Antibacterial Effect of Zinc Oxide and Copper Oxide Nanoparticles as Substitute of Antibiotics against Fowl Typhoid in Broilers
Antibacterial Effect of Zinc Oxide and Copper Oxide Nanoparticles as Substitute of Antibiotics against Fowl Typhoid in Broilers
Muhammad Atif Raza1, Muhammad Tariq Javed2, Muhammad Fiaz1*,
Muhammad Shakeel3, Muhammad Shahbaz Ul Haq2, Amna Kanwal2,
Syeda Maryam Hussain1 and Muhammad Zubair Siddiqi4
1Department of Livestock Production and Management, Faculty of Veterinary and Animal Sciences PMAS-Arid Agriculture University, Rawalpindi 44000, Pakistan
2Deprtment of Pathology, Faculty of Veterinary Science, University of Agriculture Faisalabad, Pakistan
3Department of Clinical Studies, Faculty of Veterinary and Animal Sciences, Pir Mehr Ali Shah, Arid Agriculture University, Rawalpindi 44000, Pakistan
4Department of Biotechnology, Hankyong National University, 327 Jungang-ro Anseong-si, Gyonggi-do 17579, Republic of Korea
ABSTRACT
Fowl typhoid has revolved into a pathogenic hazard to the poultry industry and extensive use of antibiotic is leading to the induction of antimicrobial resistance in microorganism. Main aim of this study was to find out substitute of antibiotic against Fowl Typhoid in broiler in terms of immunological, serum biochemistry and lipid profile parameters. Broiler chicks (Age = 1 d and n = 90) were kept under uniform management conditions. Chicks were divided randomly into six groups on day ten of their age with 15 replicates in each; control negative (CN), control positive (CP) and four treatments (T1, T2, T3 and T4). Wheras challenge infection of Salmonella gallinarum was given on nineteen days of age to all experimental birds except of those in CN group. On 22nd day, infected birds in T1 were given Florfenicol antibiotic. Whereas infected birds in groups; T2, T3 and T4 were given concentration of nanoparticles zinc oxide and copper oxide at different rate; 25+10, 37.5+15 and 50+20 mg/kg/d, respectively. Collected data were analyzed using complete randomized design through ANOVA technique. Mortality percent was found minimum 13.3% in birds under T3 group. Effect of all nanoparticle levels was not different (P>0.05) to that of antibiotic in terms of total serum protein and lipid profile; total glycerides, very low-density lipids, high density lipids and total cholesterol. It was concluded on the basis of findings of current study that nanoparticles zinc oxide and copper oxide mixture (37.5 + 15 mg/Kg/d) was found optimum alternate to Florfenicol antibiotic against Salmonella gallinarum infection in broiler birds. Hence, Zinc oxide and copper oxide nanoparticles could be an adequate alternative treatment replacing antibiotics against Fowl Typhoid in broilers.
Article Information
Received 17 October 2022
Revised 29 October 2022
Accepted 09 November 2022
Available online 13 January 2023
(early access)
Published 25 March 2024
Authors’ Contribution
MAR conducted research trial. MTJ designed this study and supervised this research work. MSU and MAK were involved in data and sample collection. MF was involved in writing of this manuscript, supervised the work. MS and SMH provided technical services to execute hematological analysis and also provided research material required for lab analysis. MZS conducted statistical analysis of collected data.
Key words
Fowl typhoid, ZnO and CuO nanoparticles, Florfenicol antibiotic, Immunoglobulins and serum biochemistry
DOI: https://dx.doi.org/10.17582/journal.pjz/20221017061044
* Corresponding author: [email protected]
0030-9923/2024/0003-1049 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Poultry has become a dynamic livestock sub-sector that plays a crucial role in the economies of developing countries. It fulfills not only daily protein requirements of increasing human population in terms of high-quality food items like chicken meat and eggs but also triggers generation of employment sources. Globally, it has become a major source of food supplies around the world (Hussain et al., 2015). However, despite its rapid growth, the poultry industry faces numerous problems and bird mortality in particular is one of the main issues affecting the sustainability of poultry production worldwide. The mortality of birds might be due to spread of infectious diseases (Ahmed et al., 2022). These infectious diseases have become a huge risk to the poultry industry in terms of drug costs and consequently bird morbidity and mortality, resulting in high economic losses for any country (Abbas et al., 2015).
Fowl typhoid caused by a bacterium, Salmonella gallinarum, is one of the pathogenic infectious diseases. The infectious diseases including fowl typhoid pose heavy economic losses to the poultry industry (Yasmin et al., 2019). Incubation period of this disease is about 4-6 days and occur in all types of birds with no exception of chicken of all ages. The birds suffering from fowl typhoid show depression, anorexia, dyspnea, weakness, droopy wings, ruffled feathers, huddling, adherence of droppings to the vent and diarrhea (Brenner et al., 2000). The birds shed the bacteria in droppings that cause the contamination of food and water (Nair et al., 2015). The disease can transmit both horizontally and vertically. The young chicks die within 5-10 days of hatching and mortality can reach up to 80% (Bhatti et al., 2013).
