Comparative Effectiveness of Chlorantraniliprole and Neem Leaf Extract against Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae)
Research Article
Comparative Effectiveness of Chlorantraniliprole and Neem Leaf Extract against Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae)
Nimra Altaf1, Muhammad Arshad1, Muhammad Zeeshan Majeed1, Muhammad Irfan Ullah1*, Hamza Latif1, Muhammad Zeeshan1, Gulfam Yousuf1 and Muhammad Afzal2
1Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan; 2Baba Guru Nanak University Nankana Sahib, Pakistan.
Abstract | Fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is a destructive insect pest of various economic crops. This species was first reported in Pakistan during 2019, and is now an emerging threat to Pakistan’s Agriculture. The main objective of the study was to evaluate the efficacy of a synthetic insecticide Chlorantraniliprole in comparison to different concentrations of single botanical, i.e., neem leaf extract @ (50 ppm and 100 ppm) against FAW larvae in maize. Our findings showed a significant effect (P < 0.001) of these chemicals on the mortality and consumption rate of FAW larvae. Results show that mortality increased in all treatments with time. Recommended dose of chlorantraniliprole (50 ml/100 litre water) insecticide showed 71.0% mortality of FAW on 5th day, which increased up to 82.0% on the 7th day after application of treatments. No significant (P > 0.05) difference in larval mortality was observed for chlorantraniliprole (recommended dose) and neem at 100ppm on the 5th and 7th day after application. Higher concentration (100ppm) of neem showed 59.0% mortality of FAW larvae on 5th and 72.0% on the seventh day of application. Chlorantraniliprole reduced 62.9% food consumption on the 5th day and 71.9% on the 7th day, while 43.5% on the 5th day and 51.6% on the 7th day reduction in food consumption occurred at 100 ppm when compared to the control group. While using 50ppm concentration of neem extract, 20.90% and 27.35% consumption rate was reduced at 5th and 7th day respectively. Our findings suggest chlorantraniliprole as an effective insecticide to control FAW larvae in maize crop; however, neem extract at 100 ppm concentration also performed well against this pest. As botanicals have less harmful to humans and the environment than synthetic insecticides, neem leaf extract can be used in integrated pest management programs of FAW.
Received | June 06, 2021; Accepted | January 19, 2022; Published | July 04, 2022
*Correspondence | Muhammad Irfan Ullah, Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan; Email: Muhammad.irfanullah@uos.edu.pk
Citation | Altaf, N., M. Arshad, M.Z. Majeed, M.I. Ullah, H. Latif, M. Zeeshan, G. Yousuf and M. Afzal. 2022. Comparative effectiveness of Chlorantraniliprole and neem leaf extract against Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sarhad Journal of Agriculture, 38(3): 833-840.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.3.833.840
Keywords | Spodoptera frugiperda, Consumption rate, Botanical, Mortality, Chlorantraniliprole
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Noctuidae: Lepidoptera) is a destructive insect pest of various economic crops worldwide (Georgen et al., 2016; Naeem-Ullah et al., 2019). It feeds on more than 80 plant species in which maize, cotton, rice, millet, and sorghum are preferred hosts (Montezano et al., 2018; FAO, 2018; Abrahams et al., 2017; Clark et al., 2007; Prowell et al., 2004; Sena et al., 2003). The most damaging FAW stage is larvae that feed on leaves, and lateral instars damage every part of the plant (Abrahams et al., 2017). Due to its attack, the plant’s photosynthetic area reduces, and it also directly damages the grains (Chimweta et al., 2020). The first time FAW was reported in tropical and subtropical regions of America (FAO, 2018). During 2016, FAW caused severe yield losses of the corn crop in the different areas of Africa (Rose et al., 2000; Goergen et al., 2016; Abrahams et al., 2017; Cock et al., 2017), and about 98% of corn growers were affected (Day et al., 2017, Cock et al., 2017). This pest causes a loss of 400 million dollars every year in Brazil (Cruz, 2008). In India, it was reported in May 2018, and its presence was also confirmed in Thailand and Myanmar (Kalleshwaraswamy et al., 2018; Guo et al., 2018). In April 2019, FAW was first observed in the Sindh province of Pakistan (Naeem-Ullah et al., 2019) So, the management of this devastating pest is of utmost importance.
