Assessment of Genetic Variability in Onion against Purple Leaf Blotch Disease under Field Conditions and its Management
Research Article
Assessment of Genetic Variability in Onion against Purple Leaf Blotch Disease under Field Conditions and its Management
Muhammad Younas1*, Khalid Hussain1, Abdul Ghaffar1, Muhammad Atiq2, Niaz Hussain1, Wasim Abbas3, Muhammad Azeem Khan4, Muhammad Nadeem1, Muhammad Irshad1, Nasir Ahmad Khan2 and Muhammad Zubair5
1Arid Zone Research Institute, Bhakkar, Punjab, Pakistan; 2Department of Plant Pathology, University of Agriculture Faisalabad, Pakistan; 3Vegetable Research Institute, Ayub Agricultural Research Institute, Faisalabad, Pakistan; 4Department of Weed Science and Botany, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 5Agricultural Research Station (ARS), Bahawalpur, Pakistan
Abstract | Purple blotch disease is exerting a severe threat on onion production. The current study was conducted for screening of accessions against purple blotch disease and determining the source of resistance against Purple blotch disease of onion caused by Alternaria porri. Potential of 50 onion accessions was evaluated against A. porri during 2017 and 2018 under field conditions. After that effect of some fungicides and plant extracts was determined for disease suppression on a susceptible variety of onion. During both years of study, eight accessions (Phulkara, Texas Early, Cylon, Sunset, Red Gystal, Rubi F1, Red Flame, ON-14133) exhibited resistant response with minimum percent disease index (PDI) which ranged between 5-10%, followed by Mirpurkhas, Nasarpuri, HON-1069, Pania, Rubi F2, GSL-132, Red Moon, PK-1032, F-1122, Red Imposta, Early Red, Desi Large and Vrio-4 which showed moderately resistant response to A.porri with PDI of 11-20%, whereas, the four accession including Golden Arab, Desired, Sultan F1 and Red Nasik expressed Moderately resistant response with PDI of 21-40%. Moreover, six accessions (Yellow Gystal, Vrio-9, Vrio-5, Vrio-4, Vrio-1, and Vrio-3) expressed susceptible response with high value of PDI 41-60% whereas; nine accessions (CBS-130, ON-14121, Pink Panther, Vrio-7, Vrio-3, F1 Zeus, SE-16, Vrio-8 and Marvi) showed maximum value (Above 60%) of PDI and expressed highly susceptible response to A.porri. Among fungicides and botanical extracts, Chlorostrobin and Azadirachta indica extract expressed significant reduction in disease severity. It was concluded that out of 50, only eight accessions were resistant which might be grown successfully for higher crop production. Moreover, use of Chlostrobin and extract of Azadirachta indica may be used for disease management.
Received | December 30, 2021; Accepted | March 22, 2022; Published | September 26, 2022
*Correspondence | Muhammad Younas, Arid Zone Research Institute, Bhakkar, Punjab, Pakistan; 2Department of Plant Pathology, University of Agriculture Faisalabad, Pakistan; Email: younasali643@gmail.com
Citation | Younas, M., K. Hussain, A. Ghaffar, M. Atiq, N. Hussain, W. Abbas, M.A. Khan, M. Nadeem, M. Irshad, N.A. Khan and M. Zubair. 2022. Assessment of genetic variability in onion against purple leaf blotch disease under field conditions and its management. Sarhad Journal of Agriculture, 38(4): 1273-1278.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.4.1273.1278
Keywords | Allium cepa, Alternaria porri, Accessions, Percent disease Index (PDI), Purple blotch
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Onion (Allium cepa L.) belongs to family Alliaceae and is cultivated all around the world for its nutritive, medicinal and health protective value. It is mainly known as “Queen of Kitchen” as it accompanies all cooking items (Mandloi, 2017). Onion carries specific taste and numerous medicinal characteristics likewise antifungal, germicide, antispasmodic and antibacterial (Griffiths et al., 2002). China and India are the leading onion producing countries (Mallor et al., 2011). In Pakistan about 147.2 hectare is under onion cultivation with 1,981.7 thousand tons production (PES, 2017-18). Onion leaves are also utilized as supplementary nourish for cattle, rabbits and poultry birds (Mba and Akueshi, 2001). Onion is attacked by numerous fungal diseases but purple blotch disease caused by Alternaria porri is the potential extortion to the successful cultivation of onion and is the potential threat all over the world (Kumar and Palakshapra, 2008).