Antibiotics like Florfenicol, Enrofloxacin, Penicillin, Erythromycin, Oxytetracycline etc. are widely used against Salmonella species infection at poultry farms which leads to the induction of antimicrobial resistance (Oloso et al., 2019). The antimicrobial resistance (AMR) is the most important consequence of antimicrobial drugs used globally against Salmonella infection in animals. The antibiotics are used as metaphylactic and prophylactic treatment in the food producing animals. These agents are also used as growth promoters in broilers and other food animals. The AMR in food producing animals is of great concern (Threlfall, 2002). The irrational use of antimicrobial agents against non-typhoid Salmonella species is leading to the induction of antimicrobial resistance in microorganism. This evolutionary process makes the virulent strains able to survive in the unfavorable drug environment (Su et al., 2004). It has been foreseen that by 2050, antibiotic-resistant pathogens may cause about 10 million deaths worldwide (Castro-Vargas et al., 2020). The salmonella species can be transferred to the human being by handling or slaughtering the infected and morbid birds (Tizard, 2004; Mouttotou et al., 2017). The discovery of alternative, preventive and treatment methods could address the problem of antimicrobial resistance (AMR), as the WHO has issued a list of bacteria that have produced AMR (Oloso et al., 2019) and in the global plan of action 2015 instructions to overcome the problem and proposed to develop new drugs as antimicrobial solutions.
Nanotechnology could be a viable alternative solution for destroying fowl typhoid bacteria. The CuO and ZnO nanoparticles have sufficient bactericidal activity against a variety of gram-positive and gram-negative bacteria (Zarrindokht and Pegah, 2011; Das et al., 2013; Khashan et al., 2016) and these metal oxides ions like Zn2+ and Cu2+ which react with the negatively charged bacterial cells. Reactive oxygen species are produced by the nanoparticles, which bind to the bacterial cell wall, enter the cell and consequently destroy the bacterial cell (Ahmed et al., 2022). The nanoparticles (NPs) can also cause bacterial cell death by destroying the vital enzymes in the bacterial cells (Dadi et al., 2019) and after entering into the bacterial cell, ZnO NPs interact with the sulphur and phosphorus containing compounds like DNA of the bacterial cell leading to bacterial cell death (Raguvaran et al., 2015).
Keeping in view the importance of ZnO and CuO nanoparticles as an adequate alternative technique, the present study was designed with main aim to find out the optimum alternative treatment solutions replacing antibiotic. The specific objective of present study was to examine the antibacterial effect of different levels of ZnO and CuO nanoparticles in comparison with Florfenicol against Salmonella gallinarum induced infection in broilers by immunological, serum biochemistry and lipid profile parameters.
MATERIALS AND METHODS
Experimental site
Experiment was conducted in Pathology Department, Faculty of Veterinary Science, University of Agriculture Faisalabad, Punjab, Pakistan. The geographical coordinates of research site are; Latitude: 31° 25’ 46.8048”. Longitude: 73° 4’ 14.3112”. Latitude: N 31° 25.7801’. Longitude: E 73° 4.2385’. Latitude: 31.429668°. Climate of the experimental site was cold with foggy nights. Ambient temperature ranged from 10 to 20°C whereas average relative humidity was 66%. The nanoparticles were synthesized in the Department of Physics. Experimental trial was conducted for a period of 30 days w.e.f. 24th December 2020 to 22nd January 2021 in poultry shed of Parasitology Department, whereas different laboratories in Faculty of Veterinary Science; Disease Diagnostic Laboratory, Physiology Laboratory and Molecular Pathology Laboratory, were used for lab analysis in this study.
Treatment groups
One day-old broiler chicks (n= 90) were taken from a local commercial hatchery. All the experimental birds were homogenous with no visible variation regarding age, weight and size. According to ethical standards, experimental birds were given freedom from hunger and thirst as well as provided an environment in which birds expressed normal behavior. Feeding was provided uniformly to all animals as per their requirements under uniform housing and management conditions. On day 10, the birds were randomly divided into six groups; control negative, control positive, treatment 1, treatment 2, treatment 3 and treatment 4 (CN, CP, T1, T2, T3 and T4). Each treatment groups comprised 15 birds and kept under different individual compartments. The vaccination of the birds was administered against ND and IB on 3rd and 14th days of age.
Induction of infection
On day 19, the challenge of Salmonella gallinarum was given to the birds of all groups except control negative (CN) group at dose 108 CFU/ml via crop route method. The group was T1 was given treatment florfenicol and groups T2, T3 and T4 were given treatments of ZnO and CuO nanoparticles at different dose level 25+10, 37.5+15 and 50+20 mg/kg/d respectively as shown in the experimental layout (Table I). The treatment was given to the birds after the appearance of clinical signs (3 days post infection).
Parameters studied and data collection
Mortality ratio of the birds were noted between 19th and 30th day, whereas three birds from each group were slaughtered for sample collection on 26th and 30th day of trial. In order to separate the serum, the blood samples were collected in 5 ml syringes and kept in a hot air oven (37° C) for 30 min. In a 1.5 ml Eppendorf tube, the serum was collected and kept at -20° C for 15 days. The total serum proteins were determined by using Bioclin Kit, Brazil, LOT-1038 and serum albumin were determined by using Bioclin Kit, Brazil, LOT-0127. The serum globulin was determined by subtracting serum albumin from total serum proteins using following equation.
