Antibacterial, Insecticidal, Antifungal and Phytochemical Screening of Alium sativum, Nigela sativa and Plantago ovata
Research Article
Antibacterial, Insecticidal, Antifungal and Phytochemical Screening of Alium sativum, Nigela sativa and Plantago ovata
Amina Rahat1, Zahin Anjum1*, Sehlina Zehra2, Fatima Umal Baneen3 and Rabia Chishti1
1College of Home Economics, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Affirmed Circle, Bowling Green, Kentucky, United States of America; 3Lahore Medical and Dental College, Harbanspura, Canal Road, Lahore, Pakistan.
Abstract | For the treatment and management of infectious pathogens, many antimicrobial and antifungal substances were previously found from synthetic and natural sources. Numerous investigations have demonstrated that the compounds coumarins, flavonoids, phenolics, alkaloids, terpenoids, tannins, essential oils, lectin, polypeptides, and polyacetylenes are present in medicinal plants. These bioactive chemicals serve as a foundation for the creation of antibiotics that are utilized to cure infectious illnesses. Allium sativum is being used traditionally since ages for many purposes. It is extensively used in numerous dishes throughout the world and due to its aromatic nature, as a fragrance and Plantago ovata is traditionally used for many therapeutic purposes; laxative anti-acidic stabliser, stomach soothing, diuretic; and it is also anticancerous. Nigela sativa is used for jaundice, paralysis, metabolic syndrome, insulin resistant syndrome, and high level of cholesterol, nerve tension problems; it stops hair fall and boost’s immune system. Based on the results of this study, it can be concluded that almost all the plant extracts showed potential antibacterial, insecticidal and antifungal activities against different. The research has focused on the potential therapeutic efficacy of AliumSativum, NigelaSativa, and Plantago Ovata in the treatment of microbial infections due to their significant antibacterial activity. Additionally, Different fractions of the A. sativum, P. ovata, N. sativa were prepared anad analyzed for antibacterial, insecticidal and antifungal activities. Based on the results of this study, it can be concluded that almost all the plant extracts showed potential antibacterial, insecticidal and antifungal activities against different microbes.
Received | February 28, 2023; Accepted | May 25, 2023; Published | June 08, 2023
*Correspondence | Zahin Anjum, College of Home Economics, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Rahat, A., Z. Anjum, S. Zehra, F.U. Baneen and R. Chishti. 2023. Antibacterial, insecticidal, antifungal and phytochemical screening of Alium sativum, Nigela sativa and Plantago ovata. Sarhad Journal of Agriculture, 39(2): 531-544.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.2.531.544
Keywords | Antifungal, Antibacterial, Nigella sativa, Metabolic syndrome, Plantago ovata
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Ever since the dawn of civilization, people have employed herbal remedies to improve their health, and many of the medications used today were first made with the help of natural resources. For the treatment and management of infectious pathogens, many antimicrobial and antifungal substances were previously found from synthetic and natural sources (Shriram et al., 2018). The availability and price of numerous currently recommended antibiotics have been further hampered by the rise of multidrug-resistant bacteria globally. It consequently lessens the efficacy of the treatment plans and raises morbidity, mortality, and medical expense rates. The development of ailments and the expansion of modern understanding of natural remedies as significant substitutes or supplemental treatments for diseases provide justification for the use of therapeutic substances derived from plants. Numerous investigations have demonstrated that the compounds coumarins, flavonoids, phenolics, alkaloids, terpenoids, tannins, essential oils, lectin, polypeptides, and polyacetylenes are present in medicinal plants. These bioactive chemicals serve as a foundation for the creation of antibiotics that are utilized to cure infectious illnesses (Kebede et al., 2021).
Allium sativumis composed of numerous fleshy cloves. It is either soft or hard neck garlic which can be grown near equator or the cooler regions (Aly et al., 2012). Alliumsativum has 500 types differing in colour, odours and taste. Allium sativum is being used traditionally since ages for many purposes. It is the main component of many dishes throughout the world and used as source of several aromatic, as a fragrance (Mohamed, 2008). Allium sativum reduces cholesterol accumulation on the vascular walls; therefore, it is useful in cardiovascular disease. It is also used against some other diseases, such as chest infections and digestive disorders (Dorant et al., 1996).
Allium sativum enhances thiamine absorption and reduces the beriberi disease. This herb is used in acquired immune deficiency syndrome (AIDS) to control toxoplasmosis (Samaranayake et al., 2000).
It can boost level of testosterone in the body. In 1985, Weishberger and Pensky reported its anticancer properties, due to the presence of allixin, which has anti-tumor property. It is effective for cancer of several types such as those of lungs, liver, prostate and breast (Powolny and Singh, 2008).
Plantago ovata is bushy herbs grow in sandy land. The plants are 60cm tall; and leaves are sessile. The flowers are tiny, borne on stalks (5-40cm) and wind pollinated. It is cultivated in cool and dry weather from October to March. Crop gets ready between 119 and 130 days (Anderson et al., 1991). Traditionally used for many therapeutic purposes; laxative anti-acidic stabliser, stomach soothing, diuretic; and it is also anticancerous. The plant is mostly used for constipation problems, help in digestive system functioning, reduce fatigue and low density lipoproteine LDL in the blood. (Mark et al., 2013). A study conducted on 60 million Americans successfully reported lowering of high level of (LDL) in the blood, while it was also used upon 26 million people of all ages in USA with type 2 diabetes in 2011. The results indicated an improvement in the glucose fasting levels after using P. ovata seeds (Liangli and Jonathan, 2002).
It is more effective under the age of 60 years and is safe even for pregnant and lactating mothers. Food and drug administration (FDA) recommends consumption of 1.7g of P. ovata seeds per serving in daily food. It is also used to control appetite because its, water absorbing and gelling capacity gives a feeling of satiety (Song et al., 2000).