Production of host specific and non-specific toxins significantly influences the level of disease incidence and these toxins cause leaf necrosis in susceptible varieties (Mamgain et al., 2013). It also destroys bulb initiation stimulus and delays bulb maturation. A. porri mainly affects bulbs and leaves of the plants which leads to heavy yield losses of up to 97% (Ravichandran et al., 2017; Kareem et al., 2012). Pathogen is soil borne and it remains viable in soil for longer period and causes severe losses to onion crop (Yaradua, 2003). Purple blotch disease is commonly initiated by moderate temperature 25-30°C and high humidity 80-90%. Numerous strategies, including the application of fungicides and plant extracts have been tested against A.porri (Jhala et al., 2017a; Yadav et al., 2017; Younas et al., 2021). Among all strategies, some are not economically applicable for the farmers because of their high cost whereas others have direct or indirect deteriorating and degrading effect on human beings and environment (Iglesias et al., 2021). Perfect method to overcome the maladies is the use of resistant onion genotypes. Therefore screening from available germplam is pre-requisite to determine the accessions which are resistant against A. porri (Thaxton and Zik, 2001). Thus, present research was aimed to find out the resistant accessions for the management of purple blotch disease under field conditions and the other component of current study was consisted on the evaluation of suitable fungicides and plant extracts for disease management in susceptible accessions.
Materials and Methods
Assessment of onion accessions against Alternaria porri under natural field conditions
In first component of the study, Onion nursery was grown at Vegetable Research Area, Ayub Agriculture Research Institute Faisalabad (AARI) on 25th and 21st of October during 2017 and 2018 respectively, whereas the transplanting was done after 45 days of sowing during both years of study. Disease free seeds of fifty accessions were used for nursery raising. The nursery was transplanted in field by following P×P and R×R distance of 22 and 60cm respectively. The experiment was laid out in Randomized complete block design (RCBD) and replicated thrice. Disease intensity were recorded after two months of transplantation by using 0-5 rating scale (Sharma, 1986) and all the accessions were grouped in different ranks depending on their resistance and susceptibility to A. porri (Pathak et al., 1986). The percent disease index (PDI) was determined by following formula, proposed by (Wheeler, 1969).
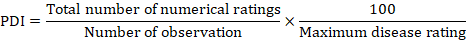
Assessment of fungicides and botanical extracts against A. porri
In second component of the study, susceptible variety of onion (Marvi) was cultivated to assess the capability of fungicides and botanical extracts against A.porri by following the same planting geometry, nursery raising and transplanting dates during both study years as in first component. The same experimental design and number of replications were followed.
Management of purple blotch through fungicides
Two fungicides Chlorostrobin and Nanok at the concentration of 1.5g/ liter of water were evaluated against purple blotch disease of onion under field conditions. Experiment was laid out in randomized complete block design (RCBD) by adopting standard row to row and plant to plant spacing. Three sprats at the interval of fifteen days were used and the data regarding disease reduction was after seven and fourteen days of each spray. First spray application was done after the appearance of characteristics symptoms.
Management of purple blotch through plant extracts
Three plant extracts (Azadirachta indica, Ocimum tenuiflorum, Allium sativum) were evaluated under natural field conditions. Three sprays of plants extracts were done whereas; the first spray was done after the appearance of initial disease symptom in onion field however, remaining sequential sprays was done at the interval of fifteen days. For the preparation of botanical extracts, 75g fresh leaves were crushed in 25 ml sterilized distilled water using a sterilized mortar and pestle. Resultant standard solution was filtered through sterilized four layered muslin cloth and whatman filter paper No.14, moreover standard arbitrarily is received
(Ilyas et al., 1996). Similarly standard concentration was prepared by mixing 100 ml of standard concentration with 100 ml of sterilized water.
Hand sprayer (IHT-401) was used for the application of chemical fungicides and botanical extracts. After the application of chemical fungicides and botanical extracts against A. porri disease reduction was calculated by using following formula.