Serum globulin = Total serum proteins - Serum albuin
The lipid profile (triglycerides, high density lipids, low density lipids, very low-density lipids and total cholesterol) was determined by using commercially available kits. The triglyceride level was determined by using LabKit, Spain, LOT: LIQ-418-A, HDL-C was determined by using Human Diagnostic Kit, Germany, LOT: 0072 and total cholesterol was determined by using Human Diagnostic Kit, Germany, LOT: 0166. The VLDL were determined by dividing the triglyceride value by 5 by using following equation.
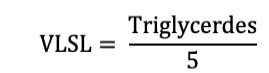
Low density lipid cholesterol was also calculated by following equation.
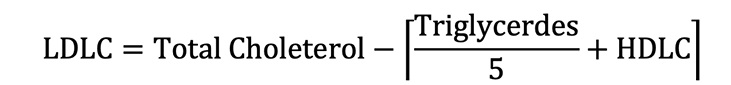
Antibody titer against sheep RBCs
The antibody response against sheep RBS was determined as described previously (Delhanty and Solomon, 1966). The 3% washed sheep RBCs were injected to the birds on 14th and 21st day of experiment. The antibody titer against sheep RBCs was determined from the serum collected from birds in all treatment groups on 21st and 28th day of trail. The sheep blood was collected in the EDTA vacutainer (Lab Vac, LOT: 07072014) from jugular vein of a sheep maintained at UAF Small Ruminant Farm using a sterile syring. After washing the sheep RBCs, a 3% (V/V) suspension of sheep RBCs was
Table I. List of experimental operations at different age days of experimental broilers under treatment groups.
|
Age (day) |
Treatment groups |
|||||
|
Control Negative |
Control Positive |
T1: Florfenicol |
Nanoparticle varying levels of ZnO and CuO (mg/kg/d) |
|||
|
T2: |
T3: |
T4: |
||||
|
10 |
Division of birds into 6 Groups |
|||||
|
14 |
1st Injection of Sheep RBCs to birds in all groups |
|||||
|
19 |
Nil |
Infection induced: Inoculation of Salmonella gallinarum at 108 CFU/ml except control positive group |
||||
|
21 |
Collection of Serum from Sheep RBCs injected Birds and 2nd Injection of Sheep RBCs to birds in all groups |
|||||
|
22 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
23 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
24 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
25 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
26 |
Sampling-I (7 days after infection of S. gallinarum): Humane Slaughtering of Birds, Collection of Blood, Serum Separation |
|||||
|
27 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
28 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
29 |
No treatment |
No treatment |
1ml/4L water |
25+10 mg/kg/d |
37.5+15 mg/kg/d |
50+20 mg/kg/d |
|
30 |
Sampling-II (11 days after infection of S. gallinarum): Humane slaughtering of birds, collection of blood, serum separation |
|||||
prepared in the normal saline. On day 14th and 21st, 1 ml of 3% sheep RBCs were injected aseptically in wing vein of birds of all groups and the serum was separated from the blood taken from the injected birds after 7-days and 14-days post injection. The antibody titer against sheep RBCs was determined by testing the collected serum samples using the titration method following the inactivation of serum in hot air oven. A volume of 50 µL of phosphate buffer saline was added in well of the row of microtitration plate for each sample. A volume of 50 µL of inactivated serum sample was added in the 1st well of the microtitration plate and incubated for 30 min at 37° C. After 30 min incubation, two-fold serial dilution was done for each sample. A volume of 50 µL of 3% sheep RBCs were added into each well and incubated at 37° C for 30 min and the titers were noted down. The treatment of collected serum with 2-merceptoethanol was done to determine the IgM and the level of IgG was determined from total antibody response minus IgM.
Statistical analysis
The collected data were analyzed under complete randomized design through ANOVA technique, whereas group mean comparison was made through Tukey’s test (Steel et al., 1997) using SAS® University Edition online software SAS 15.1.
RESULTS
Effect of Florfenicol antibiotic and varying treatment levels of nanoparticles was determined in the experimental birds with induced infection of Salmonella gallinarum in terms of following studied parameters serum biochemistry, immunoglobulins and mortality percentage in Tables II, III and Figure 1, respectively.
Mortality rate
Effect of different levels of nanoparticles with comparison of Florfenicol antibiotic in terms of mortality rate is mentioned in Figure 1. Mortality was found Nil in control negative group, whereas it was at its highest i.e., 60% in control positive group. However, 20% mortality was observed in birds treated with Florfenicol, whereas 20%, 13% and 20% mortality was found in birds treated with nanoparticle treatment groups; T2, T3 and T4, respectively. The lowest mortality was found in treatment T3: ZnO 37.5 + CuO 15 mg/kg/d.
