliar +Chelates |
69cde |
6619 |
3.31 |
53.96 |
75.48 |
19.89 |
|
|
Soil drenches |
25g |
709 |
0.35 |
95.13 |
26.68 |
6.22 |
|
|
Winter cherry (Withania somnifera) |
Foliar |
56def |
3986 |
1.99 |
72.32 |
17.16 |
15.07 |
|
Foliar +Chelates |
78bcd |
10427 |
5.21 |
27.54 |
65.39 |
66.59 |
|
|
Soil drenches |
41fg |
2979 |
1.49 |
79.28 |
19.35 |
0.37 |
|
|
Oxmyle |
Foliar |
26g |
376 |
0.19 |
97.36 |
57.95 |
16.54 |
|
Foliar +Chelates |
33g |
289 |
0.14 |
98.05 |
65.76 |
25.72 |
|
|
Soil drenches |
25g |
245 |
0.12 |
98.33 |
71.66 |
14.42 |
|
|
Untreated (control) |
--- |
--- |
--- |
--- |
--- |
--- |
|
|
Nematode only |
98a |
14374 |
7.19 |
--- |
--- |
--- |
|
|
Nematode + Chelates |
101a |
14833 |
7.42 |
--- |
44.11 |
42.30 |
|
|
Chelates only |
--- |
--- |
--- |
--- |
52.63 |
30.91 |
|
Means in each column followed by the same letters are not significantly different by (P=0.05) according to Duncan’s multiple range test. Pf: Nematode final population; Pf/Pi= Rate of nematode reproduction.
reduced plant growth (Haque and Mukhopadhyaya, 1975; Siddique et al., 2002). The involvement of minerals especially Fe, Mg, Zn and Ca in the formation of enzymes (Graham et al., 1988; Auld, 2001), may explain their role in the acquired systemic resistance by increasing the antioxidant enzymes included in the defense mechanisms that resulted in reducing nematode populations. Systemic acquired resistance can be enhanced by applying materials of different sources which suppress nematode populations and improve the growth of treated plants either directly by their effects on nematodes or by enhancing resistance of treated plants (Farahat et al., 2015). Abamectin has been evaluated in soil applications and foliar sprays for potential control of plant parasitic nematodes in several crops (Sasser et al., 1982; Cayrol et al., 1993; Jansson and Rabatin, 1997).
Novelty Statement
Developing effective methods for using natural extracts in the soil to reduce nematode populations and boost plant productivity.
Author’s Contribution
Mohammed Abdel-Mageed Abdel-Aziz Abdel-Mageed: A laboratory evaluation Meloidogyne javanica, a root knot nematode, was treated with aqueous, acetonic, and methanolic extracts.
Eman Alsayed Hammad: Corresponding author, evaluated Under greenhouse conditions, aqueous extracts of deadly nightshade, winter cherry, and chilli pepper applied to the plants as foliar sprays alone, foliar sprays with chelates and soil drenches against M. javanica were infecting tomato plants cv. Strain-B.
Nashaat Abdel-Aziz Mahmoud: Data statistical analysis.
Anas Farag El-Mesalamy: Extraction for tested material.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbasi, W.M., Ahmed, N., Zaki, J.M. and Shaukat, S.S., 2008. Effect of Barleria acanthoides Vahl. on root-knot nematode infection and growth of infected okra and brinjal plants. Pak. J. Bot., 40: 2193-2198.
Adegbite, A.A. and Adesiyan, S.O., 2005. Root extracts of plants to control root-knot nematode on edible soybean. World J. Agric. Sci., 1: 18-21.
Ardakani, A.S., 2012. Effects of Melia azedarach on Meloidogyne incognita in vitro and in vivo conditions. Nematol. Medit. 40: 55-60.
Auld, D.S., 2001. Zinc coordination share in biochemical zinc sites. Biometals, 14: 271-313. https://doi.org/10.1023/A:1012976615056
Cayrol, J.C., Djian, C. and Frankowski, J.P., 1993. Efficacy of abamectin B1 for the control of Meloidogyne arenaria. Fundam. Appl. Nematol., 16: 239–246.
Correia, M.C.C.P., 2014. Nematicidal activity of Solanum nigrum and S. sisymbriifolium extracts against the root-lesion nematode Pratylenchus goodeyi and its effects on infection and gene expression. Ph. D Thesis Doctorate in Biological Sciences, Universidade da Madeira.
Duncan, D.B., 1955. Multiple range and multiple F tests. Biometrics, 11: 1-42. https://doi.org/10.2307/3001478
El-Eslamboly, A.A.S.A., Abd El-Wanis, M.M. and Amin, A.W., 2019. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt. J. Biol. Pest Contr., 29: 1-9. https://doi.org/10.1186/s41938-019-0122-z
Farahat, A.A., Al-Sayed, A.A., Afify A.M. and Mahfoud, N.M., 2015. Inducing resistance in eggplant against Meloidogyne incognita by organic and inorganic fertilizers, plant growth regulators and amino acids. Egypt. J. Agronematol., 14: 91-115. https://doi.org/10.21608/ejaj.2015.60408
Graham, R.D., Hannam, R.J. and Vern, N.C., 1988. Manganese in soils and plants. Dev. Plant Soil Sci., 33: 125-137. https://doi.org/10.1007/978-94-009-2817-6
Haque, M.S. and Mukhopadhyaya, M.C., 1975. Influence of some micronutrients on Rotylenchulus reniformis. Indian J. Nematol., 5: 77-78.
