Cloning, Expression and Characterization of Highly Active Recombinant Thermostable Cellulase from Thermotoga naphthophila
Cloning, Expression and Characterization of Highly Active Recombinant Thermostable Cellulase from Thermotoga naphthophila
Aisha Khalid1, Muhammad Tayyab1,*, Abdual Rauf Shakoori2, Abu Saeed Hashmi1, Tahir Yaqub3, Ali Raza Awan1, Muhammad Wasim1, Sehrish Firyal1, Zaheer Hussain4 and Munir Ahmad5
Comparative analysis of cellulase from T. naphthophila str. RKU10 (Tn-ADA67783) with other members of family M42 aminopeptidase including FrvX (Ph-BAA30637), TET1 (Ph-BAA29607) and TET3 (Ph-BAA30940) from P. horikoshii str. OT3 (Russo and Baumann, 2004, 2005; Dura et al., 2009), putative fructose-lysine aminopeptidase from B. subtilis str.168 (Bs-AGA23783) (Remaut et al., 2001) and peptidase from T. maritima str. MSB8 (Tm-AHD19044) (Kapoor et al., 2010). Conserved amino acids are shown in red color. Circle above the sequence shows conserved amino acids involve in metal binding whereas the triangle above sequence indicate conserved amino acid involve in enzyme activity. The comparative analysis was developed using Clustal Omega programme.
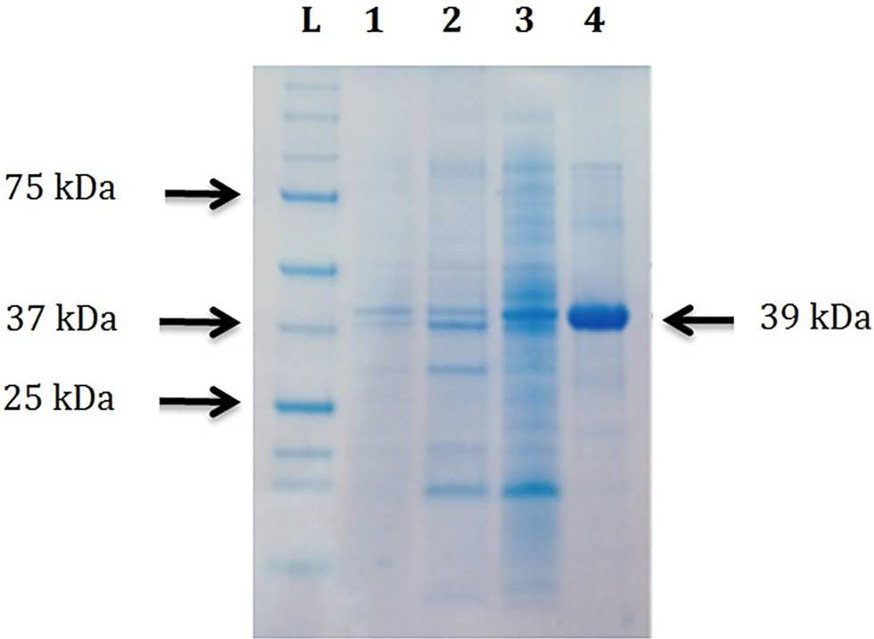
Coomassie brilliant blue stained acrylamide/bisacrylamide gel showing purification of CELTN at 25°C. Lane L, protein ladder (The Precision Plus, Bio-Rad, USA); Lane 1, the BL21 CodonPlus (DE3) total cells having pET-CEL collected after 22h of induction with final concentration of 0.5 mM IPTG at 25°C; Lane 2, insoluble part after lysis of cells in Lane 1; Lane 3, soluble part after lysis of cells in Lane 1; Lane 4, purified CELTN after Ni-NTA affinity column chromatography.
