Biodiversity, Seasonal Abundance, and Population Dynamics Assessment of Coccinellidae (Coleoptera) from Maize Crops
Biodiversity, Seasonal Abundance, and Population Dynamics Assessment of Coccinellidae (Coleoptera) from Maize Crops
Naveed Akhtar, Hafiz Muhammad Tahir*, Azizullah, Aamir Ali
Department of Zoology, Government College University Lahore, Pakistan.
Abstract | Coccinellids play a pivotal role as natural predators providing dynamic services in agriculture. The coccinellid fauna in the agricultural ecosystem of Pakistan, as well as their composition, drivers of assemblages, and species richness, lack comprehensive understanding and remain poorly documented. We studied biodiversity, species richness, and seasonal dynamics of coccinellids in two major maize-growing districts of the Punjab province, i.e., Kasur and Lahore, in 2018-2019. The coccinellids were collected from February to June during both cropping seasons. Visual counting, handpicking, sweep nets, sticky traps, and pitfall traps were employed for collection of specimens fortnightly. In aggregate, 10637 specimens representing 21 species, 16 genera, and 3 subfamilies were identified based on diagnostic morphological characteristics. The sub family Coccinellinae (Latreille, 1807) was the most dominant, constituting 94.72% of the total catch. Coccinella septempunctata (Linnaeus, 1758) was the most abundant species (21.09%), followed by Coccinella undecimpunctata (Linnaeus, 1758) (17.45%), and Cheilomenus sexmaculata (Fabricius, 1781). The study used various indices to estimate species richness (Menhinick and Margalef) and diversity (Shannon-Weiner and Simpson). The estimated species richness of all Coccinellidae species of both districts was about 95%. A significant change in the population dynamics of Coccinellidae was observed at different phenological stages (BBCH Principal Stage) of maize crops during different study months. The highest species density was recorded during April and May in both districts. A positive correlation of the Coccinellidae was observed with temperature and negative for rainfall and humidity. This work is the first to characterize coccinellids biodiversity from Pakistan’s maize crops. The research will aid in employing the ladybeetles as bio-control agents for effective Integrated Pest Management (IPM) of maize growing zones in Pakistan and across the globe.
Novelty Statement | In this study, the biodiversity, seasonal abundance, and population dynamics of coccinellids in maize crops are discussed for the better understanding of these predators, which would be helpful in the development of sustainable pest control practices.
Article History
Received: April 04, 2021
Revised: March 05, 2024
Accepted: March 26, 2024
Published: April 22, 2024
Authors’ Contributions
NA and HMT did the initial writing and formal analysis, as well as contributing to the concept and software. AU reviewed and edited the work.
Keywords
Coccinellids, Biodiversity, Population dynamics, Spring-maize Crop, Shannon-wiener index
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Hafiz Muhammad Tahir
To cite this article: Akhtar, N., Tahir, H.M., Azizullah and Ali, A., 2024. Biodiversity, Seasonal abundance, and population dynamics assessment of coccinellidae (Coleoptera) from maize crops. Punjab Univ. J. Zool., 39(1): 33-44. https://dx.doi.org/10.17582/journal.pujz/2024/39.1.33.44
Introduction
Pakistan’s agriculture sector is the backbone and critical component of economy as it contributes a substantial 22.7% to the Gross Domestic Product (GDP)- (Govt. of Pakistan, 2021). It serves as a significant contributor to both employment opportunities and foreign exchange earnings. The sustainable development of this sector is essential to ensure food security and promote rural development (Ahmad and Farooq, 2010). In Pakistan, the principal crops include wheat, rice, maize, sugarcane and cotton. Among these crops, maize has been imparting a critical role by providing food for humans, feed for livestock and poultry, and as industrial raw material (Rehman et al., 2015). Furthermore, maize is utilized to produce a range of dietary products, such as bread, corn flakes, corn starch, corn syrup and corn oil (Khan et al., 2022). Among the major crops of Pakistan, maize holds the third position after wheat and rice regarding the cultivated area and yield (Ali et al., 2017). During the year 2021-2022, maize was cultivated on an area of 1,653 thousand hectares with 10.635 million tons of production. It contributes 3.2% of the total value added in the national agricultural sector and accounts for 0.7% of the Gross Domestic Product (GDP) (GoP, 2021).
Maize crop yield is reduced by multiple biotic and abiotic factor. The damage caused by insect pest is one of the primary factors leading to reduced crop production (Adeyinka et al., 2018). Globally, 22.5% of maize is lost by insect pests and pathogens (Savary et al., 2019; Norgrove, 2021). In Pakistan, maize production has been largely susceptible to various kinds of biological and climatic risks (Akhtar et al., 2018). Various insect pests frequently invade this crop and have continuously changing feeding preferences from seedlings to crop maturity (Chaudhary et al., 2014). Control and management of these damaging insect pests have always been a global challenge due to various biological and climatic factors (Prasanna et al., 2022). Use of chemical pesticides is considered a quick solution to manage these insect pests (Poudel et al., 2020). However, their extensive and irregular use has led to the mortality of non-targeted insects including useful natural predatory insects (Sánchez-Bayo and Wyckhuys, 2019; Brühl and Zaller, 2019). The unwise utilization of synthetic pesticides in agriculture poses a significant risk to the effective application of bio-control plans (Mubushar et al., 2019).
Arthropods, including spiders, ladybugs (Coccinellids), green lacewings, and syrphid flies, serve as natural predators in agroecosystems (Basha et al., 2021). They offer valuable biological control services by preying on insect pests (Losey and Vaughan, 2006). The higher densities of these natural predators ensure the suppression of pests in the agroecosystems (Nadeem et al., 2023). Different species of natural predators utilize various hunting strategies to target pests at various stages of their life cycle resulting in enhanced biological pest control (Hajek and Eilenberg, 2018). The diverse hunting techniques by these predators contribute to more effective management of pest populations (Snyder, 2019). Even if natural predators may not be able to completely suppress the pest fauna below the threshold level, they can still have a significant impact by reducing the reproductive capacity of pests (Michalko et al., 2019).