The management strategy, primarily used to control FAW is the use of Genetically Modified Crops and synthetic insecticides (Sisay et al., 2019), but resistant development in this pest has been reported against several groups of synthetic insecticides (Abrahams et al., 2017) due to repeated applications of insecticides (Gutierrez-Moreno et al. 2019). Synthetic insecticides are available resources to control this pest, which is a significant achievement of modern agricultural practices and improves crop yield. A novel insecticide, chlorantraniliprole target on the ryanodine receptors of insects. In the result of ryanodine receptors binding, Ca+2 extensively produced. Due to high production of Ca+2 insects paralyzed and leads to death (Lahm et al., 2005; Cordova et al., 2007). Lepidopteran insect pests are being controlled by chlorantraniliprole (Liu et al., 2017). Indiscriminate use of pesticides to produce and protect plants causes toxic effects by contact, inhalation, and food exposure, leading to carcinogenesis, reproductive problems, and mutagenesis in humans (Kazem and El-Shereif, 2010; Lozowicka et al., 2015). These conditions have led to searching for other valuable and eco-friendly approaches, especially natural plant sources (Fetoh and Asiry, 2012). Various botanicals are readily available and inexpensive and available to the agricultural community; they are safer for humans and the environment with little residual effect and are less toxic to mammals (Isman, 2006; Koul et al., 2008).
Neem plant (Azadirachta indica A. Juss) belongs to the family Meliaceae is originated from Southeast Asia and the Indian subcontinent (Brahmachari, 2004; Campos et al., 2016). Each part of neem plants produces active secondary compounds used previously to control insect pests (Amaral et al., 2018). This plant has more than 100 active biological compounds, such as terpenoids, and azadirachtin (Hossain et al., 2013; Campos et al., 2016), which have insecticidal, repellent, and antifeedant properties and are very useful to suppress the population of insects (Isman, 2006; Mordue and Nisbet, 2000; Martinez, 2002). This plant has been considered a very useful in integrated pest management program (De Franca et al., 2017). Keeping in view the importance of the neem plant as a botanical insecticide, its efficacy was compared with new chemistry insecticide chlorantraniliprole against FAW larvae.
Materials and Methods
Rearing of Spodoptera frugiperda (J.E. Smith 1797)
FAW larvae and egg batches were collected from the maize field nearby the University of Sargodha, Pakistan (32°09’04.0”N 72°43’26.4”E). To establish a large colony, FAW larvae were fed on fresh young leaves of maize. However, once the colony was established, an artificial diet was provided to larvae. The artificial diet was prepared using maize leaf powder, bean powder, brewer’s yeast, sorbic acid, ascorbic acid, vitamin E tablets, methyl-p-hydroxybenzoate, sucrose, agar, and formaldehyde by using the method suggested by Parsanna et al. (2018). The larvae were fed on an artificial diet till pupation. The pupae were then placed in a plastic jar lined with cotton. The adult pairs were kept in the adult rearing cages and fed with 10% (w/v) sugar solution. The egg batches were separated and placed in glass Petri dishes that were sterilized before use. Regularly, egg batches were observed until hatching. Neonate larvae were provided with soft foliage of maize leaves to feed. The 3rd instar larvae were placed in the plastic petri dishes individually to avoid the cannibalism. The F3 generation larvae were used in the experiment.
Insecticide
A new chemistry insecticide, chlorantraniliprole 20 SC, was purchased from the local market of Sargodha, and the recommended dose (50 ml/100litre water) was tested against FAW larvae in lab.