Nigella sativa is a flowering annual herb. It grows 20-30 cm tall, has fine thread like leaves, pale flowers with, 5-10 blue and white petals, and large capsular fruits and having 3-7 united follicles with numerous seeds. It has many names, like black seed, fennel flower, Habbat al Barakah (blessed seed) (Warner and Ramankutty, 2004). Ibn-e-Sina in his famous book Cannon of medicine wrote about the benefits of N. sativa due to its multi healing properties (Meddah et al., 2009). This plant has 100 healing properties, it has bronchodilactory and anti spasmodic effects and also useful in asthma and whooping cough (Kanter and Demir, 2003). N. sativa is also known as “miracle cure”. It regulates female sexual hormone (Entela et al., 2012) helps in pregnancy, used in menopause problems (Salem, 2005). Useful for Helicobacter pylori infection reduces elevated blood sugar level and useful for different heart problems and allergies (Dadgar et al., 2005).
N. sativa is used for jaundice, paralysis, metabolic syndrome, insulin resistant syndrome, and high level of cholesterol, nerve tension problems; it stops hair fall and boost’s immune system. N. sativa is an expectorant and stimulates body to recover fatigue. The seed oil is used as a soothing agent for stomach (Mashhadian and Rakhshandeh, 2005).
N. sativa is useful in controlling erythrocytes deformity and shows antibacterial activities Plant seed extract is used against methicillin resistant Staphylococcus aureus (MRSA) A study conducted on rats and guinea pigs, having pancreatic cancer reported antitumor activity of, crude methanolic extract, it is, useful for gastric ulcers and decreases the pus cells in urinary tract infection (Suboh and Aburjai, 2004). In recent Scenario there is a dire need to gain knowledge prior the utilization of medicinal plants in any kind of herbal formulations, which are widely practiced worldwide, as well as in Pakistan. Therefore, the current study intended to evaluate and assess the potential medicinal properties of Allium sativum, Nigella sativa and Plantago ovata and also to compare the results with published data. Morever this study will contribute significanly in future plants based formualations in the field of food science and future prospects in the field of medicinal plants utilizations.It is imperative to investigate different plant species for their medicinal and curative prospectus in order to reach to the conclusions regarding their safety,efficacy and utilization of these herbs.As per estimates of World Health Orginization, about 80% of the World’s population residing in underdeveloped and Developing Countries depends on herbal Formulations as a medicine for their primary health care. Pakistan is among one of them and hence it have created avenues for reseachers to work on Etheno-Medicinal aspects of various herbs with the help of their antibacterial, insecticidal, antifungal and phytochemical Screening and this study is a distinctivecontribution in the field of food science and will provide knowledge about herbal attributes of Allium sativum, Nigella Sativa and Plantago ovata.
Materials and Methods
This research aimed to design the antibacterial, antifungal and insecticidal activity of Allium sativum, Nigella sativa and Plantago ovata.
Chemicals and experimental conditions
The materials required for insecticidal assay included test insect (T. castaneum).
Plant material
The herbs A. sativum, P. ovata, N. sativawere purchased from the local herbal market in Peshawar and identified by Prof. Dr. Farrukh Hussain, Department of Botany, UOP, Pakistan. The herbs were shade-dried and ground to powder using an electric grinder.
Extraction
Each herb (10kg) was soaked in methanol with occasional shaking at room temperature for 15 days. The prepared suspensions were then filtered followed by heating at 40ºC temperature in a rotary evaporator for making it concentrated by evaporating the methanolic extract (Siddiqui and Ali, 1997).
Fractionation of Allium sativum
The crude MeOH extract of A. sativum (860g) was suspended in 500ml distilled water which was then partitioned with 1500mL CHCl3, n-hexane and EtOAc to their respective fractions as follows: aqueous @ 210g, CHCl3 @ 160g, n-hexane @ 170g and EtOAc @ 150g fractions (Oloyede and Adebooye, 2005). About 90g of the crude. MeOH extract Ext. were stored for biological/ pharmacological investigations.
Fractionation of Nigella sativa
The Cr. MeOH Ext of N. sativa (725g) was suspended in 500 mL distilled water which was then partitioned with 1500mL of CHCl3, n-hexane and EtOAc to their respective fractions as follows: aqueous @ 190 g, CHCl3 @ 135g, n-hexane @ 140g and EtOAc @ 130g fractions. About 90g of the Cr. MeOH Ext. were stored for biological/pharmacological investigations (Ebi and Ofoefula, 1997) (Figure 1).
Fractionation of Plantago ovata
The Cr. MeOH Ext. ofP. ovata (550g) was suspended in 500 ml distilled water which was then partitioned with 1500 mL CHCl3, n-hexane and EtOAc to their respective fractions as follows: CHCl3 @ 150g, n-hexane @ 160g and EtOAc @ 140g fractions. About 90g of the crude Cr. MeOHextractwasstored for biological investigations (Flow Chart 1).
Phytochemical investigations
Tannin: 500mg of sample was suspended in a 250mL plastic bottle, and 50 mL of distilled water was addedto increase up to 100 mL. The filtrate (5mL) was pipetted out and mixed in a test tube containing 3 mL of 0.1M FeCl3, 0.1N HCl and 0.008 M potassium ferrocyanide. The absorbance of the sample was measured at a wavelength of 120 nm using a spectrophotometer within 10 minutes time interval. The change in absorbance of the sample was compared with prepared standard; using tannic acid at 100 ppm to calculate of the quantity of tannin in the sample (Obadoni and Ohuko, 2001).
Alkaloid: Five grams of sample was added to 200mL of 20% acetic acid in ethanol in a 250mL beaker and kept for 4 hr. It was then filtered and concentrated using a water bath to a quarter of the original solution. Ammonium hydroxide was added dropwise, until a precipitate was formed, settled, filtered and weighed to calculate the amount of alkaloid (Boham and Kocipai, 1994).
Flavonoid: Ten grams of the sample were suspended in 100mL of 80% aqueous MeOH at room temperature. The suspension was extracted several times, and the solution was filtered through Whatman filter paper no. 42 (125 mm). The filtrate was transferred to a crucible for drying with the help of a water bath and weighed to calculate the amount of total flavonoid content present in the sample (Barakat et al., 1973).