Lady beetles or ladybugs (Coleoptera: Coccinellidae) represent a significant and diverse group of insects found in various ecosystems (Mora et al., 2020). The known taxonomy includes approximately 6,000 distinct species which are organized into 360 genera. These are widely distributed across various ecosystem globally (Pervez, 2016; Bouchard et al., 2017) and 90% of the specie functioning as predators that consume various insect pest of cereal crops (Pan et al., 2020). They are also among the most well-known generalist predators and their role in the suppression of pest populations has been distinguished for a long time (Riddick, 2023). Due to its predatory nature, lady beetles are considered valuable biological agents for regulating the population density of insect pests (Sentis et al., 2022). Coccinellids utilize a wide range of prey species, like mites, aphids, Coleoptera, and Lepidoptera, as well as non-prey (Hodek and Honěk, 2009; Obrycki et al., 2009; Evans, 2009; Biddinger et al., 2009). Coccinellids contribute to pest suppression by preying upon both larvae and adult pests (Sappington et al., 2018). Plant volatiles and prey abundance help in the regulation of coccinellid populations (Xiu et al., 2019). Different factors like climatic conditions, crop management practices, application of pesticides, side crops, and availability of the prey may affect the diversity and population dynamics of coccinellids in maize crops (Hassan et al., 2016).
Recently, the unwise use of synthetic pesticides especially in developing countries like Pakistan has a number of drawbacks, such as non-target specificity, long-term perseverance, bio-magnification, and loss of biodiversity (Riaz et al., 2017). Considering the harmful effects of synthetic pesticides, it is essential to promote integrated pest control programs as a means to conserve biodiversity and decrease pesticide use. Despite of the ecological and economic importance of coccinellids in agroecosystems, no significant research has been conducted to assist with use of coccinellids in pest control (Rafi et al., 2005; Dhawan and Peshin, 2009). Our objective is to address the knowledge gap concerning coccinellids within the maize agro-ecosystem of Pakistan. Our study objective was to investigate biodiversity, seasonal abundance, and population dynamics of the coccinellids in maize crops. Future IPM strategies can use this information for sustainable practices, improving pest management, and reducing the need for insecticides.
Materials and Methods
Study area and field layouts
The study was conducted in two primary maize-growing districts i.e., Kasur (31.1179° N, 74.4408° E) and Lahore (31.367°N 74.367°E) in the province of Punjab, Pakistan. The sites selected from district Kasur are Khudian Khas (30.9906° N, 74.2708° E), Chunian (30.9698° N, 73.9712° E, and Pattoki (31.0249° N, 73.8479° E), whereas from Lahore are Mustafabad (30.8903° N, 73.4998° E), Pakki Haveli (31.1188° N, 74.3302° E), and Khana Nou (31.3731° N, 74.3617° E). From each district, the study area of 8,094 square meters was split into four sub-sites, each containing three plots measuring 674 square meters. The study sites in Kasur district were generally situated at an average distance of 20 to 25 kilometers from each other. In contrast, the average distance between the two sites in Lahore district was 25 to 30 kilometers. The combined average distance between the two sites in both districts was 70 kilometers.
Crop management practices
The seedbed was prepared using one deep plow and followed by two cultivations using a tractor-mounted cultivator during both cropping seasons (2018 and 2019). The hybrid maize variety Pioneer-P2848W was chosen and the maize was sown between February 14th and February 25th in both 2018 and 2019. Maize was manually sown with ridges spaced 75 cm apart and with a plant-to-plant distance of 30 cm. Standard N treatments were followed for nitrogen fertilizer application and 172 kg per hectare of phosphorus was applied. During the whole cycle of the maize crop, 12 irrigations (especially water-need-based stages like tasseling, silking, cob, and grain development) were applied through a manual tube well. Manual weed management was applied using hand picking and hoeing. No herbicide was applied at any experimental sites. Notably in 2018, the study sites in the Kasur district were characterized by a monoculture of maize, while in 2019, they were surrounded by alfalfa. Conversely, in the Lahore district, the study sites were surrounded by alfalfa in 2018 and maize monoculture 2019.
Sampling of coccinellids
To study the population dynamics of coccinellids at various growth stages of maize crops, the BBCH principal scale (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie) was employed. This scale is well-known for offering a standardized description of plant developmental stages, primarily determined by their phenological characteristics (Lancashire et al., 1991). Table 1 represents a complete account of the date of observation along with the BBCH scale of maize growth. Data collection began after the germination of the maize seed from March 1, 2018, to March 3, 2019. For data recording, 20 rows of maize plants with 15 plants in each row were selected from each plot. The whole plant was visually observed for the collection of the coccinellids fortnightly from 10 am to 2 pm. First, visual counting was performed for sampling the maize plants. The outer surfaces of plants, including leaf whorls, axils, tassels, and ears were visually inspected on each sampling date for the detection of eggs, larvae, pupae, and adults. The least exposed plant structure was also separated to expose any hidden beetles. Sweep net sampling was also performed to estimate the flying ladybeetles. A 15-inch diameter sweep net was swung in a 180o arc twice from each plant. The sampling was carried out carefully to minimize disturbance and avoid disrupting ladybug activity (Hesler and Kieckhefer, 2008). At least five sweeps from each plant were taken. Hand picking method was also used for the collection of specific ladybugs species from different part of maize plant. Sticky tarps of 200 cm2 were hung in the air to capture coccinellids (Kemp and Cottrell, 2015).
Table 1: Sampling date and their corresponding BBCH Code for maize growth stages.
|
Sampling period |
BBCH code |
BBCH description |
|
03-March |
BBCH-16 |
5 Leaves or more unfolded |
|
18-March |
BBCH-19 |
10 Leaves or more unfolded |
|
03-April |
BBCH-29 |
Maximum of tillers |
|
18-April |
BBCH-39 |
9 or more nodules detectable |
|
03-May |
BBCH-59 |
Tassel emerges and silking |
|
18-May |
BBCH-75 |
Kernels in the middle of grains |
|
03-June |
BBCH-85 |
Early dough |
|
18-June |
BBCH-89 |
Fully ripening |
In order to collect coccinellids located in various parts of maize plots, a three-layered arrangement (outer, middle, and center) of 25 glass jars was also set up in selected fields. The jars were filled with 85% alcohol and small amount of detergent to decrease surface tension. This sampling method ensured thorough coverage of targets located in the field’s margins, middle, and central areas. On each sampling day, data regarding the climate was recorded to establish a correlation between the population dynamics of lady beetles and the prevailing weather conditions.
Samples preservation and data storage
Coccinellid samples were transferred into 20 ml vials containing 95% alcohol. Each sample bottle was carefully labeled with a unique field number, collection date, and collector. All specimens were washed with 70% alcohol to remove debris attached to their bodies. Geographical coordinates, elevation data, and other notes of ecological importance such as temperature (LM-8000), humidity (R6001 Thermo-Hygrometer), rainfall data, etc. were also noted using a portable GPS device (Garmin 010-02256-00) and environmental data recorder. The preserved specimens were transferred to the Department of Zoology (Entomology Section) at Government College University Lahore for further examination.