Table II. Antibacterial effect of varying levels of mixed zinc oxide and copper oxide nanoparticles and Florfenicol on Salmonella gallinarum induced infection in broiler in terms of serum biochemistry and lipid profile at 7th day and 11th day post infection.
|
Serum biochemical parameters |
Treatments |
||||||
|
Control negative |
Control positive |
T1: Florfenicol |
Nanoparticle levels of ZnO and CuO (mg/kg/d) |
||||
|
T2: (25 + 10) |
T3: (37.5 + 15) |
T4: (50 + 20) |
|||||
|
Total serum proteins (mg/dl) |
S1 |
4.42 ± 1.35a |
1.45 ± 0.39b |
4.34 ± 0.65a |
3.42 ± 0.59a |
3.48 ± 0.46a |
3.28 ± 0.31ab |
|
S2 |
4.43 ± 0.97a |
1.86 ± 0.34b |
3.5 ± 0.55ab |
3.52 ± 0.52ab |
3.43 ± 0.59ab |
3.83 ± 0.79a |
|
|
Serum albumin (mg/dl) |
S1 |
3.50 ± 0.73a |
1.11 ± 0.11c |
3.22 ± 0.72ab |
1.79 ± 0.32c |
1.76 ± 0.34c |
1.99 ± 0.11bc |
|
S2 |
3.51 ± 0.52a |
1.60 ± 0.5b |
3.11 ± 0.31ab |
2.41 ± 0.95ab |
2.38 ± 0.54ab |
3.02 ± 0.63ab |
|
|
Serum globulin (mg/dl) |
S1 |
0.92 ± 0.70ab |
0.34 ± 0.37b |
1.12 ± 0.21ab |
1.62 ± 0.37a |
1.71 ± 0.17a |
1.29 ± 0.29ab |
|
S2 |
0.93 ± 0.54a |
0.26 ± 0.19a |
0.39 ± 0.26a |
1.11 ± 0.44a |
1.05 ± 0.05a |
0.81 ± 0.27a |
|
|
Total glycerides (mg/dl) |
S1 |
482.05 ± 38.71ab |
251 ± 38.71c |
446.15 ± 15.38b |
533.33 ± 47.0ab |
548.71 ± 38.71ab |
569.23 ± 55.47a |
|
S2 |
476.92 ± 46.15a |
174.35 ± 58.24b |
456.41 ± 125.3a |
528.2 ± 64.05a |
528.2 ± 54.02a |
533.3 ± 62.17a |
|
|
Very low density lipids (mg/dl) |
S1 |
96.41 ± 7.74ab |
50.25 ± 7.74c |
89.23 ± 3.07b |
106.6 ± 9.4ab |
109.74 ± 7.74ab |
113.84 ± 11.09a |
|
S2 |
95.38 ± 9.23a |
34.87 ± 11.64b |
91.28 ± 25.06a |
105.64 ± 12.81a |
105.64 ± 10.8a |
106.6 ± 12.43a |
|
|
High density lipids (mg/dl) |
S1 |
93.07 ± 27.13a |
31.92 ± 10.25b |
65.89 ± 8.48ab |
94.42 ± 16.47a |
83.55 ± 9.33a |
101.22 ± 27.13a |
|
S2 |
113.45 ± 16.97a |
38.72 ± 12.39b |
78.12 ± 12.45ab |
90.35 ± 15.43a |
93.75 ± 14.26a |
110.73 ± 17.33a |
|
|
Total cholesterol (mg/dl) |
S1 |
277.17 ± 26.76a |
135.86 ± 44.89b |
231.88 ± 22.13ab |
289.85 ± 38.46a |
294.38 ± 46.61a |
304.34 ± 32.94a |
|
S2 |
308.87 ± 78.49a |
103.26 ± 15.12b |
275.36 ± 31.72a |
270.83 ± 36.18a |
286.23 ± 27.75a |
297.1 ± 44.84a |
|
|
Low density lipid (mg/dl) |
S1 |
87.69 ± 44.7a |
53.68 ± 30.34b |
76.75 ± 22.61ab |
88.75 ± 30.27a |
101.08 ± 56.84a |
89.27 ± 7.71a |
|
S2 |
100.04 ± 94.16a |
29.66 ± 24.56a |
105.95 ± 15.47a |
74.83 ± 49.13a |
86.84 ± 25.19a |
79.7 ± 39.01a |
|
abcd Mean Values in rows with various superscripts are different (P<0.05), S1 and S2 indicates Sampling 1 and Sampling 2.
Table III. Antibacterial effect of varying levels of mixed zinc oxide and copper oxide nanoparticles and Florfenicol on Salmonella gallinarum induced infection in broiler in terms of Ig, IgG and IgM.
|
Response |
Parameters |
Treatments |
|||||
|
Control Negative |
Control Positive |
T1: Florfenicol |
Nanoparticle levels of ZnO and CuO (mg/kg/d) |
||||
|
T2: (25 + 10) |
T3: (37.5 + 15) |
T4: (50 + 20) |
|||||
|
Response After 1st Injection |
Total Ig |
5.5 ± 0.71a |
4.5 ± 0.71a |
5.5 ± 0.71a |
6.5 ± 0.71a |
6.5 ± 0.71a |
6.5 ± 0.71a |
|
IgG |
4.5 ± 0.71a |
3.0 ± 1.41a |
4.5 ± 0.71a |
5.0 ± 0a |
5.5 ± 0.71a |
5.5 ± 0.71a |
|
|
IgM |
1 ± 0a |
1.5 ± 0.71a |
1.0 ± 0a |
1.5 ± 0.7a |
1.0 ± 0a |
1.0 ± 0a |
|
|
Response After 2nd Injection |
Total Ig |
6.0 ± 0a |
4.5 ± 0.71a |
6.0 ± 1.41a |
6.5 ± 0.71a |
5.0 ± 1.41a |
5.5 ± 0.71a |
|
IgG |
4.5 ± 0.71ab |
3.5 ± 0.71b |
4.5 ± 0.71ab |
5.5 ± 0.71ab |
4.5 ± 2.12a |
4.5 ± 0.71ab |
|
|
IgM |
1.5 ± 0.71a |
1.0 ± 0a |
1.5 ± 0.71a |
1.0 ± 0a |
0.5 ± 0.71a |
1.0 ± 0a |
|
abcd Mean Values in rows with various superscripts are different (P<0.05).