Red= Reduction, DS= Disease Severity.
Statistical analysis
The recorded data from each experiment were statistically analyzed through Fisher’s analysis of variance (ANOVA) technique by using Statistix 8.1 software. The treatments means were compared by using Least Significant Difference (LSD) test at 5% probability level.
Table 1: Disease data was noted by visual observation and rating scale as described by (Sharma, 1986).
|
Disease ratings |
Descriptions |
Response |
|
0 |
No symptoms |
I |
|
1 |
Few spots on tip with 10% covered area |
R |
|
2 |
Purplish brown patches with covering of 20% leaf area |
MR |
|
3 |
Patches with paler outer region covering 40% leaf area |
MS |
|
4 |
Up to 75% leaf area covered with leaf streaks |
S |
|
5 |
Complete leaves are dried |
HS |
I: Immune/ highly resistant; R: Resistant; MR: Moderately Resistant; MS: Moderately Susceptible; S: Susceptible; HS: Highly Susceptible.
Results and Discussion
Assessment of onion accessions against Alternaria porri under natural field conditions
The current study was conducted for two experimental years during 2017 and 2018 under natural epiphytotic conditions. Assessment and evaluation of fifty onion accessions was carried out against purple leaf blotch disease and results are presented in Table 2. Disease severity (DS) ranged from 5% to79% among onion accessions. Different degrees of resistance were recorded among fifty accessions (Tables 2 and 3). The results revealed that there was not a single accession that showed immune response to Alternaria porri during both experimental years (2017-2018). However, during 2017 eight accessions were found resistant and thirteen accessions showed moderately resistant response. Moreover, eleven accessions were moderately susceptible whereas, nine accessions expressed susceptible response. Furthermore, remaining nine screened accessions demonstrated highly susceptible reaction to A. porri with percent disease index above 60%. Minimum disease severity was recorded in Phulkara (4.28%) whereas the maximum disease severity was observed in Marvi (79.00%).
Table 2: Response of onion accessions to purple blotch disease under field conditions during (2017).
|
Sr. |
Accessions |
PDI (%) |
Response |
Sr. |
Accessions |
PDI (%) |
Response |
|
1 |
Phulkara |
4.28l |
R |
26 |
Desi Black |
26.31v |
MS |
|
2 |
Texas Early |
4.85l |
R |
27 |
Robina |
26.31v |
MS |
|
3 |
Cylon |
5.87k |
R |
28 |
Husri |
28.12u |
MS |
|
4 |
Sunset |
6.30k |
R |
29 |
S-4466 |
32.43t |
MS |
|
5 |
Red gystal |
7.14j |
R |
30 |
Pussa Red |
33.90s |
MS |
|
6 |
Rubi F1 |
8.10i |
R |
31 |
Vrio-06 |
35.99r |
MS |
|
7 |
Red Flame |
8.85hi |
R |
32 |
Vrio-10 |
38.14q |
MS |
|
8 |
ON-14133 |
9.09h |
R |
33 |
SV-748NP |
41.35p |
S |
|
9 |
Mirpurkhas |
10.98g |
MR |
34 |
Perma |
42.74o |
S |
|
10 |
Nasarpuri |
11.66fg |
MR |
35 |
FSD Red |
45.32n |
S |
|
11 |
HON-1069 |
11.85ef |
MR |
36 |
Yellow gystal |
48.31m |
S |
|
12 |
Pania |
12.59e |
MR |
37 |
Vrio-09 |
51.75l |
S |
|
13 |
RubiF2 |
13.73d |
MR |
38 |
Vrio-05 |
54.93k |
S |
|
14 |
GSL-132 |
14.82c |
MR |
39 |
Vrio-04 |
55.49k |
S |
|
15 |
Red Moon |
15.88b |
MR |
40 |
Vrio-1 |
56.66j |
S |
|
16 |
PK-1032 |
16.260b |
MR |
41 |
Vrio-3 |
58.78i |
S |
|
17 |
F-1122 |
16.266b |
MR |
42 |
CBS-130 |
62.77h |
HS |
|
18 |
Red Imposta |
17.83a |
MR |
43 |
ON-14121 |
65.51g |
HS |
|
19 |
Early Red |
18.06a |
MR |
44 |
Pink Panther |
67.54f |
HS |
|
20 |
Desi Large |
18.90z |
MR |
45 |
Vrio-07 |
69.62e |
HS |
|
21 |
Vrio-4 |
19.71y |
MR |
46 |
Vrio-03 |
70.61d |
HS |
|
22 |
Golden Arab |
22.24x |
MS |
47 |
F1 Zeus |
71.41d |
HS |
|
23 |
Desired |
23.86w |
MS |
48 |
SE-16 |
72.58c |
HS |
|
24 |
Sultan F1 |
24.07w |
MS |
49 |
Vrio-08 |
75.66b |
HS |
|
25 |
Red Nasik |
25.89v |
MS |
50 |
Marvi |
77.72a |
HS |
|
LSD(0.05) |
0.79 |
||||||
|
SE |
0.40 |
||||||
*Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P≤0.05).
