Anti-Cancerous and Anti-Inflammatory Activities of some Novel Schiff Bases Derived from 2-[(1,3-benzothiazol-2-yl)sulfanyl]-N- [4-(hydrazinecarbonyl)phenyl]acetamide
Anti-Cancerous and Anti-Inflammatory Activities of some Novel Schiff Bases Derived from 2-[(1,3-benzothiazol-2-yl)sulfanyl]-N- [4-(hydrazinecarbonyl)phenyl]acetamide
Aqsa Gulzar1, Tariq Mahmud1,*, Rubina Munir2 and Asma Anjum3
1Institute of Chemistry, University of the Punjab, Lahore 54590
2Department of Chemistry, Kinnaird College for Women, Lahore
3Division of Science and Technology, University of Education, Lahore
ABSTRACT
The anti-cancerous and anti-inflammatory potential of two novel Schiff bases was carried out by MTT - colorimetric protocol on HeLa cells (cervical disease) and Luminol-improved chemiluminescence assay, respectively. The novel Schiff bases 4a and 4b have been synthesized by the reaction of 2-[(1,3-benzothiazol-2-yl)sulfanyl]-N-[4-(hydrazinecarbonyl)phenyl]acetamide with 3-nitro and 4-nitrobenzaldehyde. Characterization of the synthesized compounds was done by using various spectroscopic techniques namely FTIR, proton and carbon-13 NMR, Mass spectrometry and elemental analysis. The results were in great consistency with the structures of the synthesized compounds and confirmed the formation of the targeted compounds. It was found that the compound 4a was potentially anti-inflammatory active while the compound 4b was found more active anti-cancerous agent as compared to 4a.
Article Information
Received 30 September 2017
Revised 24 December 2017
Accepted 19 February 2018
Available online 19 April 2018
Authors’ Contribution
TM and RM conceived and designed the experimental scheme. AG executed the experimental work, including characterization and article writing. TM and AA helped in data analysis.
Key words
Schiff base, Anti-cancerous activity, IC50 value, Anti-inflammatory activity, Proton NMR, Carbon-13 NMR.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.3.1003.1009
* Corresponding author: tariqm06@yahoo.co.uk
0030-9923/2018/0003-1003 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Cancer is a disease, in which a group of cells exhibits uncontrolled growth, and damages adjacent tissues, spreading to other parts of the body via lymph or blood. Now a days, It is one of the most leading causes of death in the world and many efforts have been made to discover potent anticancer agents (Sloane, 2009). Efforts have been made to screen actinomycetes of various ecological niches in Pakistan in order to know their cytotoxic nature (Usman et al., 2016). While some scientists focused on the synthesis of anti-cancer agents like Schiff bases. Schiff bases are a class of organic compounds exhibiting many biological applications including anti-cancerous activity. Likewise Schiff bases that have the ability to inhibit the production of both isozymes cyclooxygenase and 5-lipooxygenase are widely explored as potent pain-killer and anti-inflammatory drugs in comparison to non-steroidal anti-inflammatory drugs (Almasirad et al., 2005). Numerous biological applications of Schiff bases encouraged the scientists to synthesize these Schiff bases for the last few decades. The conventional method for the synthesis of Schiff bases is by mixing aldehydes or ketones with primary amines in equimolar quantities (Patai, 1970). Schiff bases are among the important class of organic chemistry showing wide range of biological activities. The study of novel biologically active Schiff bases has been gaining interest of chemists and pharmacists to a large extent. Schiff bases are of extraordinary interest for chemists since they can be used as beginning material in the industrial items synthesis. A lot of Schiff bases had been far and wide synthesized due to their pharmaceutical and industrial applications (Mirkhani et al., 2008) they can also act as catalyst for various reactions (Rayati et al., 2008; Yamada et al., 2006; Chen et al., 2007). Schiff bases synthesized from acyl-hydrazide (Greenfield et al., 1990) having an extensive variety of biological activities i.e. Adriamycin immune conjugates, anti-parasitic proteinase inhibiting activity against Trypanosoma brucei, which is the major cause of sleeping sickness in people (Cafferey et al., 2002) antimyco-bacterial (Kucukguzel and Rollas, 2002) insecticidal and anti-leishmanial (Bernardino et al., 2006; Sawada et al., 2003) and distinctive derivatives of hydrazide of N-cyanoethyl likewise demonstrated activity against β-glucuronidase catalyst (Khan et al., 2002).