Total serum proteins
At 7th day post infection, the total serum protein was decreased (p<0.05) in control positive group as compared to control negative group. The total serum protein was higher in the group treated with antibiotic (Florfenicol) therapy. The total serum proteins were higher (p<0.05) in T1 as compared to that of control positive group. The total serum proteins in the birds in groups treated with different levels of ZnO and CuO nanoparticles T2, T3 and T4 were found comparable (p>0.05) to that of treatment group T1 (Florfenicol). At 11th day post infection, the total serum protein was decreased (p<0.05) in control positive group in comparison to control negative group. The total serum proteins in the treatment groups (T1, T2, T3) were found not different (p>0.05) to that of control positive group whereas total serum proteins of only treatment group T4 were found different as compared to that of control positive group. However, the total serum proteins of nanoparticles treated groups (T2, T3 T4) were found comparable (p>0.05) to that of T1 (Florfenicol) group.
Serum albumin
At 7th day post infection, serum albumin was decreased (p<0.05) in control positive group as compared to control negative group. The serum albumin was higher through antibiotic (Florfenicol) therapy. The serum albumin was higher (p<0.05) in T1 as compared to that of control positive group. The serum albumin of treatment groups T2, T3 and T4 was found not different (p>0.05) to that of control positive group. At 11th day post infection, the serum albumin was decreased (p<0.05) in control positive group as compared to control negative group. The serum albumin in T1 (Florfenicol) and T4 was improved numerically. The serum albumin in treatment groups T2, T3 and T4 was found comparable (p>0.05) to that of T1.
Serum globulins
At 7th day post infection, the serum globulins of groups T2 and T3 were found higher (p<0.05) as compared to that of control positive group while the serum globulins of T1 and T4 was found numerically higher (p>0.05) to that of control positive group. The serum globulin concentration in nanoparticle treated groups (T2, T3 and T4) was found comparable (p>0.05) to that of treatment group T1 (Florfenicol). However, at 11th day post infection, the serum globulins were found not different (p>0.05) in all groups (CN, CP, T1, T2, T3 and T4).
Total glycerides
At 7th day post infection, the total glycerides were decreased (p<0.05) in control positive group as compared to control negative group. The total glycerides were recovered through antibiotic (Florfenicol) therapy. The total glycerides were higher (p<0.05) in T1 as compared to that of in control positive group. The total glycerides in the groups T2, T3 and T4 were found lower (p<0.05) than that of control positive group. The total glycerides of treatment groups T2 and T3 were found comparable to that of T1 (Florfenicol). At 11th day post infection, the total glycerides were decreased (p<0.05) in control positive group as compared to control negative group. The total glycerides were recovered through antibiotic (Florfenicol) therapy. The total glycerides were higher (p<0.05) in T1 as compared to that of in control positive group. The total glycerides in the groups T2, T3 and T4 were found higher (p<0.05) to that of control positive group. The total glycerides of treatment groups T2, T3 and T4 were found comparable (p>0.05) to that of T1 (Florfenicol).
Very low-density lipids
At 7th day post infection, very low-density lipids were decreased (p<0.05) in control positive group as compared to control negative group. Very low-density lipids were improved through antibiotic (Florfenicol) therapy. Very low-density lipids were higher (p<0.05) in T1 as compared to that of in control positive group. Very low-density lipids of nanoparticles treated (T2, T3 and T4) groups were found higher (p<0.05) as compared to that of control positive group. Very low-density lipids of nanoparticles treated (T2 and T3) groups were found comparable (p<0.05) to that of group T1 (Florfenicol) whereas very low-density lipids of treatment group T4 was found higher (p<0.05) as compared to that of T1 (Florfenicol). At 11th day post infection, very low-density lipids were decreased (p<0.05) in control positive group as compared to control negative group. Very low-density lipids were recovered through antibiotic (Florfenicol) therapy. Very low-density lipids were higher (p<0.05) in T1 as compared to that of in control positive group. Very low-density lipids of nanoparticles treated (T2, T3 and T4) groups were found different (p<0.05) as compared to that of control positive group. Very low-density lipids of nanoparticles treated (T2, T3 and T4) groups were found comparable (p<0.05) to that of group T1 (Florfenicol).
High density lipids
At 7th day post infection, high density lipids were decreased (p<0.05) in control positive group as compared to control negative group. High density lipids were improved numerically in the group T1. High density lipids in treatment groups T2, T3 and T4 were found different (p<0.05) to that of control positive and comparable to that of T1 (Florfenicol). At 11th day post infection, high density lipids were decreased (p<0.05) in control positive group as compared to control negative group. High density lipids were improved numerically in the group T1. High density lipids in treatment groups T2, T3 and T4 were found higher (p<0.05) to that of control positive and comparable (P>0.05) to that of T1 (Florfenicol).