Table 3: Response of onion accessions to purple blotch disease under field conditions during (2018-19).
|
Sr. |
Accessions |
PDI (%) |
Response |
Sr. |
Accessions |
PDI (%) |
Response |
|
1 |
Phulkara |
5.30E |
R |
26 |
Red Nasik |
27.50QR |
MS |
|
2 |
Texas Early |
5.60E |
R |
27 |
Husri |
27.57QR |
MS |
|
3 |
Cylon |
6.95D |
R |
28 |
Robina |
27.92Q |
MS |
|
4 |
Sunset |
7.31CD |
R |
29 |
S-4466 |
33.32P |
MS |
|
5 |
Red Gystal |
8.20BC |
R |
30 |
Pussa Red |
35.17O |
MS |
|
6 |
Rubi F1 |
9.08AB |
R |
31 |
Vrio-6 |
36.26N |
MS |
|
7 |
Red Flame |
9.19AB |
R |
32 |
Vrio-10 |
39.49M |
MS |
|
8 |
ON-14133 |
9.42A |
R |
33 |
SV-789NP |
42.26L |
S |
|
9 |
Mirpurkhas |
11.27Z |
MR |
34 |
Fsd Red |
43.93K |
S |
|
10 |
HON-1069 |
12.69Y |
MR |
35 |
Perma |
44.92K |
S |
|
11 |
Nasarpuri |
12.71Y |
MR |
36 |
Yellow Gystal |
49.48J |
S |
|
12 |
Pania |
13.29Y |
MR |
37 |
Vrio-9 |
54.48I |
S |
|
13 |
Rubi F2 |
15.73X |
MR |
38 |
Vrio-4 |
55.04I |
S |
|
14 |
GSL-132 |
15.96X |
MR |
39 |
Vrio-5 |
55.29I |
S |
|
15 |
Red Moon |
17.09W |
MR |
40 |
Vrio-1 |
58.94H |
S |
|
16 |
PK-1032 |
17.32W |
MR |
41 |
Vrio-3 |
59.87H |
S |
|
17 |
F-1122 |
17.70W |
MR |
42 |
CBS-130 |
64.85G |
HS |
|
18 |
Early Red |
19.21V |
MR |
43 |
ON-14121 |
65.59G |
HS |
|
19 |
Vrio-4 |
19.28V |
MR |
44 |
Pink Panther |
68.27F |
HS |
|
20 |
Red Imposta |
19.50V |
MR |
45 |
Vrio-8 |
70.70E |
HS |
|
21 |
Desi Large |
19.89V |
MR |
46 |
Vrio-3 |
72.65D |
HS |
|
22 |
Golden arab |
21.07U |
MS |
47 |
F1 Zeus |
73.18D |
HS |
|
23 |
Sultan F1 |
25.21T |
MS |
48 |
SE-16 |
74.91C |
HS |
|
24 |
Desired |
25.92ST |
MS |
49 |
Vrio-8 |
77.80B |
HS |
|
25 |
Desi Black |
26.67RS |
MS |
50 |
Marvi |
79.67A |
HS |
|
LSD (0.05) |
1.060 |
||||||
|
SE |
0.53 |
||||||
*Mean values in a column sharing similar letters do not differ significantly as determined by the LSD test (P ≤ 0.05).