In the view of the above information we synthesized two novel Schiff bases to evaluate them for activity against cervical cancer as well as their anti- inflammatory potential. In this article, the synthesis, characterization, anti-inflammatory and anti-cancerous activity of two novel Schiff bases (4a and 4b) is described.
MATERIALS AND METHODS
The synthetic scheme for the synthesis of the hydrazide and its Schiff bases is shown in Figure 1.
Chemicals used in this project were of analytical grade and purchased from E. Merck (Germany), Sigma-Aldrich (USA) and BDH.
Synthesis of compound-1
In 10 mL DCM added benzocain (5.0 mmol, 0.825 g) and stirred till all the benzocain dissolved in DCM, then added potassium carbonate (6.0 mmol, 0.828 g) and stirred the mixture using ice bath. Then fitted the dropping funnel to the round bottom flask and poured the chloroacetyl chloride (6.0 mmol, 0.9 ml) and added drop wise to the mixture in the round bottom flask., stirred the reaction mixture at 25°C temperature for 18 h. After completion of the reaction, checked by TLC, evaporated the DCM, added ice to this reaction mixture, stirred and filtered the precipitates. The precipitates collected over the filter paper were washed with plenty of water. The product in the pure form was obtained by recrystalization from boiling water. (yield 95-97%).The desired ester (compound-1) which separated out was reported earlier (Misra et al., 1976).
Synthesis of compound-2
A mixture of 2-mercaptobenzothiazole (1.0 mmol) in dry acetone (20 ml), anhydrous K2CO3 (1.1 mmol), and the compound-1 (1.0 mmol) obtained in the previous step was stirred at room temperature for 15-16 h. The mixture was filtered to remove the salt. Excess of the acetone present in the filtrate was distilled; the crude product, was collected by filtration, washed with water, and re-crystallized by using ethanol (yield 70-75%).
Synthesis of compound-3
To the mixture of the s-alkylated 2-mercapto benzothiazole (compound-2) (1.0 mmol) obtained from the above mentioned step dissolved in absolute ethanol (20 ml), added hydrazine hydrate (80%, 3.2 mmol), and then reflux the reaction mixture with continuous stirring for 11-12 h. After completion of the reaction, confirmed by the disappearance of the reactant spot in TLC, the reaction mixture was poured in cold distilled water and stirred for 10-15 minutes. Precipitates (white in color) formed were collected by filtration, and then washed with water, dried, and re-crystallized from ethanol (yield 58-60 %).
Synthesis of Schiff bases
1.0 mmol of Hydrazide (compound-3) obtained in the previous step was treated with 1.0 mmol of each aldehyde in absolute ethanol (10 ml), 1-2 drops of glacial acetic acid were added and reflux the mixture with continuous stirring for 4-5 h. After confirmation of the reaction completion by TLC, the precipitates formed during the reaction were filtered while hot and washing was also done by using hot ethanol. The product obtained was dried in air and recrystallized by using ethanol (yield 71-73 %).
(E)-2-(benzo[d]thiazol-2-ylthio)-N-[4-{2-(3-nitrobenzylidene)hydrazine-1- carbonyl}phenyl]acetamide (4a)

Molecular formula: C23H17N5O4S2, light yellow solid, Yield: 71%, m.p: 232°C.
Molecular weight: 491.54 g/mole.
FTIR (cm-1): 1191: ʋ(C-N-C), 1629: ʋ(C=N), 1665: ʋ(C=O Amide), 3195: ʋ (-CH2).
Proton NMR (300 MHz, DMSO-δ): 4.51 (2H,s,S-CH2), 7.22(1H, t, J=8.1Hz, Ar-H), 7.38 (1H,t, J= 7.2 Hz, Ar-H), 7.69 (1H d, J = 8.1 Hz, Ar-H), 7.77(1H, t, J= 7.8 Hz , Ar-H), 7.87(1H, d, J = 7.8 Hz, Ar-H), 7.92-8.00 (4H , m, Ar-H), 8.17 (1H,d, J = 7.2 Hz, Ar-H), 8.27 ( 1H,dd, J1 = 8.3 Hz, J2= 1.2Hz Ar- H), 8.55-8.58 (2H,m,N=CH, Ar-H ), 10.86 (1H,s,NH), 12.04 (1H,s,NH).