Morphological identification
Coccinellid specimens were identified by carefully inspecting their physical characteristics using an IRMECO GmbH (IM-SZ-500) microscope mounted with a digital camera. With the help of the available published literature, the sampled specimens were identified (Kapur, 1955; Bielawski, 1972; Majerus and Kearns, 1989; Canepari and Milanese, 1997; Zahoor et al., 2003; Inayatullah et al., 2005; Rafi et al., 2005; Bahlai et al., 2015; Poorani, 2023). Voucher specimens were deposited at the Stephenson Natural History Museum, GC University, Lahore, following appropriate tagging and numbering to facilitate future reference.
Data analysis
Shapiro-Wilk test was used to determine whether the data from the current study had a normal distribution before being subjected to further analysis (Shapiro and Wilk, 1965). Since there was no significant difference (P > 0.05) in coccinellid populations at selected locations during of both cropping seasons (i.e., 2018-2019), it was combined together for further analysis. SPDIVERS.BAS program was used to generate species accumulation curves (Ludwig and Reynolds, 1988). A logarithmic tendency curve (Colwell and Coddington, 1994) was also used to visualize the increase in the number of species. As the biodiversity data collection is time consuming and laborious process, a small portion of the community is characterized by rare species, often which are mostly single-tones (Lawton et al., 1998). Most biodiversity surveys fail to detect them (Chao et al., 2009). To estimate the total species richness of the coccinellid fauna of both districts, the two most widely used estimators Chao 1 and Chao 2 non-parametric richness test were computed in Estimate S 9.1 (Colwell and Elsensohn, 2014). Inventories completeness was analyzed using the ratio between the Chao 1 estimate so that completeness value was comparable with the previous values (Chao, 1984; Hortal et al., 2006; Chao et al., 2009). Chao 1 was used as it is an abundance-based estimator, which can be applied to a single sample. Chao 1 is the most conservative estimator of species richness when there are many single-tone and double-tone species:
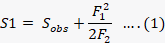
Where Sobs represent species count in the sample, F1 shows number of single tone species in the sample and F2 represent double tone species in the sample (Chao, 1984).
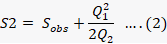
Where Sobs is the number of species in the sample, Q1 the number of single tone species in the sample and Q2 is the number of double tone species in the sample (Colwell and Coddington, 1994). The diversity of coccinellids at various selected sites was computed using commonly employed diversity indices. viz., the Shannon-Wiener index, which is sensitive to changes in the abundance of rare species within the community, as well as the Simpson index, which is sensitive to the most abundant species in the community (Solow, 1993). The following formula was used to calculate the Shannon-Wiener index (H`):
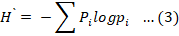
Where; pi = n/N
Species richness was also computed by using Margalef Index, which is based on the relationship between species richness (S) and the total number of individuals observed (N).
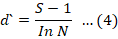
The Menhinick index was employed to determine the relationship between the species present in the sample and the total number of individuals in the samples (Whittaker and Levin, 1977). The evenness index describes how evenly species are distributed within the sample. High value of the evenness index shows that all species in the sample are equally distributed. A decreasing evenness value approaching zero indicates that the relative abundance of species in the sample has shifted away from an even distribution. The modified Hill’s Ratio (E-5) is considered the most reliable evenness index because it is not influenced by the number of species in the sample.
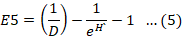
Here, D represents Simpson’s index, and H’ stands for the Shannon-Wiener index.
Diversity indices were calculated using the statistical software SPDIVERS. BAS (Ludwig and Reynolds, 1988). Cluster analysis was employed to determine the degree of association among the sampling sites. It is a valuable data reduction technique that can be helpful in analysis of grouping of objects. The cluster analysis was performed through MSVP. Version.3.22. The similarity estimates were analyzed using UPGMA, and the resulting clusters were depicted as dendrograms (Mercado et al., 2014).
Results and Discussion
Over two years (2018-2019), a total of 10,637 coccinellid specimens, comprising 21 species, 16 genera, and 3 subfamilies, were documented in districts Kasur and Lahore. Figure 1 indicates the overall relative abundance of coccinellids during sampling districts in 2018 and 2019. The sub family Coccinellinae (Latreille, 1807) was reported as the most abundant, representing 94.72% of the total catch followed by Chilocorinae (Mulsant, 1846)
Table 2: The relative abundance (%) of ladybeetles of the two major maize producing district of Punjab, Pakistan.
|
Family |
Sub-Family |
Name of Species |
Kasur |
Lahore |
Total |
R.A% |
|
Coccinellidae |
Epilachnini Mulsant 1846 |
Epilachna varivestis (Mulsant, 1850) |
55 |
34 |
89 |
0.84 |
|
Coccinellidae |
Chilocorini Costa, 1849 |
Brumoides suturalis (Fabricius, 1798) |
32 |
16 |
48 |
0.45 |
|
Coccinellidae |
Chilocorini Costa, 1849 |
Chilocorus nigrita (Fabricius, 1798) |
94 |
51 |
145 |
1.36 |
|
Coccinellidae |
Chilocorini Costa, 1849 |
Exochomus quadripustulatus (Linnaeus, 1758) |
57 |
213 |
270 |
2.54 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Adalia bipunctata (Linnaeus, 1758) |
47 |
77 |
124 |
1.17 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Adalia tetraspilota (Hope, 1831) |
62 |
99 |
161 |
1.51 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Anegleis cardoni (Weise,1892) |
104 |
79 |
183 |
1.72 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Cheilomenus sexmaculata (Fabricius, 1781) |
866 |
302 |
1168 |
10.98 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Coccinella septempunctata (Linnaeus, 1758) |
987 |
1256 |
2243 |
21.08 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Coccinella transversalis (Fabricius, 1781) |
81 |
765 |
846 |
7.95 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Coccinella trifasciata (Linnaeus, 1758) |
198 |
131 |
329 |
3.09 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Coccinella undecimpunctata (Linnaeus, 1758) |
823 |
1033 |
1856 |
17.45 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Cycloneda sanguinea (Linnaeus, 1763) |
78 |
181 |
259 |
2.43 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Harmonia dimidiata (Fabricius) |
89 |
234 |
323 |
3.04 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Hippodamia convergens (Guerin-Meneville, 1842) |
34 |
110 |
144 |
1.35 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Hippodamia variegata (Goeze, 1777) |
91 |
345 |
436 |
4.09 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Lemnia bissellata (Mulsant, 1850) |
23 |
56 |
79 |
0.74 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Menochilus sexmaculatus (Fabricius, 1781) |
68 |
142 |
210 |
1.97 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Micraspis allardi (Mulsant, 1866) |
88 |
73 |
161 |
1.51 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Oenopia oncina (Olivier, 1808) |
453 |
65 |
518 |
4.87 |
|
Coccinellidae |
Coccinellini Latreille, 1807 |
Psyllobora bisoctonatata (Mulsant) |
698 |
347 |
1045 |
9.82 |
|
Total |
5028 |
5609 |
10637 |
100 |
which constituted 4.35%, while Epilachninae (Mulsant, 1846) accounted for 0.84% of the total population. Coccinella septempunctata was the most abundant species (21.09%), followed by C. undecimpunctata (17.45%), Cheilomenus sexmaculata (10.98%), Psyllobora bisoctonatata (9.82%), C. transversalis (7.95%), Oenopia oncina (4.87%), and Hippodamia variegate (4.09%). Furthermore, Brumoides suturalis constituted the smallest proportion (0.45%) among the Coccinellidae fauna (s). More coccinellids were captured from the district Lahore (n= 5609) than Kasur (n=5028). Table 2 provides a comprehensive list of coccinellid species identified based on their morphological diagnostic characteristics.