The result of the experimental year (2018) revealed that all accessions maintained their status and exhibited consistency in their response (Resistant, Moderately Resistance, Moderately Susceptible, Susceptible and Highly Susceptible) to A. porri however, an increased percent disease index in all tested accessions was recorded in comparison to the experimental year 2017 (Table 3).
Management of purple blotch disease of onion through fungicides and plant extracts
The results of second component of study revealed that chemical fungicide Chlorostrobin caused maximum disease inhibition up to 83%, followed by Nanok (77.40%), Azadirachta indica (68%), Ocimum tenuiflorum (65%) and Allium sativum (61.33%) (Table 4).
Table 4: Assessment of botanical extracts and fungicides against Alternaria porri under field conditions.
|
Sr. |
Treatment |
Active ingredients |
Disease reduction (%) |
|
1 |
Chlorostrobin |
Azoxystrobin + Chlorothalonil |
83.00a |
|
2 |
Nanok |
Azoxystrobin + Flutriafol |
77.40b |
|
3 |
Azadirachta indica |
Azadirachtin, Azadirachtin |
68.00 c |
|
4 |
Ocimum tenuiflorum |
Apigenin, Polyphenols, Anthocyanins and luteolin |
65.00d |
|
5 |
Allium sativum |
Alicin |
61.33e |
|
6 |
Control |
Water |
0.00f |
|
LSD (0.05) |
1.12 |
||
|
SE |
0.48 |
||
Purple leaf blotch is the most disparaging disease of onion which inflicts severe damage to aerial parts of the plant (Bal et al., 2019) and lowers the yield potential to great extent (up to 50%) (Jhala et al., 2017b). Disease development depends on the presence of susceptible host, favorable environmental conditions (temperature, rainfall, relative humidity and wind speed) and virulence strain of the pathogen. Under such scenario the economical and long lasting way to save crop from Alternaria porri is the use of resistant genotypes. Although the best possible and foremost probable solution is the development of resistant cultivars by inclusion of resistant genes, but it is time taking journey. Therefore, screening of the available accessions is the short term and easy way to identify resistant source. Keeping in view the above facts, in present study fifty accessions of onion were examined against A. porri under natural field conditions for two consecutive years. Results of the contemporary study revealed that there was not a single accession that showed immune/ highly resistant response towards onset of disease. Among all accessions Phulkara exhibited resistant response whereas the Marvi showed highly susceptible response. Results of present study are similar as the findings of Mansha et al. (2019) that found nonetheless of the varieties immune to disease whereas, the tested cultivar Phulkara displayed resistant response to A. porri. Results of our study are also supported by the previous studies of Behera et al. (2013) and Ulhaq et al. (2014) that there is not a single genotype which have resistance towards A. porri. In contemporary studies, Chlorostrobin expressed maximum disease reduction as it contains chlorothalonil and azoxystrobin which inhibits multi sites of different enzymes. Nanok is an effective fungicide against Basidiomycetes, Oomycetes, Ascomycetes and Deuteromycetes, moreover, it has curative and protectant characteristics and is highly systemic leading to the long term efficacy. It inhibits mycelial growth, respiration, spore germination and maintains normal leaf area which leads to maximum average yield potential (Younas et al., 2021). Azadirachta indica has a complex of numerous constituents likewise, nimbin, nimbolide, nimbidin and limonoids and such ingredients play a pivotal role in disease management by the modulation of different genetic pathways and other activities. ß-sitosterol and Quercetin were the first polyphenolic flavonoids purified from neem leaves and were known to have antifungal activities (Govindachari et al., 1998). Furthermore, studies revealed that antimicrobial role of neem aqueous extract significantly inhibits the growth of seed borne fungi i.e., Rhizopus and Aspergillus and sporulating fungi including C. lunata, C. gloeosporioides and H. pennisetti (Mondali et al., 2009) and the results of study revealed that aqueous extract of Azadirachta indica showed growth inhibition against Alternaria porri. Results are also in line with the findings of Younas et al. (2021) that Chlorostrobin exhibits maximum reduction in disease severity. Findings of current study are also in line with Islam et al. (2020) who studied the efficacy of different plant extracts against A. porri.