Ascorbic acid (Vitamin C): Five grams of the sample were taken in an extraction tube and 100mL of ethylene diamine tetraacetic acid (EDTA)/ Trichloroacetic Acid (TCA) (2:1) was added to it. It was shaken for 30 min, centrifuged at 3000 rpm for 20 min and transferred to a 100 mL volumetric flask 20 mL of the extract was pipetted out in to a flask, mixed with 1% starch indicator, and titrated against 20% CuSO4 until the dark colour was obtained (Kim et al., 2000).
Biological evaluation
Phytotoxic activity : The screening of the phytotoxicity of the plant extracts was according to a reported protocol (Bashir et al., 2011). Healthy 16 L. minor plants with three fronds rosette were placed in each flask. These flasks were then incubated at 27 ºC for 7 days in growthchamber. The results were obtained by counting damaged number plants after seven days of incubation.
The results were obtained by counting the damaged number of plants after seven days of incubation.
Antibacterial activity
The plant crude methanolic extract and various fractions were screened for pathogens according to the method reported in the literature (Bashir et al., 2011). The nutrientbroth was first prepared and then autoclaved. The broth was then poured into sterile test tubes and then stored for one day in an incubator at 37 ºC. The broth medium was analyzed for any occurrenceof contaminations. Aseptically the nutrient broth was inoculatedwith the tested culture by spreading equally the sample on an agar medium in a laminar airflow cabinet. Wells of 6mm were made on the nutrient agar with a sterile borer. The zones of inhibitions were recorded after 24 hr of incubation at 37 oC.
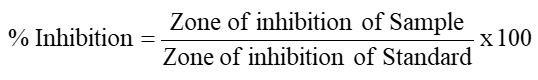
Antifungal activity
The antifungal activity was performed with stock solutions (24mg/mL) of Cr. Met.Ext and fractions in DMSO. Aliquots of 4 mL of SDA were taken in test tubes and autoclaved. After sterilization as the media temperature reach around 50ºC from the stock solution 66.6 μL of solution was transferred in each tube. The solution was left to cool down and then each fungal species that was culturedfor 7 days was inoculated in these test tubes.
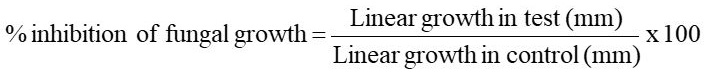
Insecticidal activity
The pests were reared with sterile plastic bottles with uniform age and size of insects .
The contact toxicity assay was adapted for insecticidal bioassay. On the first day, the filter paper was placed in Petri dishes of 9 cm. The crude methanolicextract and fraction from the samplesolution were loaded in petridishes separately with help of a sterile micropipette. The sample plates were left overnight for the organic solvents to evaporate. This was followed by 10 healthy insects transferred to the plate with a clean brush. The percent mortality of insects treated with the test samples wascalculated according to the formula:
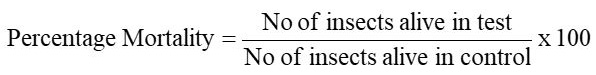
A standardinsecticidal drug (Permethrin) was used as positive control.
Statistical analysis
All data obtained from different activities were analyzed using SPSSstatistical package version 16. Two-way and, one-way analysis of variance (ANOVA), chi-square, and least significance difference test (LSD) was used for analysis.
Results and Discussion
Phytotoxic activity of A. sativum
The phytotoxicity of the test samples was determined against L. minor. The results of phytotoxic activity of Crd. MeOH Ext. and various fractions of A. sativum against L. minor are summarised in Table 1 and presented in Figure 1. The Crd. MeOH Ext. showed (25, 15 and 5%) growth regulation at 1000, 100 and 10µg/mL respectively, while no phototoxic activity was observed in n-hexane, CHCl3, EtOAc and aqueous fractions.
Phytotoxic activityof N. sativa
Herbicides of plant origin are environment friendly. Lemna minor is sensitive to bioactive compounds containing aquatic monocot small plants. The phytotoxicity assay results of crude. MeOH extract and various fractions of N. sativa against- L. minor is summarised in Table 2 and presented in Figure 2. The crude MeOHextract (75, 50 and 40%), CHCl3(10 and 5 %) and aqueous (60, 50 and 30%) extract showed growth regulation at 1000, 100 and 10µg/mL, respectively, while no activity was observed in n-hexane and EtOAc fractions.
Table 1: Phytotoxic activity of Cr. Meth. Ext. and various fractions of A. sativum.
|
Name of Plant |
Conc. of sample (μg/mL) |
No. of fronds killed |
Conc. of Stnd. drug * (μg/mL) |
|||||
|
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
Control |
|||
|
Lemna minor |
1000 |
5 |
0 |
0 |
0 |
0 |
20 |
0.015 |
|
100 |
3 |
0 |
0 |
0 |
0 |
20 |
||
|
10 |
1 |
0 |
0 |
0 |
0 |
20 |
||
|
Percent growth regulation |
Standard |
|||||||
|
1000 |
25 |
0 |
0 |
0 |
0 |
100 |
||
|
100 |
15 |
0 |
0 |
0 |
0 |
100 |
||
|
10 |
5 |
0 |
0 |
0 |
0 |
100 |
||
*Paraquat was used as standard drug.
Table 2: Phytotoxic activityofCr. Meth. Ext. and various fractions of N. sativa.
|
Name of plant |
Concentration of sample (μg/mL) |
No. of fronds killed |
Concentration of standard drug * (μg/mL) |
|||||
|
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
Control |
|||
|
Lemna minor |
1000 |
15 |
0 |
2 |
0 |
12 |
20 |
0.015 |
|
100 |
10 |
0 |
1 |
0 |
10 |
20 |
||
|
10 |
8 |
0 |
0 |
0 |
6 |
20 |
||
|
Percent growth regulation |
Standard |
|||||||
|
1000 |
75 |
0 |
10 |
0 |
60 |
100 |
||
|
100 |
50 |
0 |
5 |
0 |
50 |
100 |
||
|
10 |
40 |
0 |
0 |
0 |
30 |
100 |
||
Table 3: Phytotoxic activity of Cr. Meth. Ext. and various fractions of P. ovata.
|
Name of plant |
Conc. of sample (μg/mL) |
No. of fronds killed |
Conc. of Stand drug * (μg/mL) |
|||||
|
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
Control |
|||
|
Lemna minor |
1000 |
4 |
2 |
0 |
0 |
0 |
20 |
0.015 |
|
100 |
2 |
1 |
0 |
0 |
0 |
20 |
||
|
10 |
1 |
0 |
0 |
0 |
0 |
20 |
||
|
Percent growth regulation |
Standard |
|||||||
|
1000 |
20 |
10 |
0 |
0 |
0 |
100 |
||
|
100 |
10 |
5 |
0 |
0 |
0 |
100 |
||
|
10 |
5 |
0 |
0 |
0 |
0 |
100 |
||
*Paraquat was used as standard drug.