The pooled species accumulation curves were drawn for coccinellids of both study districts over a period of two years. A positive correlation was observed between the sample size and the number of species in both districts. Figure 2 clearly illustrates that with an increase in sample size, there is also a consistent rise in the number of captured species. The rate of species increase was prominently reduced after the capture of 4,000 specimens. Initially, there was a sharp increase in the total number of species, but in both districts, the accumulation curves did not reach asymptote i.e., additional sampling will not yield any additional species (Colwell et al., 2004). The species richness calculated by Chao-2 estimate of coccinellids for the district Kasur was 22.25 and 21.66 for Lahore, while the average % completeness of both districts was 95% (Table 3).
The total abundance, diversity, richness, and evenness indices were computed using the data combined from the two-year in both Kasur and Lahore districts (Table 4). The Shannon-Wiener index and Simpson indices exhibited nearly identical values for both districts, suggesting comparable levels of diversity and evenness in the populations
Table 3: Species diversity and inventory completeness for insect pests collected from Kasur and Lahore districts.
|
Parameters |
District Kasur |
District Lahore |
|
No. of specimens |
5028 |
5609 |
|
Observed richness |
21 |
21 |
|
Estimated richness |
||
|
No of singletons |
3 |
2 |
|
No of Doubletons |
3 |
2 |
|
Chao 1 |
21.75 |
21.33 |
|
Chao 2 |
22.25 |
21.66 |
|
% Completeness |
94 |
95 |
of coccinellids within the study areas. The Margalef richness index was slightly higher for district Kasur (2.35) than Lahore (2.32). The dynamics of coccinellids fluctuated throughout the sampling period in both study districts (2018-2019). Coccinellid population increased in March (BBCH-12 to BBCH-39), peaked in May (BBCH-51 to BBCH-65), and gradually decreased from late May (BBCH-69) to June (BBCH-89) during the ripening stage (Figure 3). The species evenness (E-5) was 0.94 for district Kasur and 0.95 for Lahore indicating a uniform distribution of species abundances, contributing to a more diverse and balanced ecosystem. The species abundance based analysis revealed two main clusters (Figure 4). Coccinellid abundance demonstrated a positive correlation with temperature (r = 0.703, P = 0.023), while negative correlations with humidity (r = -0.710, P = 0.022) and rainfall (r = -0.699, P = 0.024) (Table 5).
Table 4: Total abundance, richness, diversity, and evenness indices for the lady beetles collected from different areas of Kasur and Lahore districts during 2018 and 2019.
|
Parameters |
Study areas |
|
|
Kasur |
Lahore |
|
|
Margalef index |
2.35 |
2.32 |
|
0.296 |
0.284 |
|
|
Diversity indices |
||
|
3.44 |
3.58 |
|
|
0.127 |
0.120 |
|
|
Evenness (E-5) |
0.94 |
0.95 |
Table 5: The association of the coccinellids abundance with temperature, rainfall and humidity during maize growing seasons (2018-2019).
|
Temperature |
Rainfall |
Humidity |
|
|
Coccinellids |
r = 0.703 |
r = -0.699 |
r = -0.710 |
|
P = 0.023 |
P = 0.024 |
P = 0.022 |
* Correlation is significant at the 0.05 level
Increased agriculture production through intensive farming has resulted in more pest problems leading to lower crop yields (Maqbool et al., 2020). Despite pesticide use, crop losses have almost doubled (Wilson and Tisdell, 2001). Now, there is a worldwide resurgence of awareness and understanding regarding the hazards of pesticide use (Oerke, 2006). This growing realization has encouraged a significant decrease in pesticide usage. Also, the gradual shift towards more sustainable and environmentally friendly pest management practices has been noted in the recent past (Wezel et al., 2009). Therefore, it is necessary to adopt a strategy that would help to control pest populations using local natural predators (Deguine et al., 2021).
The ladybeetles of two districts showed dominance from the sub family Coccinellinae (Latreille, 1807) which accounted for 96.62% of total catch during the sampling period (two years). Among these, C. septempunctata (Linnaeus, 1758) was the most common and dominant species throughout the sampling period and this finding is in accordance with the earlier research on Coccinellinae distribution, conducted in the other agricultural crops (rice, wheat, and cotton) in Pakistan (Rahatullah and Inayatullah, 2010; Abbas et al., 2013). Members of this sub family are aphidophagous feeders showing dominance throughout the crop cycle. They are found in all habitats and agriculture ecosystems globally feed on aphids, mealybugs, psyllids, and thrips (Rahat et al., 2012; Zare Khormizi et al., 2013; Sanjta and Chauhan, 2018).
