Antibacterial Effect of Bay Leaf (Laurusnabilis) Aqueous Extract and its Nano-Emulsion on Some Pathogenic Bacteria
Suhair Sh. Al-Siraj1, Jihan M. Badr2 and Dalia M.A. El-Masry3*
1Biology Department, Faculty of Science, Mustansiriyah University, Bagdad, Iraq; 2Poultry Diseases Department, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Giza, Egypt; 3Nanotechnology Research unit, Animal Health Research institute, Agricultural Research center (ARC), Giza, Egypt.
*Correspondence | Dalia M.A. EL-MASRY, Nanotechnology Research Unit, Animal Health Research Institute, ARC, Giza, Egypt; Email:
[email protected]
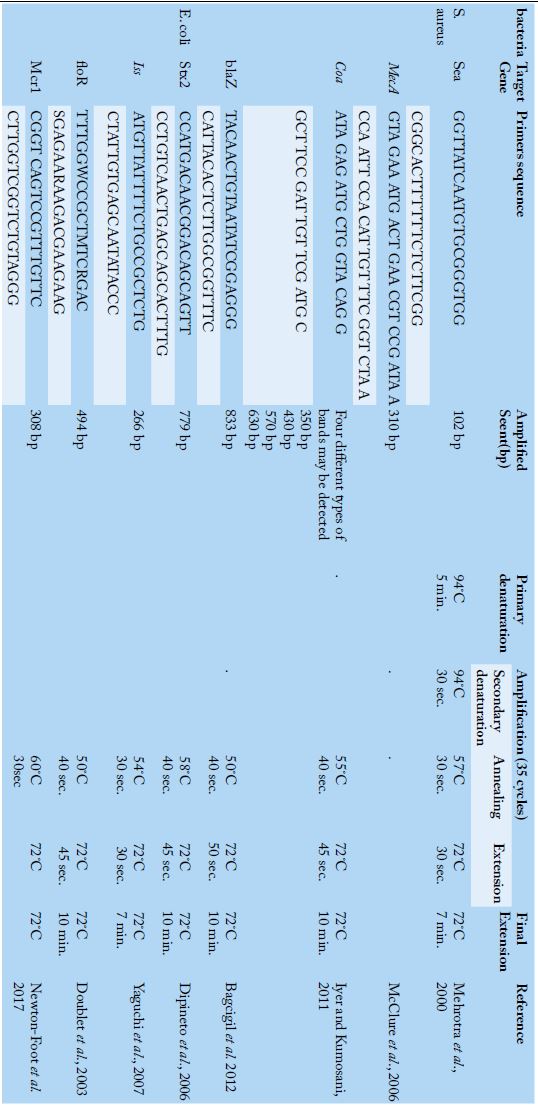
Table 1:
Primers sequences, target genes, amplicon sizes and cycling conditions.
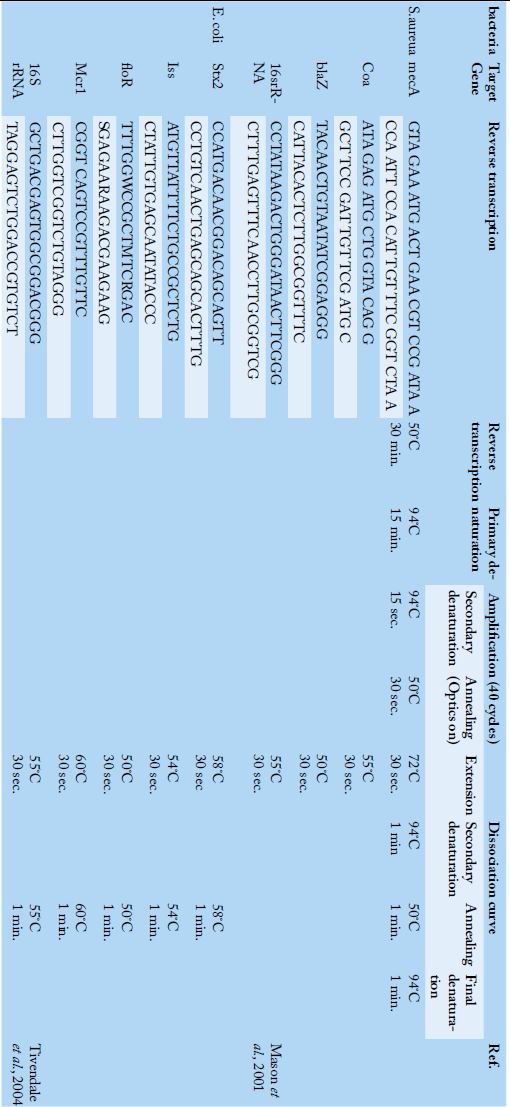
Table 2:
Primers sequences, target genes and cycling conditions for SYBR green rt-PCR.