Phytotoxic activity of P. ovata
The results of phytotoxic activity of crude methanolic extract and various fractions of P. ovata against L. minor are summarised in Table 3 and presented in Figure 3. The crude methanolic extract (20, 10 and 5%) and n-hexane (10, 5 and 0%) showed growth regulation at 1000, 100 and 10µg/mL, respectively while no phototoxic activity was observed in CHCl3, EtOAc and aqueous fractions (Patel et al., 2015).
Anti-bacterial activity
Anti-bacterial activity of A. sativum: The Crd. MeOHExt.and various fractions of A. sativum were screened against test pathogens for antibacterial activity. The results are summarised in Table 4 and presented in Figure 4.
The crude MeOH extract. exhibited significant antibacterial activity against P. mirabilis (89%) low activity against E. coli (25%), B. cereus (14%) and X. oryzae (18%), n-hexane fraction exhibited significant antibacterial activity against- B. cereus (86%) and E. coli (89%), moderate activity against X. oryzae (50%) and low activity against P. mirabilis (12%). TheCHCl3 fraction showed significant antibacterial activity against X. oryzae (92%) and P. mirabilis (89%) and low activity against E. coli (12%) and B. cereus (8%). Similar findimgs were reported by Safithri et al. (2012). TheEtOAc fraction showed low activity against E. coli (7%), P. mirabilis (5%), B. cereus (7%) and X. oryzae (8%). The aqueous fractions showed significant activity against E. coli (93%) low activity against P. mirabilis (23%), B. cereus (29%) and X. oryzae (35%). Microbial cultures of X. oryzae, P. mirabilis, E. coli and B. cereus were obtained from the Centre of Biotechnology and Microbiology, University of Peshawar (COBAM, UOP).
Fungal cultures including Verticilliumlecanii, P. oxalicum, Acremoniumstrictum and Trichodermaharzianum species were obtained from the COBAM, UOP. These fungal were tested for antifungal activities of the herb extracts.
The Two-way ANOVA test showed (P > 0.05) insignificant difference for different bacterial pathogens but a significant difference for (P <0.05) different fractions (Supplementary Table 1).
Anti-bacterial activity of N. sativa
The Crd. MeOH Ext. and various fractions of N. sativa were screened against the-test pathogens for antibacterial activity. The results aresummarised in Table 5 and presented in Figure 5. The Crd. MeOH Ext. showed significant activity against E. coli (89%), P. mirabilis (96%), B. cereus (89%) and X. oryzae (88%). The n-hexane fraction exhibited significant antibacterial activity against X. oryzae (92%), B. cereus (82%), P. mirabilis (85%) and low activity against E. coli (7%). CHCl3 fractions showed significant activity against X. oryzae (96%), E. coli (93%) and P. mirabilis (89%) and low activity against B. cereus (17%). The EtOAc fractions showed moderate activity against X. oryzae (50%), B. cereus (93%), E. coli (89%) and low activity against P. mirabilis (12%). The aqueous fractionshowed significant activity against B. cereus (93%), X. oryzae (92%), E. coli (86%) and P. mirabilis (85%). The Two-way ANOVA test showed (P>0.05) insignificant difference for different bacterial pathogens but significant difference for (P <0.05) different fractions (Supplementary Table 2). LSD results showed significant difference between different fractions compared with the negative control.
The Crd. MeOH Ext. and various fractions of P. ovata were screened against test pathogens for antibacterial activity similar findings about N. sativa was affirmed earlier by (Ali and Blunden, 2003). The two-way ANOVA test calculation showed highly significant difference between the fractions (P= 0.000) and insignificant difference between bacterial test samples (P = 0.091) (Supplementary Table 3).
Table 4: Cr. Antibacterial activities of Meth. Ext. and various fractions of A. sativum.
|
Name of Bacteria |
Zone of inhibition of standard (amoxi-cillin) 10μg |
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
|||||
|
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhib-ition (%) |
||
|
E. coli |
29 |
7.3 |
25 |
26 |
89 |
3.5 |
12 |
2.3 |
7 |
27 |
93 |
|
P. mirabilis |
28 |
25 |
89 |
3.5 |
12 |
26 |
89 |
1.5 |
5 |
6.5 |
23 |
|
B. cereus |
29 |
4.3 |
14 |
25 |
86 |
2.5 |
8 |
2.3 |
7 |
8.5 |
29 |
|
X. oryzae |
27 |
5 |
18 |
13.5 |
50 |
25 |
92 |
2.3 |
8 |
9.5 |
35 |
Table 5: Antibacterial activities of Cr. Meth. Ext. and various fractions of N. sativa.
|
Name of Bacteria |
Zone of inhibition of standard (amoxi-cillin) 10μg |
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
|||||
|
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inh-ibition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
||
|
E. coli |
29 |
26 |
89 |
2.3 |
7 |
27 |
93 |
25 |
89 |
25 |
86 |
|
P. mirabilis |
28 |
27 |
96 |
24 |
85 |
25 |
89 |
3.5 |
12 |
24 |
85 |
|
B. cereus |
29 |
26 |
89 |
24 |
82 |
5 |
17 |
27 |
93 |
27 |
93 |
|
X. oryzae |
27 |
24 |
88 |
25 |
92 |
26 |
96 |
13.5 |
50 |
25 |
92 |
Table 6: Antibacterial activities of Cr. Meth. Ext. and various fractions of P. ovata.