Furthermore, C. undecimpunctata (Linnaeus, 1758) was the second dominant species (17.45%) collected from the maize crops. Many other researchers also reported the dominance of this species in various studies worldwide (Kılınçer et al., 2010). The primary reason for their abundance in the agroecosystems could be due to the availability of plentiful prey like aphids. However, this species also feeds on host species like psyllids and small insects (Rahat et al., 2012). The 4th instar and adults (both male and female) are reported to attack equally on leaf aphids and their supremacy leads to the efficacy of pest suppression in field crops (Cabral et al., 2009). In maize crops, C. undecimpuncta is reported to be the most abundant during the month of May and the least abundant in June (Mahmoud et al., 2021). Cheilomenus sexmaculata (Fabricius, 1781), was the third most abundant species (10.98%) during both cropping seasons and similar results were also reported from other cereal crops in Punjab, Pakistan (Bodlah et al., 2021). This species feeds on aphids including Rhopalosiphum maidis (Fitch), Aphis gossypii (Glover) and Lipaphis erysimi (Kaltenbach). The aphid consumption is highest in 4th instar grubs. The high feeding potential of Che. sexmaculata makes it an excellent biological control agent. Epilachna varivestis (Mulsant, 1850) was observed during the early tasseling stage of maize crops and it is among the main pest of the maize and bean (Ranum et al., 2014). Moreover, this species exhibited a significant increase in population dynamics, transitioning from maize crops to surrounding weeds. C. transversalis (Fabricius, 1781) is a polyphagous coccinellid in agriculture fields of the Oriental regions. It feed on a wide range of aphids (Omkar and Bind, 1993; Navodita et al., 2011).
Variation in the number of coccinellid species was associated with the combined species curves. The results indicated as the sample size increased, the number of coccinellids species also increased. The species accumulation curve for coccinellids across all sites in both districts did not reach an asymptote suggesting there might be more species not yet found. This could be due to different periods of activity for different coccinellid species (Willott, 2001). Around 94–95% of the coccinellid species in the region were identified during the two-year sampling period, while the remaining 5–6% consists of a few uncommon or rare species. These species may not have been recorded during our sampling due to their distinct activity times (Willott, 2001; Gatti and Carneiro, 2019). Research shown that different species of ladybeetles show their predacious activity during different times of the day due to their varying feeding habits (Siddiqui and Mishra, 2023). This behavior might be due to signals from their prey, which change over the course of the day (Ferreira et al., 2022).
Our study highlights the correlation between ladybeetle abundance and richness with both the local habitat and landscape factors. The landscape design and surrounding habitat highly influence both the composition and diversity of the lady beetles (Egerer et al., 2017). The success of biological pest control with Coccinellidae is intricately linked to the diversity of species within this group (Michaud, 2012). Varied dietary preferences, temporal activity patterns, and habitat adaptations among coccinellid species collectively support pest control efficacy (Kheirodin et al., 2022). A diverse community ensures coverage across different pests, seasons, and locations, as well as mitigates the risk of pest resistance, and enhances the resilience of control efforts against changing environmental conditions (Skendžić et al., 2021).
The crop growth stages and climatic variables like air temperature, humidity level, and precipitation also influence the diversity and richness of coccinellids (Skendžić et al., 2021). Aphidophagous coccinellids face challenges arising directly due to climate changes which may alter prey population (Sloggett, 2021). During the current study, the coccinellid abundance increased from the vegetative stage to the tasseling stage (BBCH-19 to BBCH-65). A rapid decrease was observed at the start of the ripening stage (BBCH-70 to BBCH-89) in both districts. The peak density of coccinellids might be due to the high availability of the aphid population (Obrycki et al., 2009). These results coincide with previous findings that indicate abundance reached its highest point during the vegetative and tasseling stages, but declined during the ripening stage (Svobodová et al., 2015; Pan et al., 2020). Even under high temperature, C. septempunctata shows the highest density among the captured coccinellids fauna. Mounting evidence indicates advocated the high abundance of coccinellids even under high temperatures as the maize whorls provide microhabitat refuge to predator lady beetles (Pan et al., 2020). Low temperatures and higher humidity were observed inside the whorl as compared to the outside. Understanding the mechanisms used by natural enemies to look for and capture their prey is essential for the development of successful biological control strategies.
Conclusions and Recommendations
Due to their substantial success in the biological control of various harmful insects especially aphids, coccinellid beetles are considered to hold great economic importance within agroecosystems. The current study aimed to identify and document the coccinellid fauna of the maize crops so that they may be employed for the successful implementation of Integrated Pest Management plans. These outcomes will ultimately pave the way for the development of conservation focused biological strategies for the indigenous plant protection programs. In addition, this research will help to lay the groundwork for a cost effective and eco-friendly pest control strategy suitable for use in developing countries like Pakistan.
Acknowledgments
The authors want to thank the authorities of ORIC, GC University, Lahore and Agriculture Department, Government of the Punjab for providing necessary facilitation to complete this project.
Funding
This study was conducted solely self-funded.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Abbas, M.N., Kausar, S. and Rana, S.A., 2013. Diversity and distribution of ladybird beetles (Coccinellidae) in the cropland of Faisalabad district. Int. J. Adv. Res., 1: 27-33.
Adeyinka, O.S., Tabbassum, B., Sharif, M.N., Bhatti, M.U., Nasir, I.A. and Husnain, T., 2018. A lag in the advancement of biotechnology: reliable control of maize stem borers in Africa. J. Pl. Prot. Res., 58: 8-24.
Ahmad, M. and Farooq, U., 2010. The state of food security in Pakistan: Future challenges and coping strategies. Pak. Dev. Rev., 49: 903-923. https://doi.org/10.30541/v49i4IIpp.903-923
Akhtar, S., LI, G.C., Ullah, R., Nazir, A., Iqbal, M.A., Raza, M.H., Iqbal, N. and Faisal, M., 2018. Factors influencing hybrid maize farmers’ risk attitudes and their perceptions in Punjab Province, Pakistan. J. Integr. Agric., 17: 1454-1462. https://doi.org/10.1016/S2095-3119(17)61796-9
Ali, S., Liu, Y., Ishaq, M., Shah, T., Abdullah, Ilyas, A. and Din, I.U., 2017. Climate change and its impact on the yield of major food crops: Evidence from Pakistan. Foods, 6: 1-19. https://doi.org/10.3390/foods6060039
Bahlai, C.A., Colunga-Garcia, M., Gage, S.H. and Landis, D.A., 2015. The role of exotic ladybeetles in the decline of native ladybeetle populations: Evidence from long-term monitoring. Biol. Invasions., 17: 1005-1024. https://doi.org/10.1007/s10530-014-0772-4
Basha, H.A., Mostafa, E.M. and Eldeeb, A.M., 2021. Mite pests and their predators on seven vegetable crops (Arachnida: Acari). Saudi J. Biol. Sci., 28: 3414-3417. https://doi.org/10.1016/j.sjbs.2021.03.004
Biddinger, D.J., Weber, D.C. and Hull, L.A., 2009. Coccinellidae as predators of mites: Stethorini in biological control. Biol. Contr., 51: 268–283. https://doi.org/10.1016/j.biocontrol.2009.05.014
Bielawski, R., 1972. Die Marienkäfer (Coleoptera: Coccinellidae) aus Nepal. Fragm. Faun., 18: 283-312. https://doi.org/10.3161/00159301FF1972.18.16.283
Bodlah, M.A., Bodlah, I., Tariq Rasheed, M., Fareen, G., Ikram, K., Iqbal, Z. and Zada, R., 2021. Coccinellidae beetles (Coleoptera) fauna of district Layyah (Punjab), Pakistan. Asian J. Agric. Biol., 1: 1-8. https://doi.org/10.35495/ajab.2020.05.299
Bouchard, P., Smith, A.B., Douglas, H., Gimmel, M.L., Brunke, A.J. and Kanda, K., 2017. Biodiversity of coleoptera. Insect Biodiv. Sci. Soc., pp. 337-417. https://doi.org/10.1002/9781118945568.ch11
Brühl, C.A. and Zaller, J.G., 2019. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci., 7: 2013–2016. https://doi.org/10.3389/fenvs.2019.00177
Cabral, S., Soares, A.O. and Garcia, P., 2009. Predation by Coccinella undecimpunctata L. (Coleoptera: Coccinellidae) on Myzus persicae Sulzer (Homoptera: Aphididae): Effect of prey density. Biol. Contr., 50: 25–29. https://doi.org/10.1016/j.biocontrol.2009.01.020
Canepari, C. and Milanese, S.D., 1997. Stuttgarter beiträge zur naturkunde Serie A (Biologie): Coccinellidae (Coleoptera) from the Nepal Himalayas.