Carbon-13 NMR (75 MHz, DMSO-δ): 38.25 (S-CH2), 117.38-148.02 (All aromatic carbons), 144.23 (C=N), 145.15(C-NO2), 152.27 (C=O), 161.56 (C-S) and 167.83 (C=O).
Mass spectra: (m/z): 271 (M+- C9H7N2OS2, 3.0 %), 120 (M+− C15H11N2O2S2, 100 %).
CHN: Anal. Calculated for C23H17N5O4S2: C, 56.20; H, 3.49; N, 14.25; O, 13.02; S, 13.04; Found: C, 56.18; H, 3.47; N, 14.23; O, 13.01; S, 13.03.
(E)-2-(benzo[d]thiazol-2-ylthio)-N-[4-{2-(4-nitrobenzylidene)hydrazine-1-carbonyl}phenyl]acetamide (4b)
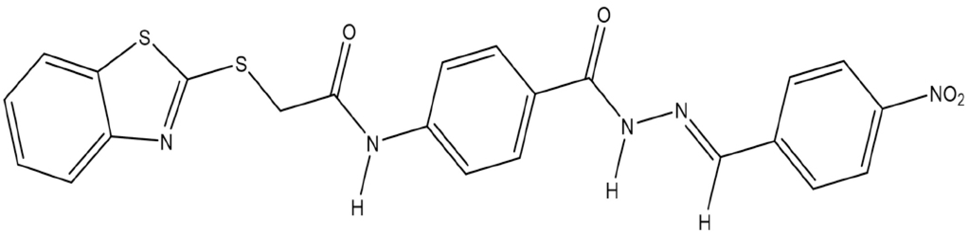
Molecular formula: C23H17N5O4S2, Yellow solid, Yield: 73%, m.p: 230°C.
Molecular weight: 491.54 g/mole.
FTIR (cm-1): 1141: ʋ(C-N-C), 1643: ʋ(C=N), 1665: ʋ(C=O Amide), 3195: ʋ (-CH2), Proton NMR (300 MHz, DMSO-δ): (DMSO) δ: 3.57 (2H, s, S-CH2), 7.15(1H, t, J=7.2Hz, Ar-H), 7.33(1H,t, J= 7.8 Hz, Ar-H), 7.73 (2H d, J = 8.7 Hz, Ar-H), 7.90-7.99 (4H, m, Ar-H), 8.25-8.32 (4H, m, Ar-H), 8.54 (1H,s,N=CH), 10.47 (1H,s,NH), 12.07 (1H,s,NH).
Carbon-13 NMR (75 MHz, DMSO-δ): 38.28 (S-CH2), 117.38-152.27 (All aromatic carbons), 148.02 (C=N), 150.20(C-NO2), 152.27 (C=O), 161.56 (C-S) and 167.83 (C=O).
Mass spectra: (m/z): 417(M+- C6H5), (271 (M+- C9H7N2OS2, 7.0 %), 120 (M+− C15H11N2O2S2, 100 %).
CHN: Anal. Calculated for C23H17N5O4S2: C, 56.20; H, 3.49; N, 14.25; O, 13.02; S, 13.04.
Found: C, 56.18; H, 3.47; N, 14.23; O, 13.01; S, 13.03.
Elecro-analytical characterization
All the reactions were examined with pre-coated aluminum TLC cards. Melting and decomposition points were taken by using melting point apparatus (Gallenkamp). FTIR spectrum was obtained using nujol mull technique on VENUS PHARMA/Agilent technologies Cary 630 FTIR spectrophotometer. NMR spectrum was obtained in DMSO on Brücker /Avance NMR (300 MHz) and internal standard used was TMS. Chemical shifts are obtained in δ (ppm). Recording of E-I+ Mass spectra was done on a Jeol-600H-2 instrument. Conductivity measurements were made with the inoLab Cond 720 conductometer using DMSO as a solvent at room temperature. To evaluate the changes in biological activities of the Schiff bases anti-cancerous and anti-inflammatory activities was carried out and compared their IC50 values, by using standard drugs.