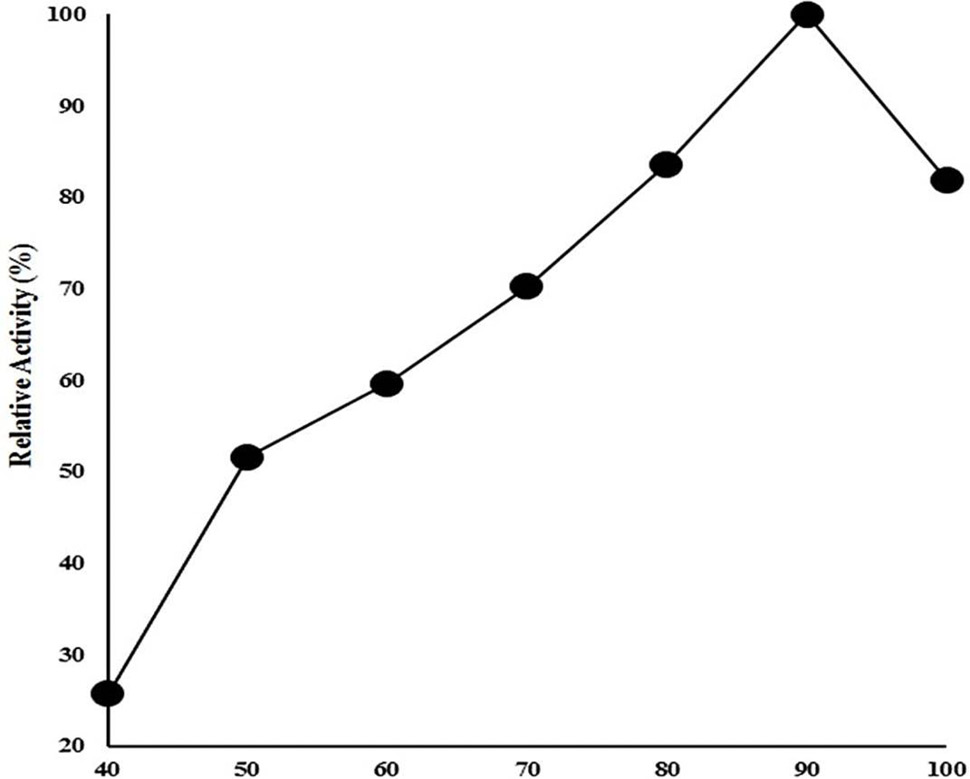
Effect of temperature on CELTN activity. Enzyme activity was examined at wide range of temperature from 40 to 100°C in 50 mM acetate buffer of pH 4.8 and 1% sodium carboxymethyl cellulose as substrate. The data on X-axis shows the temperature (°C) whereas on Y-axis shows relative enzyme activity (%).
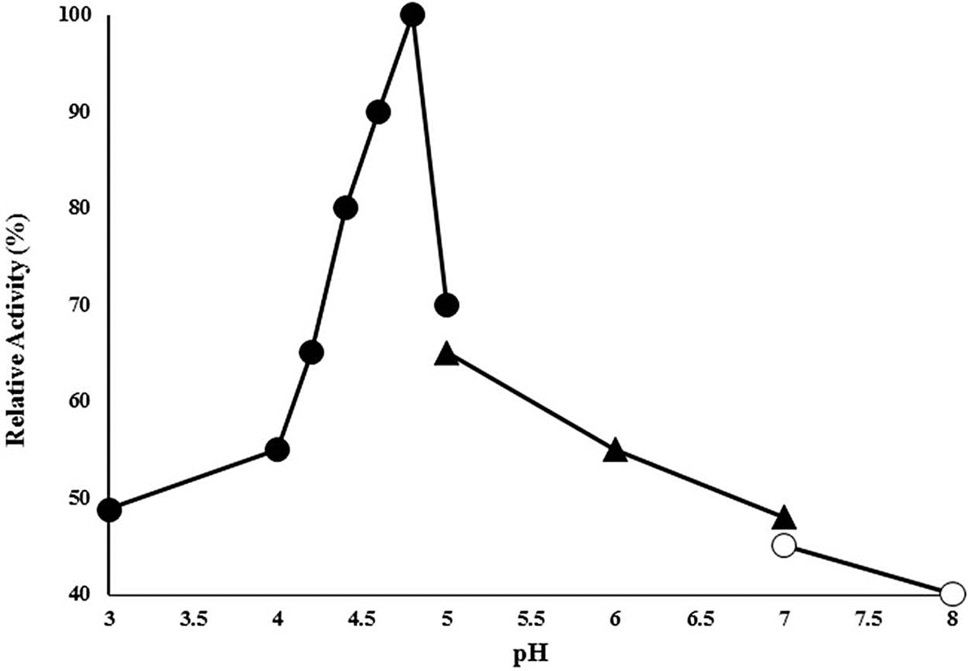
Effect of pH on CELTN activity enzyme activity was analyzed in 50 mM of each of acetate buffer range (3-5), phosphate buffer (5-7) and Tris HCl buffer (7-8) at 90°C using 1% sodium carboxymethyl cellulose as substrate. X-axis shows the pH while Y-axis presents the relative activity (%).