Conclusions and Recommendations
From the above findings of screening of accessions and evaluation of fungicides and plant extracts it was concluded that the eight cultivars which were resistant to this disease can be grown for better crop production. Moreover, Chlorostrobin and extract of Azadiracta indica can be used for disease suppression in susceptible accessions.
Novelty Statement
Identification of resistant accessions may be helpful in further studies and breeding programs. Moreover, recommended fungicide and botanical extract may also help farmers for timely management of purpe leaf blotch disease and higher crop production.
Author’s Contribution
Muhammad Younas: Collection of the data and manuscript writing.
Khalid Hussain: Conceived idea, Literature review.
Abdul Ghaffar: Analyzed and compiled the data.
Muhammad Atiq: Provide Resources.
Niaz Hussain: Supervised the whole research.
Muhammad Azeem Khan: Data analysis.
Wasim abbas: Designed research methodology.
Muhammad Nadeem: Helped in data Collection.
Muhammad Irshad: Data Interpretation.
Nasir Ahmad Khan: Ediedt the manuscript
Muhammad Zubair: Literature review
Conflict of interest
The authors have declared no conflict of interest.
References
Bal, S., T.K. Maity, A.B. Sharangi and A. Maji. 2019. Screening of onion (Allium cepa L.) germplasm against purple blotch disease. J. Pharmacogn. Phytochem., 8(6): 546-548.
Behera, S., P. Santra, S. Chattopadhyay, S. Das and T.K. Maity. 2013. Variation in onion varieties for reaction to natural infection of Alternaria porri (Ellis) Ciff. and Stemphylium vesicarium (Wallr.). Bioscan, 8(3): 759-761.
Govindachari, T.R., G. Suresh, G. Gopalakrishnan, B. Banumathy and S. Masilamani. 1998. Identification of antifungal compounds from the seed oil of Azadirachta indica. Phytoparasitica, 26(2): 109-116. https://doi.org/10.1007/BF02980677
Griffiths, G., L. Trueman, T. Crowther, B. Thomas and B. Smith. 2002. Onions a global benefit to health. Phytotherap. Res., 16(7): 603-615. https://doi.org/10.1002/ptr.1222
Iglesias, L., M.J. Havey and B.A. Nault. 2021. Management of onion thrips (Thrips tabaci) in organic onion production using multiple IPM tactics. Insects, 12(3): 207. https://doi.org/10.3390/insects12030207
Ilyas, M.B., M.A. Khan and M.U. Din. 1996. Evaluation of some fungicides against Fusarium oxyporium f. sp. lini and linseed wilt. Pak. J. Phytopathol., 8:42-54.
Islam, M.M., F. Begum, N. Nahar, U.A. Habiba and K.M. Fakruzzaman. 2020. In vivo and in vitro management of purple blotch of onion by using fungicides and plant extracts. Int. J. Sci. Res., 9(10): 930-938. https://doi.org/10.21275/SR201008003034
Jhala, P., B.L. Mali and M.K. Meena. 2017a. Effective management of purple blotch of onion caused by Alternaria porri (Ellis) through host resistance, fungicides and botanicals. Int. J. Cur. Microb. Appl. Sci., 6: 1737-1745. https://doi.org/10.20546/ijcmas.2017.605.188
Jhala, P., M.K. Meena and B.L. Mali. 2017b. Impact of abiotic factors and age of host plant on purple blotch of onion caused by Alternaria porri (Ellis) and estimation of yield losses. J. Plant Dev. Sci., 9(5): 447-451.
Kareem, M.A., K.V.M. Murthy, A.N. Hasansab and M.A. Waseem. 2012. Effect of temperature, relative humidity and light on lesion length due to Alternaria porri in onion. Bioinf. A Quart. J. Life Sci., 9(3): 264-266.
Kumar, P.T. and M.G. Palakshapra. 2008. Management of purple blotch of onion through bioagents. Karnat. J. Agric. Sci., 21: 306‒308.