Anti-cancer assay
Anti-cancer activity of the compounds was assessed by using 96 well level bottomed micro plates utilizing the standard MTT (3-[4, 5-dimethyl-2-yl]-2,5-diphenyl-tetrazolium bromide) colorimetric protocol (Mosmann, 1983). Therefore HeLa cells (cervical disease) were refined in Minimum Essential Medium Eagle, provided with 5.0 % of fetal bovine serum (FBS), penicillin (100 IU/mL) and streptomycin (100 µg/mL) in 75 cm2 flakes, and kept at 37°C in 5 % carbon dioxide incubator. Quickly developing cells were reaped tallied with haemocytometer and dilution was done by using a specific medium. Cell culture with the convergence of 6×104 cells/mL was arranged and presented (100 µL/well) into 96-well plates. Medium was removed after the incubation of one night, and 200 micro liter of new medium was included with the compounds of different concentrations (1-30 µM). After 48 h, 200 µL MTT (0.5 mg/mL) was supplied to each well and further incubated for next 4 h. Accordingly, 100 µL of dimethyl sulphoxide was introduced into each well. The degree of MTT diminishment to formazan inside cells was computed by absorbance measurement at 570 nm, utilizing a smaller scale plate peruser (Spectra Max in addition, Molecular Devices, CA, USA). Cytotoxic results were obtained as their concentration responsible for 50% growth retardation (IC50) for HeLa. The % inhibition was computed by using the accompanying equation:
% inhibition
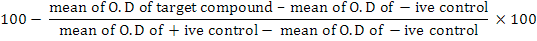
The outcomes (% inhibition) were handled by utilizing Soft-Max software (Molecular Device, USA).
Anti-inflammatory assay
Luminol-improved chemiluminescence measure was performed, as depicted in literature (Helfand et al., 1982) Precisely 25 µL of low concentration entire sample of blood Hanks Balanced Salt Solution, comprising of CaCl2 and MgCl2 (Sigma, St. Louis, USA) with 25 µL of 03 different concentrations of the tested compounds (1, 10 and 100 µg/mL), was incubated, each in triplicate manner. Control wells got Hanks Balanced Salt Solution, comprising of CaCl2 and MgCl2 and cells only without any compound. Test was performed in half zone (white) 96-well plates (Costar, NY, USA), which was incubated for 15 min at 37 ºC, in the indoor regulator chamber of luminometer (Labsystems, Helsinki, Finland). When incubation has done, 25 µL of serum opsonized zymosan (SOZ) (Fluka, Buchs, Switzerland) and 25 µL of intracellular responsive oxygen species identifying test, luminol (Research Organics, Cleveland, OH, USA) were included into each well, away from clear wells (having just HBSS++). The level of the ROS was noted in luminometer in term of light units (RLU). The reference drug utilized for comparison is Ibuprofen having IC50 = 11.2 ± 1.9.
Molecular docking studies
Docking studies have been executed to anatomize the interactions between DNA-Ligand using AutoDock v4.2 and AutoDock tools v1.5.6 (Morris et al., 1998). To determine best possible conformation of anti-cancerous active Schiff base in DNA, molecular docking was carried out.
Results and discussion
The synthesized Schiff bases were further characterized by using various spectroscopic techniques, including FTIR, UV-VIS spectroscopy, proton and carbon-13 NMR and mass spectrophotometer etc.
FTIR
The appearance of a peak due to (–CH=N) group in FTIR spectra in the frequency range of 1629-1643 cm-1 and absence of NH2 and carbonyl peak confirmed the target compound formation.
Proton NMR
The proton NMR spectrum of Schiff bases (4a-b) showed singlet at 12.04-12.07 δ due to NH protons, one singlet due to azomethine proton appeared at 8.54-8.55 δ. The peaks due to twelve aromatic protons (12H) were observed in the region of 7.15 - 8.58 ppm as doublets, triplets and multiplets. The absence of peak due to the protons of NH2 group was also give confirmation to the formation of targeted Schiff bases (4a-b). The confirmation of the Schiff base formation was also obtained by the appearance of singlet peaks in the range of 3.57-4.51 ppm and 10.47-10.86 ppm for the protons of S-CH2 and NH functionality. Appearance of a multiplet due to two protons in the spectra of ‘4a’ was due to merging of a singlet of azomethine proton with the proton of the ortho carbon to the carbon bearing the electron withdrawing NO2 group at meta position.
Carbon-13 NMR
In their Carbon-13 NMR spectra, the peaks due to carbonyl group was appeared around 144.23-148.08 ppm. S-alkylation of 2-Mercaptobenzothiazole was confirmed by the appearance of a signal in the range of 38.25 -38.28ppm in the Carbon-13 NMR spectra of all the Schiff bases.