Total cholesterol
At 7th day post infection, total cholesterol was decreased (p<0.05) in control positive group as compared to control negative group. High density lipids were improved numerically in the group T1. The total cholesterol of treatment groups T2, T3 and T4 was found different (p<0.05) as compared to that of control positive group and comparable (p>0.05) to that of T1 (Florfenicol) treatment group. At 11th day post infection, total cholesterol was decreased (p<0.05) in control positive group as compared to control negative group. Total cholesterol was higher (p<0.05) in the group T1 as a result of antibiotic (Florfenicol) therapy. The total cholesterol of treatment groups T2, T3 and T4 was found higher (p<0.05) as compared to that of control positive group and comparable (p>0.05) to that of T1 (Florfenicol) treatment group.
Low density lipids
At 7th day post infection, low density lipids were decreased (p<0.05) in control positive group as compared to control negative group. Low density lipids were improved numerically in the group T1. Low density lipids of treatment groups T2, T3 and T4 was found higher (p<0.05) as compared to that of control positive group and comparable (p>0.05) to that of T1 (Florfenicol) treatment group. At 11th day post infection, low density lipids of all groups were found not different (p>0.05).
Antibody titer against sheep RBCs
The log antibody titer (Ig, IgG and IgM) of all treatment groups (T1, T2, T3 and T4) including control negative and control positive against sheep RBCs after 1st and 2nd injection was found not different (p>0.05) as mentioned (Table III). However, IgG in birds treated with T3 was found higher (p<0.05) than that of control positive treatment in case of 2nd injection of washed Sheep RBCs. While in case of treatment groups T1, T2 and T4, the IgG level was found comparable (p>0.05) to that of control positive group. The IgG level in nanoparticles treated groups (T2, T3 and T4) was comparable to that of treatment group T1 (Florfenicol).
DISCUSSION
In continuation of previous efforts, findings of this study also substantially endorsed nanotechnology as a reasonable substitute of antibiotic treatment against fowl typhoid. Preliminary findings like clinical signs appeared in response to induced infection of Salmonella gallinarum like fatigue, loss of appetite, ruffled feathers, sunken eyes, yellow diarrhea and significant mortality were in line to with the previous studies (Shivaprasad, 2000; Shah et al., 2013; Chiroma et al., 2017; Birhanu et al., 2020). In addition to above, gross pathological signs like bronze colored liver, splenomegaly and necrotic foci on visceral organs; liver, spleen and heart were also validated by Kumari et al. (2013). Followed by signs, significant decline in level of serum biochemistry parameters like total serum proteins, serum albumin and serum globulins was reported by previous workers (Kokosharov, 2006; Shah et al., 2013; Fotouh et al., 2014; Biazus et al., 2017). This decile might be due to damaged liver resulting in decreased synthesis of plasma proteins and serum albumin (Biazus et al., 2017), whereas damaged kidney led to increased loss of proteins and decreased appetite (Coles, 1980). In fact catalase enzyme might be produced by Salmonella gallinarum which could trigger proteolysis and consequently it might reduce protein concentration in the blood (Kokosharov, 2000).
Nanoparticles might be substantiated substitute of Florfenicol antibiotic therapy due to its adequate efficacy against induced infection of Salmonella gallinarum as evident by findings of this study. Total serum proteins and globulin level was preliminary decreased in response to infection and then reinstated might be attributed to a factor of substantial response of nanoparticles under T2 and T3 treatments at 1st sampling. This might be due to bactericidal activity of nanoparticle by degenerating the bacterial cells (Dadi et al., 2019; Ahmed et al., 2022) which could prevent liver damage in case of treated birds.
Comparable efficacy of nanoparticles with that of antibiotic in terms of lipid profile was also noticed. Following pattern of serum proteins and globulin, lipid profile parameters; total cholesterol, triglycerides, high density lipids, low density lipids and very low density lipids were decreased and then reinstated. This might be attributed due to a factor that liver tissue exposed to nanoparticle treatment could significantly increase lipid peroxides formation (Syama et al., 2013). Furthermore, Ahmadi et al. (2013) also strengthened findings of this study that lipid profile in terms of cholesterol, HDL and LDL were increased by nanoparticle treatment in birds.
The log antibody titer against sheep RBCs was found not different (P>0.05) in our study in all treatment groups at the time of both samplings (7th and 11th days post infection). These findings were in line to a recent study of Ahmed et al. (2022). However, Bami et al. (2018) reported differently in contrast to findings of this study that there was an increase in the log antibody titer against sheep RBCs in birds supplemented with ZnO NPs. The reason for this divergence might be due to different methodology adopted in that study in which birds were not induced infection of Salmonella gallinarum. Further research is recommended to authenticate the efficacy of nanoparticle treatment in birds infected with Salmonella gallinarum.
It was inferred based on fact of comparable efficacy of nanoparticles with that of Florfenicol against fowl typhoid, any dose level of zinc oxide and copper oxide 25+10, 37.5+15 and 50+20 mg/kg/d could be used as substitute of antibiotic treatment. However, a combination of zinc oxide and copper oxide with concentration 37.5 + 15 mg/kg/d was found optimum level of nanoparticles based on findings regarding minimum mortality of birds infected with Salmonella gallinarum in this treatment group. In general, nanoparticles could be a potential technique to be industrialized and applied in poultry industry to save birds from the danger of fowl typhoid with no antibiotic. Replacing antibiotic with more friendly and safer treatment with nanoparticles could ensure food safety for human consumption.