Chao, A., 1984. Nonparametric estimation of the number of classes in a population. Scand. J. Stat., 11: 265–270.
Chao, A., Colwell, R.K., Lin, C.W. and Gotelli, N.J., 2009. Sufficient sampling for asymptotic minimum species richness estimators. Ecology, 90: 1125-1133. https://doi.org/10.1890/07-2147.1
Chaudhary, D.P., Kumar, S. and Langyan, S., 2014. Maize: nutrition dynamics and novel uses (Vol. 3). New Delhi: Springer India. https://doi.org/10.1007/978-81-322-1623-0
Colwell, R.K. and Coddington, J.A., 1994. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. London. Ser. B Biol. Sci., 345: 101–118. https://doi.org/10.1098/rstb.1994.0091
Colwell, R.K. and Elsensohn, J.E., 2014. Estimates turns 20: statistical estimation of species richness and shared species from samples, with non-parametric extrapolation. Ecography, 37: 609-613. https://doi.org/10.1111/ecog.00814
Colwell, R.K., Rahbek, C. and Gotelli, N.J., 2004. The mid-domain effect and species richness patterns: What have we learned so far? Am. Nat., 163: E1-E23. https://doi.org/10.1086/382056
Deguine, J.P., Aubertot, J.N., Flor, R.J., Lescourret, F., Wyckhuys, K.A. and Ratnadass, A., 2021. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev., 41: 38. https://doi.org/10.1007/s13593-021-00689-w
Dhawan, A.K. and Peshin, R., 2009. Integrated pest management: Concept, opportunities and challenges. Pest Manage. Innov. Process., 1: 51-81. https://doi.org/10.1007/978-1-4020-8992-3_2
Egerer, M.H., Arel, C., Otoshi, M.D., Quistberg, R.D., Bichier, P. and Philpott, S.M., 2017. Urban arthropods respond variably to changes in landscape context and spatial scale. J. Urban Ecol., 3: p.jux001. https://doi.org/10.1093/jue/jux001
Evans, E.W., 2009. Lady beetles as predators of insects other than Hemiptera. Biol. Contr., 51: 255-267. https://doi.org/10.1016/j.biocontrol.2009.05.011
Ferreira, J.O., Silva-Torres, C.S., Carmo, E.B., Laumann, R.A., Borges, M. and Blassioli-Moraes, M.C., 2022. Do interactions among ladybeetles affect their fitness and predatory behavior. J. Insect Behav., 35: 195–212. https://doi.org/10.1007/s10905-022-09810-7
Gatti, F.D. and Carneiro, M.A.A., 2019. Estimation of the species richness of hyperdiverse beetles (Coleoptera: Cerambycidae) in an area of atlantic forest, minas gerais, southeastern Brazil. Neotrop. Biol. Conserv., 14: 489–498. https://doi.org/10.3897/neotropical.14.e49026
Government of Pakistan, 2021. Agriculture Economic survey 2021. Minist. Financ. https://www.finance.gov.pk/survey/chapters_21/02-Agriculture.pdf
Hajek, A.E. and Eilenberg, J., 2018. Natural enemies: An introduction to biological control. Cambridge University Press. https://doi.org/10.1017/9781107280267
Hassan, K., Pervin, M., Mondal, F. and Mala, M., 2016. Habitat management: A key option to enhance natural enemies of crop pest. Univ. J. Pl. Sci., 4: 50–57. https://doi.org/10.13189/ujps.2016.040402
Hesler, L.S. and Kieckhefer, R.W., 2008. Status of exotic and previously common native coccinellids (Coleoptera) in South Dakota landscapes. J. Kans. Entomol. Soc., 81: 29-49. https://doi.org/10.2317/JKES-704.11.1
Hodek, I. and Honěk, A., 2009. Scale insects, mealybugs, whiteflies and psyllids (Hemiptera, Sternorrhyncha) as prey of ladybirds. Biol. Contr., 51: 232–243. https://doi.org/10.1016/j.biocontrol.2009.05.018
Hortal, J., Borges, P.A.V. and Gaspar, C., 2006. Evaluating the performance of species richness estimators: Sensitivity to sample grain size. J. Anim. Ecol., 75: 274–287. https://doi.org/10.1111/j.1365-2656.2006.01048.x
Inayatullah, M., Hayat, A. and Rafi, M.A., 2005. Species composition, distribution and seasonal occurrence of Coccinellidae (Coleoptera) in district Poonch, Azad Kashmir with new records. Sarhad J. Agric.,21: 97-100.