Collection and preparation of plant extract
Fresh leaves of neem, Azadirachta indica were collected from the field near by Sargodha University. Leaves were shade-dried for twenty days. Dried leaves were ground to a fine powder and kept in Laboratory at room temperature. 100 g of neem leaves powder was mixed in 1 L of water in a conical flask to make a stock solution (10% w/v). The mixture was agitated for one hour using an electric shaker (KS130, Germany). The solution was filtered using Whatman no. 1 filter paper before use. The solvent was evaporated using a rotary vacuum evaporator, and the extract was stored in the refrigerator at 4 ºC for one month before use (Kumar et al., 2011).
Leaf-Dip bioassay
The experiment was performed in the laboratory of the Department of Entomology, University of Sargodha, Pakistan. Field recommended dose chlorantraniliprole (50 ml/100 litre) and two concentrations of A. indica extract (50 ppm and 100 ppm) were used in the bioassay. Water was used in the control treatment. The treatments were replicated three times, and 15 larvae were used in each replication totaling 45 larvae/treatment. Leaf dip bioassay was used to assess the mortality of FAW larvae. Maize leaves were collected from the field and washed with distilled water. The leaves were cut into pieces and dipped in the solutions for 10 seconds and left to dry for 10 minutes at room temperature. Third instar larvae of FAW were collected from the already developed culture in lab and kept them starving for 24 hours before releasing them into the Petri dishes. Maize leaves were changed daily, and feces were removed. The weight of leaves before and after the feeding of FAW was recorded to evaluate the consumption rate. The consumption rate of FAW was recorded using the formula given by Waldbauer (1968).
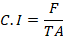
Where;
C.I: Consumption index; F: Fresh or dry weight of food eaten; T: Duration of feeding period (days); A: Mean fresh or dry weight of insects during feeding period.
Mortality data of FAW was recorded at 1, 3, 5, and 7 days after application. Mortality data was corrected using Abbott’s formula (Abbott, 1925).
Data analysis
Larval mortality and consumption data were analyzed using one-way ANOVA, and means were separated by Tukey HSD all-pairwise comparison test. All the analyses were performed using SPSS 20.0 software.
Results and Discussion
Chlorantraniliprole at recommended dose rate (50 ml/100litre water) and neem extracts at two different concentrations (50ppm and 100ppm) were tested against 3rd instar larvae of FAW under laboratory conditions.
There was a significant effect of treatments on mortality of S. furgiperda on 1st (F = 128.0, P < 0.001), 3rd (F = 561.0, P < 0.001), 5th (F = 76.9, P < 0.001) and 7th (F = 99.1, P < 0.001) day after application. Over time, mortality was increased. Chlorantraniliprole insecticide showed 71.0% mortality of FAW on the 5th day, and it was increased up to 82.0% on the 7th day. No significant (P > 0.05) difference was observed for chlorantraniliprole and neem @ 100ppm on the 5th and seventh day. Higher concentration (100ppm) of neem showed 59.0% mortality of FAW larvae on the 5th and 72.0% on the seventh day of application. Neem (50ppm) showed 35.0% mortality of FAW larvae on the 5th and 42.0% on the seventh day of application. In the control treatment, no mortality was found (Figure 1).
Larval Consumption rate was calculated and it was found that A significant effect of treatments was found on consumption rate of FAW on 1st (F = 496.0, P < 0.001), 3rd (F = 421.0, P < 0.001), 5th (F = 289.0, P < 0.001) and 7th (F = 439.0, P < 0.001) day after application. The consumption rate was found to be higher on 1st day (220.0 mg), on 3rd day (260.0 mg), on 5th day (310.0 mg) and on 7th day (385.0 mg) in control treatment. Chlorantraniliprole reduced 62.9% consumption on 5th day and 71.9% on 7th day when compared to control group. Similarly, neem 100 ppm reduced 43.5% on 5th day and 51.6% on 7th day compared to control. While using 50ppm concentration of neem extract, 20.90% and 27.35% consumption rate was reduced at 5th and 7th day respectively (Figure 2).