Mass spectra
As a final point, molecular ion peaks in MS spectra were confirmed the synthesis of the targeted Schiff bases. Mass spectra are in great consistency with the NMR results of the synthesized Schiff bases.
Conductance measurements
The conductivity measurement values of the synthesized Schiff bases listed in Table I were measured in DMSO at room temperature. Very low conductance values of the synthesized compounds revealed that all the compounds were non-electrolytes.
Electronic spectra
The electronic spectral data of Schiff bases is also listed in Table I. The electronic absorption spectra of the synthesized compounds in DMSO were obtained in UV-visible region. The absorption spectra consisted of a strong band located in the range of 339-370 nm credited to n to π* transition of the azomethine group. Another strong band of higher energy in the spectra of the compounds was associated with π to π* transition of aromatic part of the compounds.
Anti-cancer activity
Anti-cancer activity of the novel Schiff bases were also tested in order to know their cytotoxic nature. The anti-cancer activity was recorded as their IC50 values for HeLa. The results, obtained for the evaluated compounds are shown in Table II. Results of the ligands (4a and 4b) were compared with the anti-cancerous activity of the standard drug Doxorubicin.
Table I.- Conductivity and electronic spectral data of the Schiff Bases (4a-b).
| Compound |
Conductance (µS/cm) |
Absorption (Nm) |
| 4a |
6.78 |
252, 339 |
| 4b |
10.91 |
295, 370 |
Table II.- Anti-Cancerous activity of the Schiff bases (4a-b). Standard drug Doxorubicin having IC50 value = 1.2 ± 0.4.
| Compound |
Conc. ( mg/ml) |
% Inhibition |
IC50±SD |
| Doxorubicin |
30 µM |
71 |
1.2 ± 0.4 |
| 4a |
30 µg/ml |
13 |
Inactive |
| 4b |
30 µg/ml |
77 |
10.1 ±1.1 |
Anti-inflammatory activities
The synthesized Schiff bases (4a and 4b) were also evaluated for their anti-inflammatory potential and their IC50 values were recorded (Table III). The activity was observed in the following order: Ibuprofen > 4a > 4b compared with standard drug Ibuprofen having IC50 value 11.2±1.9 µg/ml.
| Compound | Conc. ( mg/ml) | % Inhibition |
IC50±SD |
| 4a | 25 µg/ml | 66 | 28.2 ±5.2 |
| 4b | 25 µg/ml | 56 | 56.6 ±0.4 |
Molecular docking simulations
Among several drug targets of anticancer therapy, chemical agents targeting DNA have been known to produce significant effects against cancer and have been very interesting area for medicinal chemists since long. Chemical agents may bind with DNA structure in three possible ways, they may bind to the major groove or to the minor groove of DNA or they may bind across the DNA helix by intercalation as in case of Doxorubicin. To rationalize the anticancer potential of our synthesized anti-cancer active Schiff base 4b, molecular docking was carried out. Schiff base ‘4b’ poses highest binding affinity of -14.81Kcal mol-1 and from visualization it is evident that it binds to the narrow minor groove of the DNA double helix.
Conclusion
The synthesized novel Schiff bases were synthesized, characterized electro-analytically and evaluated for their anti-cancerous and anti-inflammatory activities. All the spectroscopic results confirmed the formation of the targeted Schiff bases. From the anti-cancerous results of the synthesized compounds, it is concluded that the compound 4b having electron withdrawing ‘NO2’ group at its para position, was proved to be active anti-cancerous agent as compared to the compound 4a having this group at meta position to the azomethine group. While in case of anti-inflammatory activity compound 4a proved to a little bit more active as compared to 4b.
Acknowledgement
The authors are grateful to Higher Education Commission (HEC), Government of Pakistan, for access to Scientific Instrumentation and Institute of Chemistry, University of the Punjab, Lahore-Pakistan, for providing laboratory facilities.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Almasirad, A., Tajik, M., Bakhtiari, D., Shafiee, A., Abdollahi, M., Zamani, M.J. and Esmaily, H., 2005. Synthesis and analgesic activity of N-arylhydrazone derivatives of mefenamic acid. J. Pharm. Sci., 8: 419-425.