|
Name of Bacteria |
Zone of inhibition of standard (amoxi-cillin) 10μg |
Crd. MeOH Ext. |
n-hexane |
CHCl3 |
EtOAc |
Aqueous |
|||||
|
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
Zone of Inhi-bition (mm) |
Inhi-bition (%) |
||
|
E. coli |
29 |
13.3 |
45 |
26 |
89 |
0 |
0 |
0 |
0 |
0 |
0 |
|
P. mirabilis |
28 |
26 |
92 |
25 |
89 |
0 |
0 |
0 |
0 |
0 |
0 |
|
B. cereus |
29 |
25 |
86 |
25 |
86 |
0 |
0 |
0 |
0 |
0 |
0 |
|
X. oryzae |
27 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Antifungal activity
Antifungal activity of A. sativum: The Crd. MeOH Ext. and various fractions of A. sativum were screened against test pathogens for antifungal activity andresults are summarised in Table 7 and presented in Figure 7. The Crd. MeOH Ext. showed good antifungal activity against P. oxalicum (62%), A. strictum (61%) and moderate activity against V. lecanii (53%) and T. harzianum (48%). The n-hexane fraction showed moderate activity against P. oxalicum (58%), A. strictum (56%) and V. lecanii (43%) and low activity against T. harzianum (36%). The CHCl3 fraction showed significant activity against V. lecanii (87%), good activity against P. oxalicum (69%) and A. strictum (64%) and moderate activity against T. harzianum (42%). The EtOAc showed good activity against A. strictum (69%) and P. oxalicum (65%) and moderate activity against V. lecanii (50%) and low activity against- T. harzianum (30%). The aqueous fraction showed good activity against A. strictum (64%), moderate activity against V. lecanii (47%) and low activity against T. harzianum (18%) and P. oxalicum (15%).
Table 7: Antifungal activity of Cr. Meth. Ext. and various fractions of A. sativum.
|
Fractions |
V.lecanii |
P. oxalicum |
A. strictum |
T. harizinum |
|
Crude extract |
53 |
62 |
61 |
48 |
|
n-hexane |
43 |
58 |
56 |
36 |
|
CHCl3 |
87 |
69 |
64 |
42 |
|
EtOAc |
50 |
65 |
69 |
30 |
|
Aqueous |
47 |
15 |
64 |
18 |
|
Control (-) |
0 |
0 |
0 |
0 |
|
Control (+) |
100 |
100 |
100 |
100 |
Two-way ANOVA test showed highly significant difference between the fractions (P= 0.000) and between fungal strains (P= 0.03) (Supplementary Table 4). The LSD between A. sativum, T. harzianum and V. lecanii showed highly significance difference (P = 0.026). The LSD comparison between the fractions of A. sativum showed highly significance difference due to control.
Antifungal activity of P. ovata
The Crd. MeOH Ext. and various fractions of P. ovata were screened against test pathogens for antifungal activity and results aresummarised in Table 8 and presented in Figure 8.
Table 8: Antifungal activity of Cr. Meth. Ext. and various fractions of P. ovata.
|
Fractions |
V. lecanii |
P. oxalicum |
A. strictum |
T. harizinum |
|
Crude extract |
43 |
19 |
86 |
55 |
|
n-hexane |
50 |
46 |
69 |
58 |
|
CHCl3 |
0 |
0 |
0 |
0 |
|
EtOAc |
0 |
0 |
0 |
0 |
|
Aqueous |
0 |
0 |
0 |
0 |
|
Control (-) |
0 |
0 |
0 |
0 |
|
Control (+) |
100 |
100 |
100 |
100 |
The Crd. MeOH Ext. showed significant activity against A. strictum (86%), moderate activity against T. harzianum (55%) and V. lecanii (43%) and low activity against P. oxalicum (19%). The n-hexanefractions showed good activity against A. strictum (69%), moderate activity against T. harzianum (58%), V. lecanii (50%) and P. oxalicum (46%). The Two-way ANOVA test showed insignificant difference between the different strains of fungi (P = 0.179) and highly significant difference showed for fractions (P = 0.000) (Supplementary Table 5). The LSD showed significant difference between the different fractions compare with control.
Antifungal activities of N. sativa
The Crd. MeOH Ext. and various fractions of N. sativa were screenedagainst- different strains of fungi for antifungal activity and results are summarised in Table 9 and presented in Figure 9. The Crd. MeOH Ext. showed significant activity against P. oxalicum (92%), good against A. strictum (64%), moderate activity against V. lecanii (56%) and T. harzianum (42%). The n-hexanefractions showed good activity against A. strictum (69%), T. harzianum (61%), and moderate activity against- P. oxalicum (54%) and V. lecanii (53%). The CHCl3 fraction showed significant activity against P. Oxalicum (100%), T. harzianum (100%), V. lecanii (100%) and A. strictum (100%). The EtOAc fraction showed significant activity against A. strictum (80%), good activity against- V. lecanii (67%), T. harzianum (61%) and low activity against P. oxalicum (26%). The aqueous fraction showed significant activity against A. strictum (86%), good activity against- V. lecanii (60%), moderate activity against T. harzianum (58%) and low activity against P. oxalicum (19%).
Table 9: Antifungal activity of Cr. Meth. Ext. and various fractions of N. sativa.
|
Fractions |
V. lecanii |
P. oxalicum |
A. strictum |
T. harizinum |
|
Crude extract |
56 |
92 |
64 |
42 |
|
n-hexane |
53 |
54 |
69 |
61 |
|
CHCl3 |
100 |
100 |
100 |
100 |
|
EtOAc |
67 |
26 |
80 |
61 |
|
Aqueous |
60 |
19 |
86 |
58 |
|
Control (-) |
0 |
0 |
0 |
0 |
|
Control (+) |
100 |
100 |
100 |
100 |
The two-way ANOVA test showed null hypothesis is rejected for different fractions of N. sativa (P = 0.000) but accepted for different test pathogens of fungi (P = 0.349) (Supplementary Table 6). The LSD (Comparison between the fractions) showed highly significant difference between fractions compare with control (P = 0.000).