Synthetic insecticides are becoming a big problem for the environment; that is why plant-based insecticides are being used to control different insect pests, including armyworms. For the management of insect pests, the botanical extracts showed an effective control. Many plant extracts have a repellent effect against different damaging insect pests (Pang et al., 2020).
We used neem extract at 50 and 100 ppm concentrations in comparison to Chlorantraniliprole insecticide against FAW 3rd instar larvae. Our findings showed that insecticide showed higher mortality of FAW larvae than neem extract. However, no significant difference in larval mortality was observed using insecticide and higher concentration (100ppm) of neem at 5th and 7th day post treatment interval. Various Lepidopteran pests are highly susceptible to Chlorantraniliprole insecticide (Cao et al., 2010; Pereira, 2013), a registered insecticide against many Lepidopteran non-Lepidopteran pests (Lutz et al., 2018). Chlorantraniliprole attacks insects’ ryanodine receptors and disturbs the calcium homeostasis in the cells, resulting in feeding cessation, muscle paralysis, lethargy, and ultimately death of that insect (Lahm et al., 2005). Armyworm larvae had a high potential to resist Chlorantraniliprole (Muthusamy et al., 2014). Further research is needed on whether moths can quickly develop resistance to chlorantraniliprole.
Our findings showed that neem extract at 100 ppm concentration also showed effectiveness as a synthetic insecticide. There was no significant effect in mortality due to neem 100ppm and insecticide after 5 and 7 days of application. Neem plant belonging to the family Meliaceae has been reported for its potential as bio-insecticide (Verma et al., 2007; Montes-Molina et al., 2008; Javed et al., 2008; Anjorin et al., 2008; Farooq et al., 2011). The neem plant’s insecticidal properties are due to azadirachtin, a widely known, effective insecticide (Zheng et al., 2011). Neem, Azadirachtin can control many insect pests, including fall armyworm (Nisbet, 2000; Silva et al., 2015). The damaging properties of neem extract on insect pests are highly effective and complex molecule azadirachtin (Viana et al., 2007). Azadirachtin is an environment-friendly, non-mutagenic, selective, and easily decomposable, having minimal effect on mammals, and it could be an outstanding alternate for the management of FAW (Campos et al., 2012).
Chlorantraniliprole insecticide and neem extract at the concentration of 100ppm caused a significant reduction in the feeding activity of FAW. In agreement with our observations, other studies also demonstrated the deterrent effects of A. indica (Lehman et al., 2007; Montes-Molina et al., 2008; Sharma et al., 2008; Hernández-Lambraño et al., 2014). Azadirachta indica shows antifeedant properties against many lepidopteran pests (Liang et al., 2003; Roel et al., 2010).
Conclusions and Recommendations
Fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Noctuidae: Lepidoptera) is a very destructive insect pest of various economic crops. The chlorantraniliprole is an effective synthetic insecticide to control FAW larvae, however, neem extract at 100 ppm concentration also performed well against this pest. As botanicals are less harmful to humans and the environment than synthetic insecticides, neem leaf extract @ 100ppm can be used in integrated pest management programs of FAW.
Novelty Statement
The findings showed that chlorantraniliprole and neem extract at 100 ppm concentration performed well to control FAW’ larvae. As botanicals have less harmful to humans and the environment than synthetic insecticides, neem leaf extract can be used in integrated pest management programs of FAW.
Authors’ Contributions
Hamza Latif, Muhammad Zeeshan, and Gulfam Yousuf: Performed experiments.
Muhammad Irfan Ullah, Nimra Altaf and Muhammad Arshad: Wrote the manuscript.
Muhammad Afzal and Muhammad Zeeshan Majeed: Designed the experiment.
Muhammad Arshad and Nimra Altaf: Analyzed the data.