Conclusion
It was concluded on the basis of findings of current study that nanoparticles zinc oxide and copper oxide mixture (37.5 + 15 mg/Kg/d) was found optimum alternate to Florfenicol antibiotic against Salmonella gallinarum infection in broiler birds. Hence, Zinc oxide and copper oxide nanoparticles could be an adequate alternative treatment replacing antibiotics against fowl typhoid in broilers. Further research is required to authenticate findings of present study. It is also recommended that replacing antibiotic with administration of nanoparticles through water should be studied which would be more easy for farmers for implementation.
ACKNOWLEDGEMENTS
Efforts of Mr. Muhammad Huanain Ahmed, Mr. Muhammad Rashid, Mr. Arslan Shakoor, Miss Afsheen Fazil and Mr. Imran Ullah are highly appreciated for their kind support. Cooperation granted from Dr. Yasir Javed (Assistant Professor, Department of Physics, UAF) and Dr. Sohail Sajid (Associate Professor, Department of Parasitology, UAF) is also acknowledged. A huge gratitude is also extended to Dr. Manshaad Basheer (Big Bird) for sponsoring in purchase of experimental birds.
Funding
A partial funding from Dr. Manshaad Basheer (Big Bird) was received in term of free experimental birds to support researchers for execution of this research trail.
IRB approval
The experiment was carried out in line with the Responsible Conduct in Research (RCR) Training Policy (Policy No. 10.07.001) of the University of Agriculture, Faisalabad’s. The National Institutes of Health (NIH) Publication No. 8023, Revised 1978) guidelines for the welfare and housing of research animals are followed by the RCR Policy.
Ethical statement
During the whole period research trail, the bird were provided freedom from hunger, thirst and pain. A suitable environment was provided to express the natural behavior.
Statement of conflict of interests
The authors have declared no conflict of interest.
REFERENCES
Abbas G., Khan S.H., Hassan M., Mahmood S., Naz S., and Gilani, S.S., 2015. Incidence of poultry diseases in different seasons in Khushab district, Pakistan. J. Adv. Vet., 2: 141-145. https://doi.org/10.5455/javar.2015.b65
Ahmadi, F., Ebrahimnezhad, Y., Sis, N.M., and Ghiasi, J., 2013. The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. Int. J. Biosci., 3: 23-29. https://doi.org/10.12692/ijb/3.7.23-29
Ahmed, M.H., Javed, M.T., Bahadur, S.U.K., Tahir, M.H., Tariq, M.E., Khan, M.K., Naseer, M.U., Anwar, H., Tariq, N., and Hassan, U., 2022. In vivo antibacterial action of copper oxide nanoparticles against E. coli induced infection in broilers. https://doi.org/10.1007/s13204-022-02482-x
Bami, M.K., Afsharmanesh, M., Salarmoini, M., and Tavakoli, H., 2018. Effect of zinc oxide nanoparticles and Bacillus coagulans as probiotic on growth, histomorphology of intestine, and immune parameters in broiler chickens. Comp. clin. Pathol., 27: 399-406. https://doi.org/10.1007/s00580-017-2605-1
Bhatti, M.I., Ali, A.A., and Iftikhar, M., 2013. The prevalence of Salmonellosis in poultry farms in and around district Kasur, Pakistan (report). Sci. Int. (Lahore), 25: 603-604.
Biazus, A.H., Da Silva, A.S., Bottari, N.B., Baldissera, M.D., do Carmo, G.M., Morsch, V.M., Schetinger, M.R.C., Casagrande, R., Guarda, N.S., Moresco, R.N., and Stefani, L.M., 2017. Fowl typhoid in laying hens cause hepatic oxidative stress. Microb. Pathog., 103: 162-166. https://doi.org/10.1016/j.micpath.2016.12.009
Birhanu, B.T., Lee, E.B. and Park, S.C., 2020. Evaluation of the pharmacokinetic pharmacodynamic integration of marbofloxacin in combination with methyl gallate against Salmonella Typhimurium in rats. PLoS One, 15: 0234211. https://doi.org/10.1371/journal.pone.0234211
Brenner, F.W., Villar, R.G., Angulo, F.J., Tauxe, R., and Swaminathan, B., 2000. Salmonella nomenclature. J. clin. Microbiol., 38: 2465-2467. https://doi.org/10.1128/JCM.38.7.2465-2467.2000
Castro-Vargas, R.E., Herrera-Sánchez, M.P., Rodríguez-Hernández, R. and Rondón-Barragán, I.S., 2020. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World, 13: 2070. https://doi.org/10.14202/vetworld.2020.2070-2084
Chiroma, M.A., Adamu, S., Gadzama, J.J., Esievo, K.N., Abdulsalam, H., Balami, A.G., Enam, S.J., Muhammad, Y., and Atata, A.J., 2017. Some plasma biochemical changes in layers experimentally infected with Salmonella gallinarum. Afr. J. cell. Pathol., 9: 66-72. https://doi.org/10.5897/AJCPath2018.0010
Coles, E.H., 1980. Veterinary clinical pathology, 3rd edition, 3rd edition. W B Saunders Co., Philadelphia, US, Eastbourne. ISBN 9780721626444.