Insecticidal activity of A. sativum
The Crd. MeOH Ext. and various fractions of A. sativum were screened for insecticidal activity against T. castaneum. The results are summarised in Table 10 and presented in Figure 10. The Crd. MeOH Ext. showed significant insecticidal activity (90%), while low activity was found in the CHCl3 (40%), EtOAc and aqueous (20%) fractions, the similar results were generated by Waller (1987). The n-hexane fraction showed no activity against T. castaneum. Pearson’s chi-Square test provided significant association between row and column variables; Value = 27.989 and P = 0 .000 (Supplementary Table 6).
Table 10: Insecticidal activity (% Mortality) of Cr. Meth. Ext. and various fractions of the selected plants.
|
Fractions |
A. sativum |
N. sativa |
P. ovate |
|
Crude extract |
90 |
20 |
20 |
|
n-hexane |
0 |
0 |
0 |
|
CHCl3 |
40 |
70 |
0 |
|
EtOAc |
20 |
0 |
0 |
|
Aqueous |
20 |
30 |
0 |
|
Control (-) |
0 |
0 |
0 |
|
Control (+) |
100 |
100 |
100 |
Insecticidal activity of N. sativa
The Crd. MeOH Ext. and various fractions of N. sativa were screened for insecticidal activity against T. castaneum. The results are summarised in Table 10 and presented in Figure 11.
The CHCl3 showed good insecticidal activity (70%), while low activity was found in Crd. MeOH Ext. (20%) and aqueous (30%) fraction. Then-hexane and EtOAc fractions showed no activity against T. castaneum. Pearson’s chi-Square test provided significant association between row and column variables; Value is 23.750 and P = 0.000 (Supplementary Table 7).
Insecticidal activity of P. ovata
The Crd. MeOH Ext. and various fractions of P. ovata were screened for insecticidal activity againstT. castaneum. The results are summarised in Table 10 and presented in Figure 12.
The Crd. MeOH Ext. showed low activity (20%) while n-hexane, CHCl3, EtOAc and aqueous fractions showed no activity against T. castaneum. Pearson’s chi-square test provided insignificant association between row and column variables; Value = 4.286 and P = 0.117 (Supplementary Table 8). P. Ovata have important insecticidal properties due to the presence of varios compounds such as flavonoids, alkaloids, terpenoids, phenolic compounds (caffeic acid derivatives), antioxidants, vitamin C, and anti-inflammatory agents (Saghir et al., 2008).
Conclusions and Recommendations
Based on the results of this study, it can be concluded that almost all the plant extracts showed significant activities against different bacterial, fungal and insect species. In conclusion, the research has contributed to the potential therapeutic efficacy of Alium sativum, Nigela sativa, and Plantago ovata in the treatment of microbial infections due to their significant antibacterial activitives. Additionally, the findings showed that Alium sativum, Nigela sativa, and Plantago ovata have very powerful antifungal and insecticidal activities, supporting their promising usage as possible antifungal and bio-insecticides in agricultural production and preservation of leguminous plants.
It is imperative to investigate different plant species for their medicinal and curative prospectus in order to reach to the conclusions regarding their safety, efficacy and utilization of thsese herbs. As per estimates of World Health Orginization, about 80% of world’s population residing in underdeveloped and developing countries depends on herbal formulations as a medicine for their primary health care. Pakistan is among one of them and hence it have created avenues for reseachers to work on Etheno-Medicinal aspects of various herbs with the help of their antibacterial, insecticidal, antifungal and phytochemical screening.
Acknowledgement
Authors are highly obliged to Nuclear Institute for Foods and Agriculture (NIFA), Peshawar and (COBAM) Centre of Biotechnology and Molecular Biology, University of Peshawar, for their help in phytochemical and Gamma irradiation of research work. We are also thankful to Department of Agricultural Chemistry, University of Agriculture, Peshawar for performing different Pharmacological investigations.
Novelty Statement
This research study intended to evaluate and assess the potential medicinal properties of Allium sativum, Nigella sativa and Plantago ovata in the field of medicinal plants utilizations.
Author’s Contribution
Amina Rahat: Data collection, lab work and analysis.
Zahin Anjum: Writing of the manuscript and analysis.
Sehlina Zehra: Helped in manuscript literature.
Fatima Umal Baneen: Helped in samples treatments.
Rabia Chishti: Helped in statistical analysis and tabulation.
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.sja/2023/39.2.531.544
Conflict of interest
The authors have declared no conflict of interest.
References
Abdul, H., S. Sidrah and U. Muhammad. 2008. Antibacterial activity of Nigella sativa against clinical isolation of methicillin resistant Staphlococcus aureus. J. Ayub Med. Coll. Abbottabad, 20(3): 334-350.
Akram, M., 1999. Chemical composition and medicinal properties of Nigella. sativa L. J. Inflammopharmacol. Sheffield U.K., 7: 15-35. https://doi.org/10.1007/s10787-999-0023-y
Ali, B.H. and G. Blunden. 2003. Pharmacological and toxicological properties of Nigella sativa. Phytotherapy Res., 17(4): 299–305. https://doi.org/10.1002/ptr.1309
Aly, A., E. Ishak, M. Alsharari, N. Al-Muaikel and T. Bedair. 2012. Aminonaphthoquinones in Heterocyclization. J Heter. Chemis., 49(01): 9-20. https://doi.org/10.1002/jhet.639
Aly, S.M. and M.F. Mohamed. 2010. Echinacea purpurea and Allium sativum as immunostimulants in fish culture using Nile tilapia (Oreochromisniloticus). J. Anim. Physiol. Anim. Nutr., 94: 31-39. https://doi.org/10.1111/j.1439-0396.2009.00971.x
Aly, S.M., Atti, N.M.A. and M.F. Mohamed. 2008. Effect of garlic on survival, growth, resistance and quality of Oreochromisniloticus. Int. Symp. Tilapia Aquac., pp. 277-296.