Conflict of interest
All authors have declared no conflict of interest.
References
Abbott, W.S. 1925. A method of computing effectiveness of insecticide. J. Econ. Entomol., 18: 265-267. https://doi.org/10.1093/jee/18.2.265a
Abrahams, P., M. Bateman, T. Beale, V. Clottey, M. Cock, Y. Colmenarez, N. Corniani, R. Day, R. Early, J.L. Godwin, J. Gomez, P. Gonzalez-Moreno, S.T. Murphy, B. Oppong-Mensah, N. Phiri, C. Pratt, G. Richards, S. Silvestri and A. Witt. 2017. Fall Armyworm: Impacts and implications for Africa. Evidence Note (2), September 2017. Report to DFID. Wallingford, UK: CAB International.
Amaral, K.D., L.C. Martínez, M.A.P. Lima, J.E. Serrão and T.M.C. Della Lucia. 2018. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut., 243: 809-814. https://doi.org/10.1016/j.envpol.2018.09.066
Anjorin, S.T., H.A. Makun, T. Adesina and I. Kudu. 2008. Effects of Fusarium verticilloides, its metabolites and neem leaf extract on germination and vigour indices of maize (Zea mays L.). Afr. J. Biotechnol., 14: 2402-2406.
Brahmachari G. 2004. Neem – an omnipotent plant: a retrospection. Chembiochem., 5(4): 408-421. https://doi.org/10.1002/cbic.200300749
Campos, A.P., A.L. Boica Junior and D.C. Lagartas. 2012. Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) submitted to different concentrations of neem oil. Rev. bras. milho sorgo., 11 (2): 137-144. https://doi.org/10.18512/1980-6477/rbms.v11n2p137-144
Campos, E.V., J.L. de Oliveira, M. Pascoli, R. de Lima and L.F. Fraceto. 2016. Neem oil and crop protection: from now to the future. Front. Plant Sci., 7: 1-8. https://doi.org/10.3389/fpls.2016.01494
Cao, G., Q. Lu, L. Zhang, F. Guo, G. Liang, K. Wu and Y. Guo. 2010. Toxicity of chlorantraniliprole to Cry1Ac-susceptible and resistant strains of Helicoverpa armigera. Pestic. Biochem. Physiol., 98(1): 99-103. https://doi.org/10.1016/j.pestbp.2010.05.006
Chimweta, M., I. Nyakudya, L. Jimu and A.B. Mashingaidze. 2020. Fall armyworm Spodoptera frugiperda (J.E. Smith)] damage in maize: management options for flood-recession cropping smallholder farmers. Int. J. Pest Manage., 66(2): 142-154. https://doi.org/10.1080/09670874.2019.1577514
Clark, P.L., J. Molina-Ochoa, S. Martinelli, S.R. Skoda, D.J. Isenhour, D.J. Lee, J.T. Krumn and J.E. Foster. 2007. Population variation of Spodoptera frugiperda (J.E. Smith) in the Western Hemisphere. J. Ins. Sci., 7: 1-10. https://doi.org/10.1673/031.007.0501
Cock, M.J., P.K. Beseh, A.G. Buddie, G. Cafá and J. Crozier. 2017. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep., 7(1): 4103. https://doi.org/10.1038/s41598-017-04238-y
Cordova, D., E.A. Benner, M.D. Sacher, J.J. Rauh, J.S. Sopa, G.P. Lahm Y. Tao. 2007. Elucidation of the mode of action of Rynaxypyr®, a selective ryanodine receptor activator. Pesticide Chemistry, Crop Protection, Public Health, and Environmental Safety. Wiley-VCH Verlag GmbH & C, 121-126. https://doi.org/10.1002/9783527611249.ch12
Cruz, I. 2008. Manejo de pragas. In: Cruz, J. C.; Karam, D.; Monteiro, M. A.; MAGALHÃES, P. C. (Ed). A cultura do milho. Brasília: Embrapa Informação Tecnológica, 303-362.