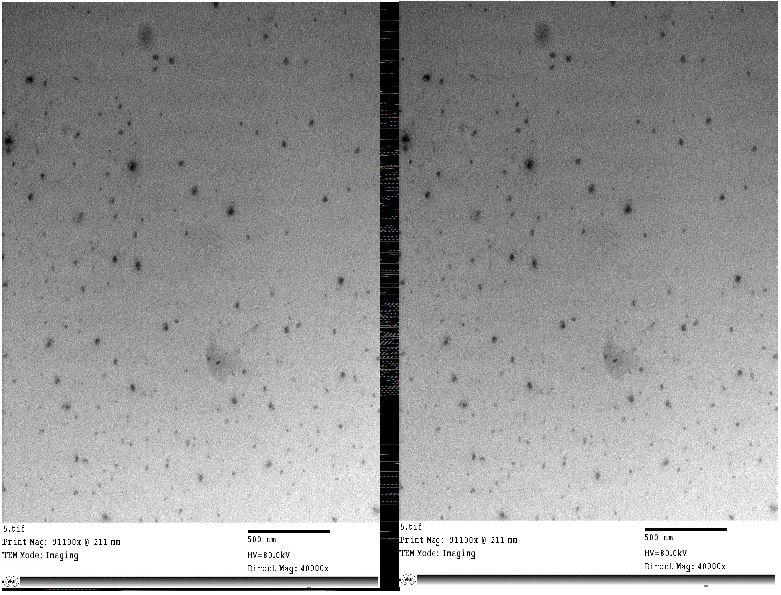
Figure 1:
HRTEM of Bay leaf (Laurus nobilis) nanoparticles showed nano sphere form, no aggregation and size (33.15) nm with Mag. 5000× to 123000× and 40000× to 91100× (Central lab. in NRC).
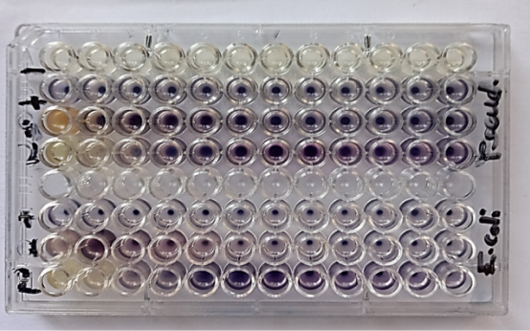
Figure 3:
Antibacterial activity of Bay leaves (laurus nobilis) Aqueous extracts and nanoemulsion against E. coli and Pseudomonas aeruginosa
1=Bay leaf (laurus nobilis) Aqueous extract , 2= Bay leaf (laurus nobilis) nano emulsion
- = negative control, + = positive control.
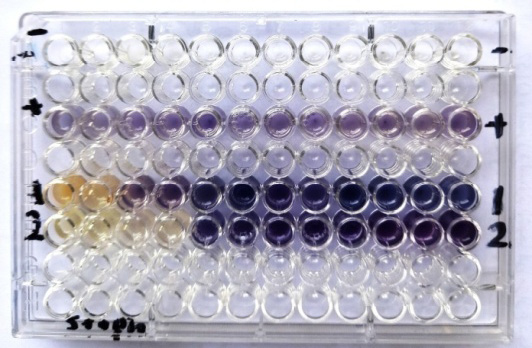
Figure 2:
Antibacterial activity of Bay leaves (laurus nobilis) Aqueous extracts and nano emulsion against S. aureus
1-Bay leaf (laurus nobilis) Aqueous extract , 2- Bay leaf (laurus nobilis) nano emulsion, - = negative control, + = positive control.
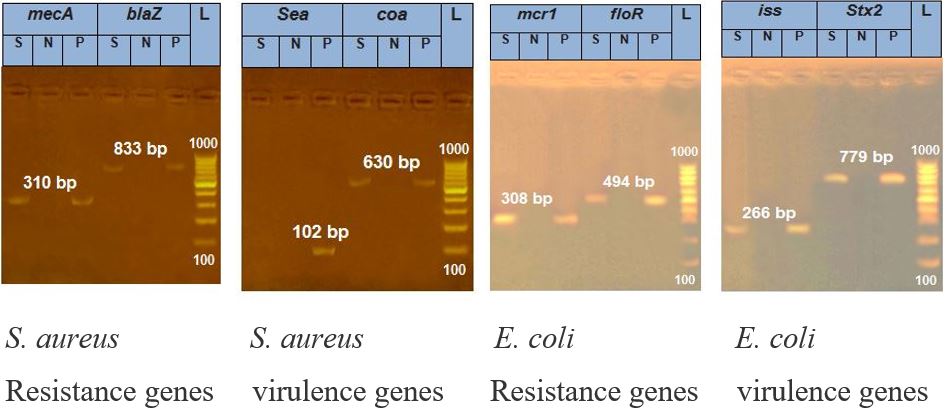
Figure 4:
Results of PCR analysis of identification of resistance and virulence genes of S. aureus and E. coli.
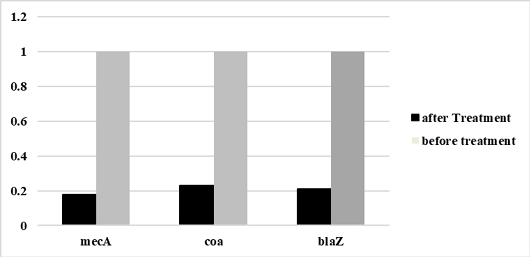
Figure 5:
Relative mecA, coa, and blaZgenes expression of S. aureus before and after treatment with laurus nobilis nanoemulsion extract .
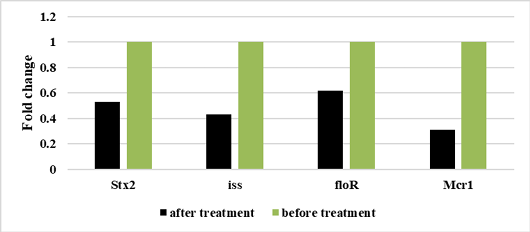
Figure 6:
Relative Stx2, iss, floR, Mcr1genes expression of E. coli before and after treatment with laurus nobilis nanoemulsion extract.