Kapur, A.P., 1955. Coccinellidae of Nepal. Rec. Zool. Surv. India, 53: 309–338. https://doi.org/10.26515/rzsi/v53/i3-4/1955/162012
Kemp, E.A. and Cottrell, T.E., 2015. Effect of lures and colors on capture of lady beetles (Coleoptera: Coccinellidae) in tedders pyramidal traps. Environ. Entomol., 44: 1395-1406. https://doi.org/10.1093/ee/nvv108
Khan, Z., Sharawi, S.E., Khan, M.S., Xing, L.X., Ali, S. and Ahmed, N., 2022. Prevalence of insect pests on maize crop in District Mansehra, Khyber Pakhtunkhwa, Pakistan. Braz. J. Biol., 84: 1–7. https://doi.org/10.1590/1519-6984.259217
Kheirodin, A., Cárcamo, H.A., Sharanowski, B.J. and Costamagna, A.C., 2022. Crop diversity increases predator abundance but not predation on cereal leaf beetles in agricultural landscapes. J. Pest Sci., 95: 1091–1110. https://doi.org/10.1007/s10340-021-01454-4
Kılınçer, N., Yiğit, A., Kazak, C., Er, M.K., Kurtuluş, A. and Uygun, N., 2010. Biological control of pests from theory to practice. Turk. J. Biol. Contr., 1: 15-60.
Lancashire, P.D., Bleiholder, H., Boom, T.V.D., Langelüddeke, P., Stauss, R., Weber, E. and Witzenberger, A., 1991. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol., 119: 5561-603. https://doi.org/10.1111/j.1744-7348.1991.tb04895.x
Lawton, J.H., Naeem, S., Thompson, L.J., Hector, A. and Crawley, M.J., 1998. Biodiversity and ecosystem function: getting the Ecotron experiment in its correct context. Funct. Ecol., 12: 848-852.
Losey, J.E. and Vaughan, M., 2006. The economic value of ecological services provided by insects. Bioscience, 56: 311-323. https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Ludwig, J.A. and Reynolds, J.F., 1988. Statistical ecology: A primer in methods and computing (Vol. 1). John Wiley & Sons.
Mahmoud, H.H., Abd El-Rahman, S.F., Naroz, M.H. and Ahmed, S.S., 2021. Effect of sowing dates on the populations of three major insect pests and associated natural enemies throughout the growth stages of maize plants. Polish J. Entomol., 90: 130-144. https://doi.org/10.5604/01.3001.0015.2381
Majerus, M. and Kearns, P., 1989. Ladybirds. Naturalists’ Handbook 10. Dorset: The Richmond Publishing Co Ltd.
Maqbool, A., Rather, S.U., Akbar, S.A. and Wachkoo, A.A., 2020. Preliminary survey of ladybird beetle composition (Coleoptera: Coccinellidae) in unmanaged apple orchard ecosystems of Kashmir Himalayas. Proc. Zool. Soc., 73: 160-174. https://doi.org/10.1007/s12595-020-00322-w
Mercado, M.I., Coll Aráoz, M.V., Manrique, I., Grau, A. and Catalán, C.A., 2014. Variability in sesquiterpene lactones from the leaves of yacon (Smallanthus sonchifolius) accessions of different geographic origin. Crop Evol., 61: 1209–1217. https://doi.org/10.1007/s10722-014-0103-8
Michalko, R., Pekár, S., Dul’a, M. and Entling, M.H., 2019. Global patterns in the biocontrol efficacy of spiders: A meta-analysis. Glob. Ecol. Biogeogr., 28: 1366-1378. https://doi.org/10.1111/geb.12927
Michaud, J.P., 2012. Coccinellids in biological control. Ecology and behaviour of the ladybird beetles (Coccinellidae), pp. 488-519. https://doi.org/10.1002/9781118223208.ch11
Mora, P., Vela, J., Ruiz-Ruano, F.J., Ruiz-Mena, A., Montiel, E.E., Palomeque, T. and Lorite, P., 2020. Satellitome analysis in the ladybird beetle Hippodamia variegata (Coleoptera, Coccinellidae). Genes., 11: 783. https://doi.org/10.3390/genes11070783
Mubushar, M., Aldosari, F.O., Baig, M.B., Alotaibi, B.M. and Khan, A.Q., 2019. Assessment of farmers on their knowledge regarding pesticide usage and biosafety. Saudi J. Biol. Sci., 26: 1903–1910. https://doi.org/10.1016/j.sjbs.2019.03.001
Nadeem, A., Tahir, H.M., Khan, A.A., Bano, N., Hassan, Z. and Khan, A.M., 2023. Species composition, seasonal abundance and population dynamics of predatory spiders from cotton field plots of irrigated and semi-arid regions of Punjab, Pakistan. Saudi J. Biol. Sci., 30: 103686. https://doi.org/10.1016/j.sjbs.2023.103686
Navodita, M., Bouchard, A., Kumar, A. and Ramteke, P.W., 2011. Duration of development and survival of larvae of Coccinella transversalis fed on essential and alternative foods. Eur. J. Environ. Sci., 1: 24–27. https://doi.org/10.14712/23361964.2015.61
Norgrove, L., 2021. Trade-offs in maize seedling losses in African grasslands. Crop Prot., 146: 105676. https://doi.org/10.1016/j.cropro.2021.105676
Obrycki, J.J., Harwood, J.D., Kring, T.J. and O’Neil, R.J., 2009. Aphidophagy by Coccinellidae: application of biological control in agroecosystems. Biol. Contr., 51: 244–254. https://doi.org/10.1016/j.biocontrol.2009.05.009
Oerke, E.C., 2006. Crop losses to pests. J. Agric. Sci., 144: 31–43. https://doi.org/10.1017/S0021859605005708
Omkar, B.R. and Bind, R.B., 1993. Records of aphid-natural enemies complex of Uttar Pradesh. II. The Coccinellids. J. Adv. Zool., 14: 96-99.
Pan, H., Xiu, C., Liu, B., Wyckhuys, K.A. and Lu, Y., 2020. Whorl-stage maize provides a microclimate refuge for predatory ladybeetles. Biol. Contr., 142: 104162. https://doi.org/10.1016/j.biocontrol.2019.104162
Pan, H., Yang, X., Romeis, J., Siegfried, B.D. and Zhou, X., 2020. Dietary RNAi toxicity assay exhibits differential responses to ingested dsRNAs among lady beetles. Pest Manage. Sci., 76: 3606–3614. https://doi.org/10.1002/ps.5894
Pervez, A., 2016. Ladybird beetles. In: Ecofriendly pest management for food security. Academic Press. pp. 281-310. https://doi.org/10.1016/B978-0-12-803265-7.00009-9
Poorani, J., 2023. An illustrated guide to lady beetles (Coleoptera: Coccinellidae) of the Indian Subcontinent. Part 1. Tribe Coccinellini. Zootaxa., 5332: 1-307. https://doi.org/10.11646/zootaxa.5332.1.1
Poudel, S., Poudel, B., Acharya, B. and Poudel, P., 2020. Pesticide use and its impacts on human health and environment. Environ. Ecosyst. Sci., 4: 47–51. https://doi.org/10.26480/ees.01.2020.47.51
Prasanna, B.M., Carvajal-Yepes, M., Kumar, P.L., Kawarazuka, N., Liu, Y., Mulema, A.A., McCutcheon, S. and Ibabao, X., 2022. Sustainable management of transboundary pests Akhtar requires holistic and inclusive solutions. Fd. Secur., 14: 1449–1457. https://doi.org/10.1007/s12571-022-01301-z
Rafi, M.A., Irshad, M. and Inaytullah, M., 2005. Predatory ladybird beetles of Pakistan. National Insect Museum & Insect Pest Informatics, IPM Programme.