Dadi, R., Azouani, R., Traore, M., Mielcarek, C., and Kanaev, A., 2019. Antibacterial activity of ZnO and CuO nanoparticles against gram positive and gram negative strains. Mater. Sci. Eng. C., 104: 109968. https://doi.org/10.1016/j.msec.2019.109968
Das, D., Nath, B.C., Phukon, P., and Dolui, S.K., 2013. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B., 101: 430-433. https://doi.org/10.1016/j.colsurfb.2012.07.002
Delhanty, J.J., and Solomon, J.B., 1966. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology, 11: 103.
Fotouh, A., Gab-Allah, M.S., Tantawy, A.A., Soufy, H., and Nasr, S.M., 2014. Alterations of blood components in broiler chicks experimentally infected with Salmonella gallinarum. Glob. Vet., 13: 787-793.
Hussain, J., Rabbani, I., Aslam, S., and Ahmad, H.A., 2015. An overview of poultry industry in Pakistan. World’s Poult. Sci. J., 71: 689-700. https://doi.org/10.1017/S0043933915002366
Khashan, K.S., Sulaiman, G.M., and Abdulameer, F.A., 2016. Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arab J. Sci. Eng., 41: 301-310. https://doi.org/10.1007/s13369-015-1733-7
Kokosharov, T., 2000. Bacterial colonization and endotoxin activity during experimental acute fowl typhoid in chickens.
Kokosharov, T., 2006. Changes in the protein profile in birds with experimental acute fowl typhoid. Bulg. J. Vet. Med., 9: 189-192.
Kumari, D., Mishra, S.K., and Lather, D., 2013. Pathomicrobial studies on Salmonella gallinarum infection in broiler chickens. Vet. World, 6: 725. https://doi.org/10.14202/vetworld.2013.725-729
Mouttotou, N., Ahmad, S., Kamran, Z. and Koutoulis, K.C., 2017. Prevalence, risks and antibiotic resistance of Salmonella in poultry production chain. In: Current topics in Salmonella and Salmonellosis, pp. 215-234. https://doi.org/10.5772/67438
Nair, A., Balasaravanan, T., Malik, S.S., Mohan, V., Kumar, M., Vergis, J., and Rawool, D.B., 2015. Isolation and identification of Salmonella from diarrheagenic infants and young animals, sewage waste and fresh vegetables. Vet. World, 8: 669. https://doi.org/10.14202/vetworld.2015.669-673
Oloso, N.O., Adeyemo, I.A., Van Heerden, H., Fasanmi, O.G., and Fasina, F.O., 2019. Antimicrobial drug administration and antimicrobial resistance of salmonella isolates originating from the broiler production value chain in Nigeria. Antibiotics, 8: 75. https://doi.org/10.3390/antibiotics8020075
Raguvaran, R., Manuja, A., and Manuja, B.K., 2015. Zinc oxide nanoparticles: opportunities and challenges in veterinary sciences. Immunome Res., 11: 1. https://doi.org/10.4172/1745-7580.1000095
Shah, S.N., Kamil S.A., Darzi M.M., Mir M.S., and Bhat S.A., 2013. Haematological and some biochemical changes in experimental fowl typhoid infection in broiler chickens. Comp. clin. Pathol., 22: 83-91. https://doi.org/10.1007/s00580-011-1371-8
Shivaprasad, H.L., 2000. Fowl typhoid and pullorum disease. Rev. Off. Int. Epizoot., 19: 405-424. https://doi.org/10.20506/rst.19.2.1222
Steel, R.G.D., Torrie, J.H. and Dickey, D., 1997. Principles and procedure of statistics. A biometrical approach. 3rd Edition, McGraw Hill, Inc. Book Co., New York. pp. 352-358.
Su, L.H., Chiu, C.H., Chu, C., and Ou, J.T., 2004. Antimicrobial resistance in nontyphoid Salmonella serotypes: A global challenge. Clin. Infect. Dis., 39: 546-551. https://doi.org/10.1086/422726
Syama, S., Reshma, S.C., Sreekanth, P.J., Varma, H.K., and Mohanan P.V., 2013. Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defense mechanisms in mouse liver. Toxicol. environ. Chem., 95: 495-503. https://doi.org/10.1080/02772248.2013.789606
Threlfall, E.J., 2002. Antimicrobial drug resistance in Salmonella: problems and perspectives in food-and water-borne infections. FEMS Microbiol. Rev., 26: 141-148. https://doi.org/10.1111/j.1574-6976.2002.tb00606.x
Tizard, I., 2004. Salmonellosis in wild birds. In: Seminars in avian and exotic pet medicine. WB Saunders. 13: 50-66. https://doi.org/10.1053/j.saep.2004.01.008
Yasmin, S., Nawaz, M., Anjum, A.A., Ashraf, K., Ullah, N., Mustafa, A., Ali, M.A., and Mehmood, A., 2019. Antibiotic susceptibility pattern of Salmonellae isolated from poultry from different Districts of Punjab, Pakistan. Pak. Vet. J., 40: 98-102. https://doi.org/10.29261/pakvetj/2019.080
Zarrindokht, E.K., and Pegah, C., 2011. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. microbiol. Res., 5: 1368-1313. https://doi.org/10.5897/AJMR10.159
To share on other social networks, click on any share button. What are these?