Day, R., P. Abrahams, M. Bateman, T. Beale, V. Clottey, M. Cock and A. Witt. 2017. Fall armyworm: impacts and implications for Africa. Outlooks Pest Manag., 28(5): 196-201. https://doi.org/10.1564/v28_oct_02
De França, S.M., M.O. Breda, D.R. Barbosa, A.M. Araujo and C.A. Guedes. 2017. The sublethal effects of insecticides in insects. Biological control of pest and vector insects, 23-39. https://doi.org/10.5772/66461
FAO. 2018. Integrated management of the Fall Armyworm on maize: A guide for Farmer Field Schools in Africa. Food and Agriculture Organization of United Nations, Rome.
Farooq, M., K. Jabran and Z.A. Cheema. 2011. The role of allelopathy in agricultural pest management. Pest. Manag. Sci., 5: 493-506. https://doi.org/10.1002/ps.2091
Fetoh, B.E.S.A. and K.A. Asiry. 2012. Toxicological and larvicidal activities of Alzanzalakhet, Melia azedarach against cucurbit fly, Dacus ciliatus at Hail Province in Saudi Arabia. Toxicol. Environ. Chem., 94: 1350-1356. https://doi.org/10.1080/02772248.2012.705466
Goergen, G., P.L. Kumar, S.B. Sankung, A. Togola and M. Tamò. 2016. First report of outbreaks of the Fall Armyworm Spodoptera frugiperda (J. E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 11(10): e0165632. https://doi.org/10.1371/journal.pone.0165632
Guo, J., J. Zhao, K. He, F. Zhang and Z. Wang. 2018. Potential invasion of the crop-devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Prot., 44:1-10.
Gutiérrez-Moreno R., D. Mota-Sanchez, C.A. Blanco, M. Whalon, H. Terán-Santofimio, J.C. Rodriguez-Maciel and C. Difonzo. 2019. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol., 112: 792-802. https://doi.org/10.1093/jee/toy372
Hernández-Lambraño, R., K. Caballero-Gallardo and J. Olivero-Verbel. 2014. Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae). Asian Pac. J. Trop. Biomed., 4: 695-700. https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0178
Hossain, M.A., W.A.S. Al-Toubi, A.M. Weli, Q.A. Al-Riyami and J.N. Al-Sabahi. 2013. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J. Taibah. Univ. Sci., 7: 181-188. https://doi.org/10.1016/j.jtusci.2013.05.003
Isman, M.B. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol., 51: 45-66. https://doi.org/10.1146/annurev.ento.51.110104.151146
Javed, N., S.R. Gowen and S.A. EL-Hassan. 2008. Efficacy of neem (Azadirachta indica) formulations on biology of root-knot nematodes (Meloidogyne javanica) on tomato. Crop Prot., 1: 36-43. https://doi.org/10.1016/j.cropro.2007.04.006
Kalleshwaraswamy, C. M., R. Asokan, H. M. M. Swamy, M. S. Maruthi, H. B. Pavithra, H. Kavita and G. Goergen. 2018. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest. Manage. Horticul. Ecosyst., 24(1): 23-29.
Kazem, M.G. and S. El-Shereif. 2010. Toxic effect of capsicum and garlic xylene extracts in toxicity of boiled linseed oil formulations against some piercing sucking cotton pests. Am. Eur. J. Agric. Environ. Sci., 8: 390-396.
Koul, O., S. Walia and G. Dhaliwal. 2008. Essential oils as green pesticides: potential and constraints. Biopestic. Int., 4: 63-84.