Hussain, M.A., Mukhtar, T. and Kayani, M.Z., 2011. Efficacy evaluation of Azadirachta indica, Calotropis procera, Datura stramonium and Tagetes erecta against root-knot nematodes Meloidogyne incognita. Pak. J. Bot., 43: 197–204.
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep., 57: 1025-1028.
Jansson, R.K. and Rabatin, S., 1997. Curative and residual efficacy of injection applications of avermectins for control of plant-parasitic nematodes on banana. J. Nematol., 29: 695–702.
Khan, A., Sayed, M., Shaukat, S.S. and Handoo, Z.A., 2008. Efficacy of four plant extracts on nematodes associated with papaya in Sindh, Pakistan. Nematol. Medit., 36: 93-98.
Meena, K.S., Sivakumar, M. and Prabhu, S., 2010. Efficacy of acetone extracts of Tagetes species on egg hatching and larval mortality of Meloidogyne incognita. Indian J. Nematol., 40(1): 88-90.
Montasser, S.A., Ahmed, M.M. and Mostafa, M.A., 1999. Effect of dry leaf powders of some medicinal and aromatic plants as soil application in controlling the root-knot nematode, Meloidogyne incognita in relation to the growth of sunflower plants. Minufiya J. Agric. Res., 24: 757-774.
Nandakumar, A., Muthu, M.V., Sundararaju P. and Udayakumar, R., 2017a. Nematicidal activity of aqueous leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens. Am. J. Entomol., 1: 39-45. https://doi.org/10.9734/AJOB/2017/34241
Nandakumar, A., Vaganan, M.M., Sundararaju, P. and Udayakumar, R., 2017b. Phytochemical analysis and nematicidal activity of ethanolic leaf extracts of Datura metel, Datura innoxia and Brugmansia suaveolens Against Meloidogyne incognita. Asian J. Biol., 2: 1-11. https://doi.org/10.9734/AJOB/2017/34241
Olanigi, M.O., Moens, M. and Moermans, M., 2005. Effect of soil amendment with herbs in the control of Meloidogyne incognita on tomato. Special 32nd Con. Edit. Niger. J. Plant Prot., 22: 140–148.
Oplos, Ch., Eloh, K., Spiroudi, U., Pierluigi, C. and Ntalli, N., 2018. Nematicidal Weeds, Solanum nigrum and Datura stramonium. J. Nematol., 50: 1-13. https://doi.org/10.21307/jofnem-2018-017
Orisajo, S.B., Okeniyi, M.O., Fademi, O.A. and Dongo, L.N., 2007. Nematicidal effects of water extracts of Acalypha ciliate, Jatropha gosssypifolia, Azadiractha indica and Allium ascalonicum on Meloidogyne incognita infection on cacao seedlings. J. Res. Biosci., 3: 49-53.
Pavela, R., 2004. Insecticidal activity of certain medicinal plants. Fitoterapia, 75: 745-749. https://doi.org/10.1016/j.fitote.2004.08.005
Ploeg, A.T. and Stapleton, J.J., 2001. Glasshouse studies on the effects of time, temperature and amendment of soil with broccoli plant residues on the infestation of melon plants by Meloidogyne incognita and M. javanica. Nematology, 3: 855–861. https://doi.org/10.1163/156854101753625353
Qamar, F., Kapadia, Z., Khan, S.A. and Badar, Y., 1995. Datura metel L., a plant with nematicidal potential. Pak. J. Sci. Ind. Res., 38: 319-321.
Saeed, M.R.M., Awadh, G.A.M., Al-Thobhani, M.A. and Al-Deen, A.T., 2015. In vitro nematicidal activity of ten plant extracts against juveniles of M. incognita. Egypt. J. Agronematol., 14: 78-90. https://doi.org/10.21608/ejaj.2015.60403
Sasser, J.N., 1980. Root-knot nematodes: A global menace to crop production. Plant Dis., 64(1): 36-41. https://doi.org/10.1094/PD-64-36
Sasser, J.N.; Kirkpatrick, T.L. and Dybas, R.A., 1982. Efficacy of avermectins for root-knot control in tobacco. Plant Dis., 66: 691–693. https://doi.org/10.1094/PD-66-691
Shahwar, D., Abid, M., Rehman, A.U., Maqbool, M.A. and Choudhary, M.I., 1995. Nematicidal compounds from D. fastuosa Eds. Rehman AU, Choudhary MA, Sheikhani MS. In the proc. 19th IURC symposium on the chemistry of natural products. HEJ Res. Inst. Chem. Univ. Karachi, Pakistan. pp. 171-179.
Siddique, I.A., Shaukat, S.S. and Hamid, M., 2002. Role of zinc in rhizobacteri amediated suppression of root-infecting fungi and root-knot nematode. J. Phytopathol., 150: 569-575. https://doi.org/10.1046/j.1439-0434.2002.00805.x
Southey, J.F., 1986. Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture Fisheries and Food. HMSO. London, UK.
Sowley, E.N.K., Kankam, F. and Adomako, J., 2014. Management of rootknot nematode Meloidogyne spp. on sweet pepper (Capsicum annuum L.) with moringa (Moringa oleifera Lam.) leaf powder. Arch. Phytopathl. Plant Prot., 47: 1531- 1538. https://doi.org/10.1080/03235408.2013.848710
Youssef, M.M.A., El-Nagdi, W.M.A. and Lashein, A.M.S., 2016. Comparative study on the effect of garlic clove and acetyl salicylic acid aqueous extracts with emphasis on inducing resistance against root knot nematode, Meloidogyne incognita on sugar beet. Int. J. Pharm Tech Res., 9: 01-07.
To share on other social networks, click on any share button. What are these?