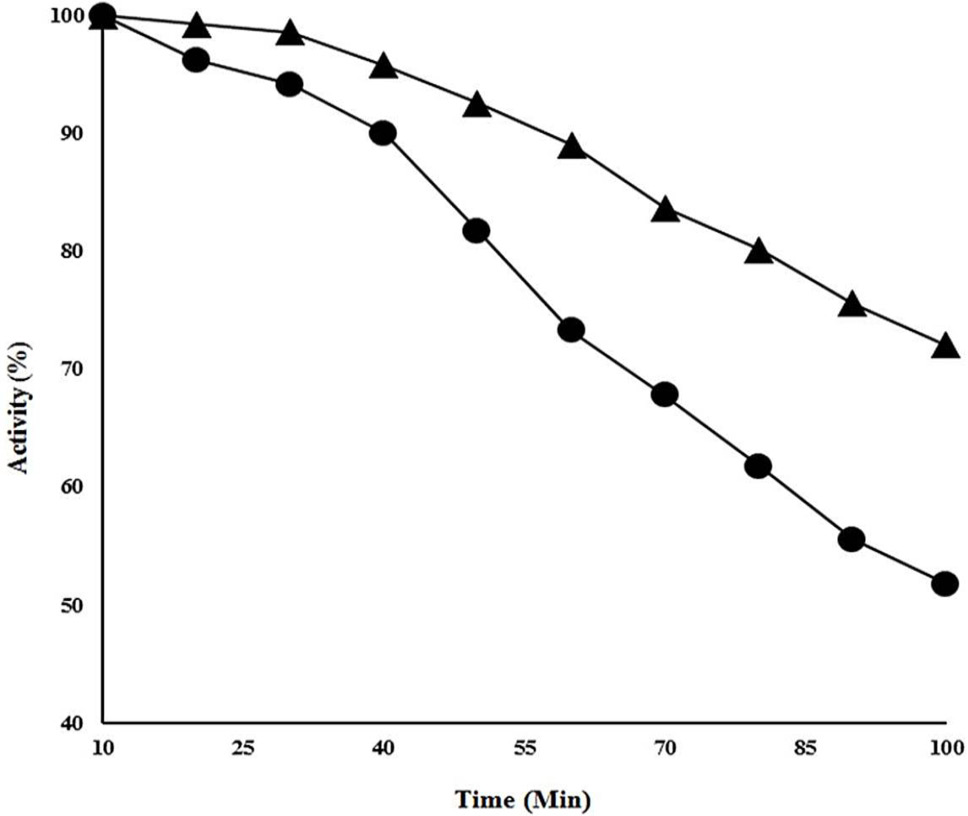
Thermo-stability studies of CELTN. The protein was incubated at 90°C in the absence or presence of 2.5 mM of Co2+. Closed circle shows the residual activity of cellulase without metal ion whereas closed triangles presents the cellulase activity at 90°C in the presence of 2.5 mM of Co2+.
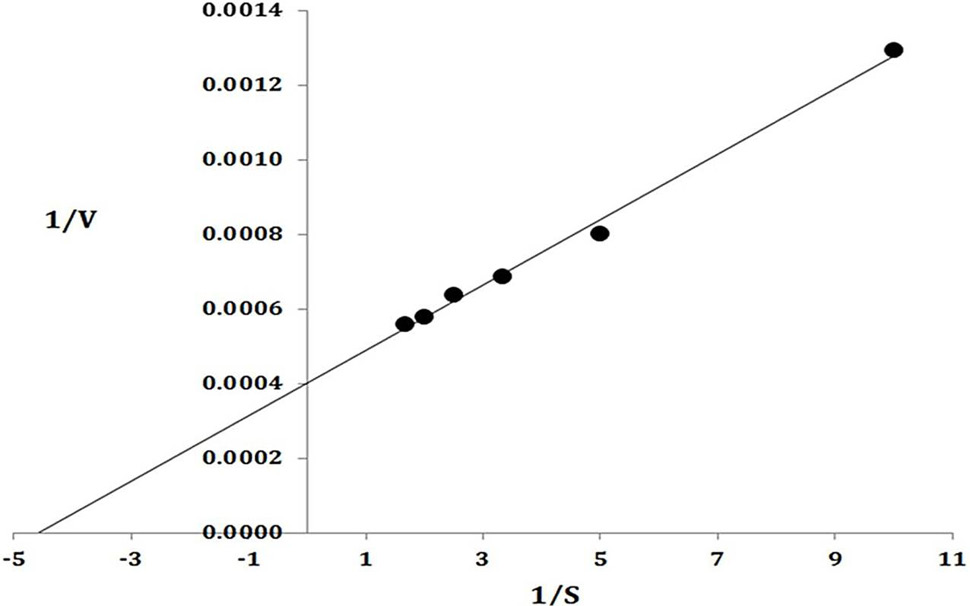
Lineweaver Burk plot. The data on X-axis indicate the 1/substrate while along Y-axis present 1/Velocity.
De-inking action of CELTN: A, ability of CELTN to remove ball point ink; B, ability of CELTN to remove pen ink.