Kumar, S., A.P. Singh, G. Nair, S. Batra, A. Seth, N. Wahab and R. Warikoo. 2011. Impact of Parthenium hysterophorus leaf extracts on the fecundity, fertility and behavioural response of Aedes aegypti L. Parasitol. Res., 108(4): 853-859. https://doi.org/10.1007/s00436-010-2126-1
Lahm, G.P., T.P. Selby, J.H. Freudenberger, T.M. Stevenson, B.J. Myers, G. Seburyamo and D. Cordova. 2005. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorgan. Med. Chem., 15: 4898-4906. https://doi.org/10.1016/j.bmcl.2005.08.034
Lehman, A.D., F.V. Dunkel, R.A. Klein, S. Ouattara, D. Diallo, K.T. Gamby and M. N’Diaye. 2007. Insect management products from Malian traditional medicine—establishing systematic criteria for their identification. J. Ethnopharmacol., 110(2): 235-249. https://doi.org/10.1016/j.jep.2006.06.016
Liang, G.M., W. Chen and T.X. Liu. 2003. Effects of three neem-based insecticides on diamondback moth (Lepidoptera: Plutellidae). Crop Prot., 22: 333-340. https://doi.org/10.1016/S0261-2194(02)00175-8
Liu, Y., Y. Gao, G. Lian and Y. Lu. 2017. Chlorantraniliprole as a candidate pesticide used in combination with the attracticides for lepidopteran moths. Plos One., 12(6): e0180255. https://doi.org/10.1371/journal.pone.0180255
Lozowicka, B. 2015. Studies of pesticide residues in tomatoes and cucumbers from Kazakhstan and the associated health risks. Environ. Monit. Assess., 187: 609. https://doi.org/10.1007/s10661-015-4818-6
Lutz, A.L., I. Bertolaccini, R.R. Scotta, M.C. Curis, M.A. Favaro, L.N. Fernandez and D.E. Sánchez. 2018. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manage. Sci., 74(12): 2817-2821. https://doi.org/10.1002/ps.5070
Martinez, S. 2002. Ação do nim sobre os insetos. In: Martinez, S.S. (Ed.). O nim — Azadirachta indica: natureza, usos múltiplos, produção. Londrina: Iapar,. 59-64.
Montes-molina, J.A., M.L. Luna-guido and N. Espinoza-paz. 2008. Are extracts of neem (Azadirachta indica A. Juss. (L.)) and Gliricidia sepium (Jacquin) an alternative to control pests on maize (Zea mays L.)? Crop Prot., 3: 763-774. https://doi.org/10.1016/j.cropro.2007.11.002
Montezano, D.G., A. Specht, D.R. Sosa-Gómez, V.F. Roque-Specht, J.C. Sousa-Silva, S.V.D. Paula-Moraes, J.A. Peterson and T.E. Hunt. 2018. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol., 26(2): 286-300. https://doi.org/10.4001/003.026.0286
Muthusamy, R., M. Vishnupriya and M.S. Shivakumar. 2014. Biochemical mechanism of chlorantraniliprole resistance in Spodoptera litura (Fab) (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol., 17: 865-869. https://doi.org/10.1016/j.aspen.2014.09.001
Naeem-Ullah, U., M.A. Ansari, N. Iqbal and S. Saeed. 2019. First authentic report of Spodoptera frugiperda (JE Smith) (Noctuidae: Lepidoptera) an alien invasive species from Pakistan. ASBE., 6(1): 13.