Kumari, A., R. Kumar, S. Maurya, J.S. Choudhary and S. Kumar. 2013. Antifungal efficacy of aqueous extracts of neem cake, karanj cake and vermicompost against some phytopathogenic fungi. Bioscan, 8(2): 671-674.
Mallor, C., M. Balcells, F. Mallor and E. Sales. 2011. Genetic variation for bulb size, soluble solids content and pungency in the Spanish sweet onion variety Fuentes de Ebro. Response to selection for low pungency. Plant Breed., 130(1): 55-59. https://doi.org/10.1111/j.1439-0523.2009.01737.x
Mamgain, A., R. Roychowdhury and J. Tah. 2013. Alternaria pathogenicity and its strategic controls. Res. J. Biol., 1: 1-9.
Mandloi, R., 2017. Studies on purple blotch (Alternaria porri (Ellis) ciff.) of Onion (Allium cepa L.) (Doctoral dissertation, Rvskvv, Gwalior (MP)).
Mansha, M.Z., S.T. Sahi, A. Habib and S. Ahmed. 2019. Variations in yield and nutritional profile of onion germplasm under the influence of purple blotch disease. Int. J. Agric. Biol., 21(1): 120-124.
Mba, M.C. and C.O. Akueshi. 2001. Some physico-chemical changes induced by A. flavus and A. niger on S. indicum and S. radiatum. Afr. J. Nat. Sci., 4: 94‒97.
Mondall, N.K., A. Mojumdar, S.K. Chatterje, A. Banerjee, J.K. Datta and S. Gupta. 2009. Antifungal activities and chemical characterization of Neem leaf extracts on the growth of some selected fungal species in vitro culture medium.
Pathak, M.A., T.B. Fitzpatrick and E.W. Kraus. 1986. Usefulness of retinoic acid in the treatment of melasma. J. Am. Acad. Dermatol., 15(4): 894-899. https://doi.org/10.1016/S0190-9622(86)70247-8
PES (Pakistan Economic Survey 2017-18) https://www.finance.gov.pk/survey_1718.html
Ravichandran, S., B.C. Kamanna, K. Jayalakshmi, V.I. Benagi and K.B. Yadahalli. 2017. Severity of purple blotch of onion caused by Alternaria porii in Northern Karnataka, India. Int. J. Curr. Microbiol. App. Sci., 6(12): 3634-3638. https://doi.org/10.20546/ijcmas.2017.612.419
Sharma, S., 1986. Effect of fungicidal sprays on purple blotch and bulb yield of onion. Indian Phytopathol., 39: 78-82.
Thaxton, P.M. and K.M.E. Zik. 2001. Bacterial blight. In: Compendium of cotton diseases, 2nd Edition, Kirkpatrick, T.L. and C.S. Rothrock (eds.). American Phytopathological Society (APS Press) St. Paul, Minnesota, USA. pp. 34‒35.
Ulhaq, I., Z. Zaman, A. Habib, N. Javed, S.A. Khan, M. Iqbal and J. Ihsan. 2014. Assessment of yield losses caused by purple blotch disease in onion (Allium cepa L.) and its management. Pak. J. Phytopathol., 26(2): 225-232.
Wheeler, B.E.J., 1969. An introduction to plant diseases. John Willey and Sons Ltd. London, UK. pp. 301.
Yadav, R.K., A. Singh, A.S. Dhatt and S. Jain. 2017. Field assessment of onion genotypes for resistance against purple blotch complex (Alternaria porri and Stemphylium vesicarium) under artificial epiphytotic conditions in Indian Punjab. Int. J. Appl. Sci. Biotech., 5(4): 498-504. https://doi.org/10.3126/ijasbt.v5i4.18637
Yaradua, A.A., 2003. Some studies on epidemiology of purple blotch of onion (Allium cepa) in Zaria, Nigeria.
Younas, M., M. Atiq, N.A. Rajput, W. Abbas, M.R. Bashir, S. Ahmad, M.S. Ullah, W.A. Bhatti, N. Liaqat and I. Ahmad. 2021. Induction of resistance in onion against purple leaf blotch disease through chemicals. Asian J. Agric. Biol., pp. 202001039. https://doi.org/10.35495/ajab.2020.01.039
To share on other social networks, click on any share button. What are these?