Anderson, J., L.D. Allgood, A. Lawrence, L.A. Altringer, G.R. Jerdack, D.A. Hengehold and J.G. Morel. 2000. Cholesterol-lowering effects of Psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: Meta-analysis of 8 controlled trials. Am. J. Clin. Nutr. 71: 472-479. https://doi.org/10.1093/ajcn/71.2.472
Anderson, J., T. Floore, P. Bazel, D. O’neil and T. Balm. 1991. Hypocholesterolemic effects of different bulk forming hydrophilic fibers as adjuncts to dietary therapy in mild to moderate hypercholesterolemia. Arch. Intern. Med., 151: 1597-1602. https://doi.org/10.1001/archinte.151.8.1597
Barakat, M.Z., S.K. Shehab, N. Danish and E. Zahermy. 1973. Determination of ascorbic acid from plants. Analyst. Biochem. J., 53: 225-245.
Bashir, A., A. Sadiq, S. Bashir, N. Ali and I. Khan. 2011. Phytotoxicity, antibacterial and haemagglutination activities of aerial parts of Mysineafricana. Afr. J. Biotech., 10: 97-102. https://doi.org/10.5897/AJB11.115
Bashir, A., A. Sadiq, S. Bashir, H. Farrukh and M.C. Iqbal. 2011. Insecticidal, brine shrimps cytotoxicity, antifungal activities of aerial parts of Myrsine. Afr. J. Biotech., 10: 1448-1453. https://doi.org/10.5897/AJB11.115
Bell, L.P., K. Hectorne, H. Reynolds, T.K. Balm and D.B. Hunninghake. 1989. Cholesterol-lowering effects of psyllium hydrophilic mucilloid, adjunct therapy to prudent diet for patients with mild to moderate hypercholesterolemia. J. Am. Med. Assoc., 261: 3419-3423. https://doi.org/10.1001/jama.261.23.3419
Belman, S., 1983. Onion and garlic oils inhibit tumor promotion. J. Carcinog., 4: 1063-1065. https://doi.org/10.1093/carcin/4.8.1063
Benhaddou-Andaloussi, A., A. Elimadi, A. Settaf, Y. Cherrah and P.S. Haddad. 2004. The petroleum ether extract of Nigella sativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J. Ethnopharmacol., 94: 251-259. https://doi.org/10.1016/j.jep.2004.04.030
Benhddou, A.L.E., A. Elimadi, A. Settaf, Y. Cherrah and P. Haddad. 2004. The petroleum ether etract of Nigellasativa exerts lipid-lowering and insulin-sensitizing actions in the rat. J. Ethnopharmacol., 94: 251-259. https://doi.org/10.1016/j.jep.2004.04.030
Block, E., S. Ahmad and E.Z. Ajoene. 1984. A potent antithrombotic agent from garlic. J. Am. Chem. Soc., 106: 8295-8296. https://doi.org/10.1021/ja00338a049
Boham, A.B. and A.C. Kocipai. 1994. Flavonoid and condensed lannins from Leaves of Hawailian vaccinium vaticulumm and vicalyciinum. Pac. Sci. J., 48: 458-463.
Childs, N.M., 1999. Marketing functional foods: what have we learned? An examination of the Metamucil, benefit, and heartwise introductions as cholesterol reducing ready to eat cereals. J. Med. Food, 2: 11-19. https://doi.org/10.1089/jmf.1999.2.11
Dadgar, T., M. Asmar, A. Saifi, M. Mazandarassi, H. Bayat, A. Moradi and M. Bazueri. 2006. Antibacterial activity of certain iranian medicinal plants again at methicillin-resistant and sensitive Staphylococcus aureus. Asian J. Plant Sci., 5: 861-6-25. https://doi.org/10.3923/ajps.2006.861.866
Dorant, E., van-den., P.A. Brandt and R.A. Goldbohm. 1996. Allium sativumvegetable consumption, garlic supplement intake, and female breast carcinoma incidence. Breast Cancer Res. Treat., 33: 163-170. https://doi.org/10.1007/BF00682723
Ebi, G.C. and S.I. Ofoefule. 1997. Investigating into folkloric antimicrobial activities of landolphia owerrience. Phytother. Res., 11: 149-151.
Edward, H., 2012. Stomach soothing use of habbat al-barakha. TWN brifing paper nogoya protocol new Delhi. Int. Code Nomenclat. Prokaryotes, 2: 2-6.
Entela, H., M. Stefano, T. Vilma, V. Silvia, Z. Paola, T. Irma and K. Henri. 2012. Antibacterial and antifungal activity assessment of Nigella sativa essential oils. World Acad. Sci. Eng. Technol., 66: 1198-1200
Gupta, A.D., S.N. Das, S.A. Dhundasi and K.K. Das. 2009. Effect of garlic (Allium sativum) on heavy metal induced alteration of serum lipid profile in male albino rats. Int. J. Environ. Res. Publ. Health, 10: 201-211.
Hsing, A.W., A.P. Chokkalingam, Y.T. Gao, M.P. Madigan, J. Deng, G. Griley and J.F. Fraumeni. 2002. Allium sativum vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer, 94: 1648-1651. https://doi.org/10.1093/jnci/94.21.1648
Kaleem, M., D. Kirmani, M. Asif, Q. Ahmed and B. Bano. 2006. Biochemical effects of Nigella sativa L seeds in diabetic rats. Indian J. Exp. Biol., 44: 745-748.
Kanter, M., I. Meral, Z. Yener, H. Ozbek and H. Demir. 2003. Partial regeneration/ proliferation of the beta-cells in the islets of Langerhans by Nigelia sativa L. in streptozotocin-induced diabetic rats. Tohoku J. Exp. Med., 201(4): 213-219. https://doi.org/10.1620/tjem.201.213
Kebede, T., E. Gadisa, A. Tufa. 2021. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS One, 16(3): e0249253. https://doi.org/10.1371/journal.pone.0249253
Kim, M.J., H.S. Yook and M.W. Byun. 2000. Effects of Gamma irradiation on microbial contamination and extraction yields of Korean medicinal herbs. Radiat. J. Phys. Chem., 57: 55–58. https://doi.org/10.1016/S0969-806X(99)00298-4
Liangli, Y. and Jonathan, P., 2002. Effects of solid-state enzyme treatments on the water absorbing and gelling properties of psyllium, Department of Food Sciene and Human Nutrition, Colorado State University, USA, 80: 139-157.