Nisbet A.J. 2000. Azadirachtin from the neem tree Azadirachta indica: its action against insects. An. Soc. Entomol. Brasil., 29: 615-632. https://doi.org/10.1590/S0301-80592000000400001
Pang, X., Y.X. Feng, X.J. Qi, Y. Wang, B. Almaz, C. Xi and S.S. Du. 2020. Toxicity and repellent activity of essential oil from Mentha piperita Linn. leaves and its major monoterpenoids against three stored product insects. Environ. Sci. Pollut. Res., 27(7): 7618-7627. https://doi.org/10.1007/s11356-019-07081-y
Pereira, N.C. 2013. Evaluation of the toxic effect of insecticide chlorantraniliprole on the silkworm (Lepidoptera: Bombycidae). Open J. Anim. Sci., 3: 343-353. https://doi.org/10.4236/ojas.2013.34051
Prasanna, B.M., J.E. Huesing, R. Eddy and V.M. Peschke. 2018. Fall armyworm in Africa: A guide for integrated pest management, 1st Edition. Fall Armyworm in Africa: A Guide for Integrated Pest Management, First Edition. Mexico, CDMX: CIMMYT
Prowell, D.P., M. McMichael and J.F. Silvain. 2004. Multilocus genetic analysis of host use, introgression, and speciation in host strains of fall armyworm (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am., 97(5): 1034-1044. https://doi.org/10.1603/0013-8746(2004)097[1034:MGAOHU]2.0.CO;2
Roel, A.R., D.M. Dourado, R. Matias, K.R.A. Porto, A.V. Bednaski and R.B. Costa da. 2010. The effect of sub-lethal doses of Azadirachta indica (Meliaceae) oil on the midgut of Spodoptera frugiperda (Lepidoptera, Noctuidae). Rev. Bras. Entomol., 54: 505-510. https://doi.org/10.1590/S0085-56262010000300024
Rose, D.J.W., C.F. Dewhurst and W.W. Page. 2000. The African Armyworm Handbook: The Status, Biology, Ecology, Epidemiology and Management of Spodoptera exempta (Lepidoptera: Noctuidae), 2nd Edition. Nat. Resour. Inst., Chatham (UK).
Sena Jr, D. G., F.A.C. Pinto, D. M. Queiroz and P.A. Viana. 2003. Fall armyworm damaged maize plant identification using digital images. Biosyst. Eng., 85(4): 449-454. https://doi.org/10.1016/S1537-5110(03)00098-9
Sharma, P.R., O.P. Sharma and B.P. Saxena. 2008. Effect of sweet flag rhizome oil (Acorus calamus) on hemogram and ultrastructure of hemocytes of the tobacco armyworm, Spodoptera litura (Lepidoptera: Noctuidae). Micron., 5: 544-551. https://doi.org/10.1016/j.micron.2007.07.005
Silva, M.S., S.M.F. Broglio, R.C.P. Trindade, E.S. Ferrreira, I.B. Gomes and L.B. Micheletti. 2015. Toxicity and application of neem in fall armyworm. Comun. Sci., 6(3): 359-364. https://doi.org/10.14295/cs.v6i3.808
Sisay, B., T. Tefera, M. Wakgari, G. Ayalew and E. Mendesil. 2019. The efficacy of selected synthetic insecticides and botanicals against fall armyworm, Spodoptera frugiperda, in maize. Insects, 10(2): 1-14. https://doi.org/10.3390/insects10020045
Verma, V.C. and S.K.A. Gond. 2007. The endophytic mycoflora of bark, leaf, and stem tissues of Azadirachta indica A. Juss (neem) from Varanasi (India). Microbial. Ecol., 1: 119-125. https://doi.org/10.1007/s00248-006-9179-9
Viana, P.A., H.T. Prates, P.E.A. Ribeiro. 2007. Effect of neem extracts and application methods on leaf damage and the development of the roundworm, Spodoptera frugiperda, in corn. Embrapa Corn and Sorghum-Article in an indexed Journal (ALICE)., 6: 17–25. https://doi.org/10.18512/1980-6477/rbms.v6n1p17-25
Waldbauer, G.P. 1968. The consumption and utilization of food by insects. In Advances in insect physiology. Acad. Press. 5: 229-288. https://doi.org/10.1016/S0065-2806(08)60230-1
Zheng, X., X. Ren and J. Su. 2011. Insecticide susceptibility of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) in China. J. Econ. Entomol., 2: 653-658. https://doi.org/10.1603/EC10419
To share on other social networks, click on any share button. What are these?