Rahat, U., Faizul, H., Habib, A., Mian, I., Kausar, S. and Shahroz, K., 2012. Morphological characteristics of ladybird beetles collected from District Dir Lower, Pakistan. Afr. J. Biotechnol., 11: 9149–9155. https://doi.org/10.5897/AJB11.1363
Rahatullah, A.H. and Inayatullah, M., 2010. Species diversity of coccinellidae of Dir valley (Doctoral dissertation, M. Phil. thesis. Department of Zoology, Hazara University, Mansehra, Pakistan.
Ranum, P., Peña-Rosas, J.P. and Garcia-Casal, M.N., 2014. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci., 1312: 105–112. https://doi.org/10.1111/nyas.12396
Rehman, A., Jingdong, L., Shahzad, B., Chandio, A.A., Hussain, I., Nabi, G. and Iqbal, M.S., 2015. Economic perspectives of major field crops of Pakistan: An empirical study. Pac. Sci. Rev. B Humanit. Soc. Sci., 1: 145–158. https://doi.org/10.1016/j.psrb.2016.09.002
Riaz, S., Kausar, S., Mohsin, M., Memon, A.M., Maqsood, I. and Abbas, M.N., 2017. Spider diversity in some common oilseed crops in central Punjab, Pakistan. Pak. J. Sci. Ind. Res. Ser. B Biol. Sci., 60: 168–175. https://doi.org/10.52763/PJSIR.BIOL.SCI.60.3.2017.168.175
Riddick, E.W., 2023. Production of coleopteran predators. In: Mass production of beneficial organisms. Academic Press. pp. 13-36. https://doi.org/10.1016/B978-0-12-822106-8.00013-0
Sánchez-Bayo, F. and Wyckhuys, K.A., 2019. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv., 232: 8–27. https://doi.org/10.1016/j.biocon.2019.01.020
Sanjta, S. and Chauhan, U., 2018. Incidence and diversity of thrips and its associated natural enemies in medicinal plants. J. Med. Plant Stud., 6: 1–2.
Sappington, T.W., Hesler, L.S., Allen, K.C., Luttrell, R.G. and Papiernik, S.K., 2018. Prevalence of sporadic insect pests of seedling corn and factors affecting risk of infestation. J. Integr. Pest Manage., 9: 16. https://doi.org/10.1093/jipm/pmx020
Savary, S., Willocquet, L., Pethybridge. S.J., Esker, P., McRoberts, N. and Nelson, A., 2019. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3: 430-439. https://doi.org/10.1038/s41559-018-0793-y
Sentis, A., Hemptinne, J.L., Magro, A. and Outreman, Y., 2022. Biological control needs evolutionary perspectives of ecological interactions. Evol. Appl., 15: 1537–1554. https://doi.org/10.1111/eva.13457
Shapiro, S.S. and Wilk, M.B., 1965. An analysis of variance test for normality (complete samples). Biometrika., 52: 591-611. https://doi.org/10.1093/biomet/52.3-4.591
Siddiqui, A. and Mishra, G., 2023. Optimising aphid biocontrol with the predator Propylea dissecta, based on experimental evolution of a predatory population. Can. Entomol., 155: e11. https://doi.org/10.4039/tce.2022.36
Skendžić, S., Zovko, M., Živković, I.P., Lešić, V. and Lemić, D., 2021. The impact of climate change on agricultural insect pests. Insects., 12: 440. https://doi.org/10.3390/insects12050440
Sloggett, J.J., 2021. Aphidophagous ladybirds (Coleoptera: Coccinellidae) and climate change: A review. Insect Conserv. Divers., 14: 709–722. https://doi.org/10.1111/icad.12527
Snyder, W.E., 2019. Give predators a complement: Conserving natural enemy biodiversity to improve biocontrol. Biol. Contr., 135: 73–82. https://doi.org/10.1016/j.biocontrol.2019.04.017
Solow, A.R., 1993. A simple test for change in community structure. J. Anim. Ecol., 62: 191–193. https://doi.org/10.2307/5493
Svobodová, Z., Skoková Habuštová, O., Hutchison, W.D., Hussein, H.M. and Sehnal, F., 2015. Risk assessment of genetically engineered maize resistant to Diabrotica spp.: Influence on above-ground arthropods in the Czech Republic. PLoS One, 10: e0130656. https://doi.org/10.1371/journal.pone.0130656
Wezel, A., Bellon, S., Doré, T., Francis, C., Vallod, D. and David, C., 2009. Agroecology as a science, a movement and a practice. A review. Agron. Sustain. Dev., 29: 503–515. https://doi.org/10.1051/agro/2009004
Whittaker, R.H. and Levin, S., 1977. The role of mosaic phenomena in natural communities. Theor. Popul. Biol., 12: 117–139. https://doi.org/10.1016/0040-5809(77)90039-9
Willott, S.J., 2001. Species accumulation curves and the measure of sampling effort. J. Appl. Ecol., 38: 484–486. https://doi.org/10.1046/j.1365-2664.2001.00589.x
Wilson, C. and Tisdell, C., 2001. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol. Econ., 39: 449–462. https://doi.org/10.1016/S0921-8009(01)00238-5
Xiu, C., Zhang, W., Xu, B., Wyckhuys, K.A., Cai, X., Su, H. and Lu, Y., 2019. Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol. Contr., 129: 1–11. https://doi.org/10.1016/j.biocontrol.2018.11.008
Zahoor, M.K., Suhail, A., Iqbal, J., Zulfaqar, Z. and Anwar, M., 2003. Biodiversity of predaceous coccinellids and their role as bioindicators in an agro-ecosystem. Int. J. Agric. Biol., 5: 555–559.
Zare Khormizi, M., Biranvand, A. and Shakarami, J., 2013. The faunistic survey of lady beetles (Coleoptera, Coccinellidae) in the Mehriz region (Yazd Province), Iran. Iran. Bull. Iraq Nat. Hist. Mus., 12: 43–51.
To share on other social networks, click on any share button. What are these?