Mark, N., A. Feinglos, D. Roger, G. Gibb, S. Rihard, B. Surwi and W.M. Johnson. 2013. Bioactive carbohydrates and dietary fiber. Science Direct Psyllium Improves glycemic control in patients with type-2 diabetes mellitus. J. Nutr., 40: 156-161. https://doi.org/10.1016/j.bcdf.2013.02.003
Mashhadian, N.V., and H. Rakhshandeh. 2005. Antibacterial and antifungal effects of Nigella sativa extracts against S. aureus. P. aeruginosa and C. albicans. Pak. J. Med. Sci., 12: 21-78.
Meddah, B., R. Ducroc, L. Mahraoui, A. Benhaddou, L.C. Martineau, Y. Cherrah and P.S. Haddad. 2009. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol., 21(3): 419-424. https://doi.org/10.1016/j.jep.2008.10.040
Mohammad, A.R., 2008. Black seed Nigella sativa deserves more attention. J. Ayub Med. Coll. Abbottabad, 20: 455-460.
Najmi, A., and R.A. Khan. 2008. Effect of Nigella sativa oil on various clinical and biochemical parameters of metabolic syndrome. https://doi.org/10.4103/0973-3930.41980
Nancy, D.Y., 2011. Screening of selected Asian Species for anti obesity related bioactivities. J. Food Chem., 126: 1724-1729. https://doi.org/10.1016/j.foodchem.2010.12.066
Obadoni, B.O. and P.O. Ohuko. 2001. Phytochemical studies and comparative efficacy of the crude extracts of some homeostatic plants in Edo and Delta States of Nigoria. Glob. J. Pure appl. Sci., 8: 203-208. https://doi.org/10.4314/gjpas.v8i2.16033
Okwu, D.E., and C. Josiah. 2006. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr. J. Biotechnol., 5: 257-361.
Oloyede, F.M. and O.C. Adebooye. 2005. Effect of season on growth, fruit yield and nutrient profile of two landraces of Trichosanthes cucumerina L. African J. Biotechnol., 4: 1040-1044.
Patel, M.J., K. Alobaidy, M.S. Farouk and S. Lokesh. 2015. Formulation design, in-vitro and biological correlation of site specific drug delivery system for distal gastro-intestinal tract. Int. J. Pharm. Sci. Res., 6(12): 5126-5133.https://doi.org/10.13040/IJPSR.0975-8232
Powolny, A.A., and S.V. Singh. 2008. Multitargeted prevention and therapy of cancer by diallyltrisulfide and related Allium sativum vegetable-derived organosulfur compounds. J. Cancer, 269: 305-314. https://doi.org/10.1016/j.canlet.2008.05.027
Saghir, S., M. Iqbal, M. Hussain, A. Koschella and T. Heinze. 2008. Structure characterization and carboxymethylation of arabinoxylan isolated from Ispaghula (Plantago ovata) seed husk. Carbohydrate Polymers. 74: 309–317. https://doi.org/10.1016/j.carbpol.2008.02.019.
Safithri, M., M. Bintang and M. Poeloengan. 2012. Antibacterial activity of garlic extract against some pathogenic animal bacteria. Media Peternakan. 34(3): 155-158. https://doi.org/10.5398/medpet.2011.34.3.155
Salem, M.L., 2005. Anti bacterial activity of Nigella sativa against clinical isolates of mtethicillin resistant Staphylococcus aureus: Immunomodulatory and therapeutic properties of Nigella sativa seed. J. Immunopharmacol., 5: 49-70.
Samaranayake, M.D., S.M. Wickramasinghe, P. Angunawela, S. Jayasekera, S. Lwai and S. Fukushima. 2000. Inhibition of chemically induced liver carcinogenesis in Wister rats by garlic (A. sativum). J. Phytother. Res., 14: 564-567. https://doi.org/10.1002/1099-1573(200011)14:7<564::AID-PTR664>3.0.CO;2-Z
Siddiqui, A.A. and M. Ali. 1997. Practical pharmaceutical chemistry; 1st Ed.; CBS Publishers and Distributors, New Delhi, pp: 126-131.
Shriram, V., T. Khare, R. Bhagwat, R. Shukla and V. Kumar. 2018. Inhibiting bacterial drug efflux pumps via phyto-therapeutics to combat threatening antimicrobial resistance. Front. Microbiol., 9: 2990. https://doi.org/10.3389/fmicb.2018.02990
Solá, R., E. Bruckert, R.M. Valls, S. Narejos, X. Luque and M. Castro-cabezas. 2010. Soluble fibre (Plantago ovata husk) reduces plasma low-density lipoprotein (LDL) cholesterol, triglyceride, insulin, oxidised LDL and systolic blood pressure in hypercholesterolaemic patients: A randomised trial. Atherosclerosis, 211: 630-637. https://doi.org/10.1016/j.atherosclerosis.2010.03.010
Song, Y.J., M. Sawamura, K. Ikeda, S. Igawa and Y. Yamori. 2000. Soluble dietary fiber improves insulin sensitivity by increasing muscle GLUT-4 content in smoke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol., 27: 41-45. https://doi.org/10.1046/j.1440-1681.2000.03198.x
Suboh, Y.Y., T.A. Bilto and Aburjai. 2004. Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother. Res. Phytother., 18: 280-284. https://doi.org/10.1002/ptr.1380
Van-Burden, T.P. and W.C. Robinson. 1981. Formation of complexes between protein and tannin acid. J. Agric. Food Chem., 1: 77-82.
Waller, G.R. 1987. Allelochemicals: Role in agriculture and forestry. ACS Symposium Series No. 330. American Chemical Society, Washington, DC: USA. pp. 606.
Warner, P.K., V.P.K. Nambiar and Ramankutty. 2004. Indian medicinal plants: A compendium of 500 species: Orient Longman Pvt. Ltd, Chennai, 4: 139-142.
To share on other social networks, click on any share button. What are these?