Bernardino, A.M.R., Gomes, A.O., Charret, K.S., Freitas, A.C.C. and Machado, G.M., 2006. Synthesis and leishmanicidal activities of 1-(4-X-phenyl)-N′-[(4-Y-phenyl)methylene]-1H-pyrazole-4-carbohydrazides. Eur. J. med. Chem., 41: 80-87. https://doi.org/10.1016/j.ejmech.2005.10.007
Cafferey, C.R., Schanz, M., Nkemgu-Njinkeng, J., Brush, M.E. and Hansell, F.E., 2002. Screening of acyl hydrazide proteinase inhibitors for antiparasitic activity against Trypanosoma brucei Antimicrob. J. Agents, 19: 227-231. https://doi.org/10.1016/S0924-8579(01)00488-5
Chen, Y., Ruppel, J.V. and Zhang, X.P., 2007. Cobalt-catalyzed asymmetric cyclopropanation of electron-deficient olefins J. Am. Chem. Soc., 129: 12074-12075. https://doi.org/10.1021/ja074613o
Helfand, S.L., Werkmeister, J.E.R.O.M.E. and Roder, J.C.,1982. Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. J. exp. Med.., 156: 492-505. https://doi.org/10.1084/jem.156.2.492
Khan, K.M., Shujaat, S., Rahat, S., Hayat, S., Rahman, A. and Choudhary, M.I., 2002. β-N-Cyanoethyl acyl hydrazide derivatives: A new class of β-Glucuronidase inhibitors. Chem. Pharm. Bull. Jpn., 50: 1443-1446. https://doi.org/10.1248/cpb.50.1443
Kucukguzel, S.G. and Rollas, S., 2002. Synthesis, characterization of novel coupling products and 4-arylhydrazono-2-pyrazoline-5-ones as potential antimycobacterial agents. Farmacology, 57: 583-587. https://doi.org/10.1016/S0014-827X(02)01253-3; https://doi.org/10.1016/j.catcom.2007.06.003
Mirkhani, V., Moghadam, M., Tangestaninejad, S., Mohammadpoor-Baltork, I., Shams, E. and Rasouli, N., 2008. Investigation of catalytic activity of cobalt–Schiff base complex covalently linked to the polyoxometalate in the alkene and benzyl halide oxidation with hydrogen peroxide. Catal. Commun., 9: 219-223. https://doi.org/10.1016/j.catcom.2008.04.014
Mirkhani, V., Moghadam, M., Tangestaninejad, S., Mohammadpoor-Baltork, I., Shams, E. and Rasouli, N., 2008. Catalytic epoxidation of olefins with hydrogen peroxide by hybrid complex containing nickel (III) Schiff base complex covalently linked to polyoxometalate. Appl. Catal., 334: 106-111. https://doi.org/10.1016/j.apcata.2007.09.041
Misra, R.S., Barthwal, J.P., Parmar, S.S., Singh, S.P. and Stenberg, V.I., 1976. Synthesis of substituted thiobenzoxazoles/benzothiazoles: Inhibition of cellular respiratory and monoamine oxidase activities and anticonvulsant property. J. pharmaceut. Sci., 65: 405-408. https://doi.org/10.1002/jps.2600650322
Morris, G.M., Goodsell, D.S., Halliday, R.S., Huey, R., Hart, W.E., Belew, R.K. and Olson, A.J., 1998. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem., 19: 1639-1662. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B
Mosmann, T., 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods, 65: 55-63. https://doi.org/10.1016/0022-1759(83)90303-4
Patai, S. (ed.), 1970. The chemistry of carbon-nitrogen double bond. John Wiley and Sons, New York., pp. 61-146. https://doi.org/10.1002/9780470771204
Rayati, S., Torabi, N., Ghaemi, A., Mohbbi, S., Wojtczak, A. and Kozakiewicz, A., 2008. Vanadyl tetradentate Schiff base complexes as catalyst for C–H bond activation of olefins with tert-butylhydroperoxide: Synthesis, characterization and structure. Inorg. Chem. Acta, 361: 1239-1245. https://doi.org/10.1016/j.ica.2007.08.004
Sawada, Y., Yanai, T., Nakagawa, H. and Tsukamoto, Y., 2003. Synthesis and insecticidal activity of benzoheterocyclic analogues of N′-benzoyl-N-(tert-butyl) benzohydrazide: Part 3. Modification of N-tert-butylhydrazine moiety. Pest Manage. Sci., 59: 49-57. https://doi.org/10.1002/ps.605
Sloane, D., 2009. Cancer epidemiology in the United State: Racial, social and economic factors. Meth. mol. Biol., 471: 65-83. https://doi.org/10.1007/978-1-59745-416-2_4
Usman, A., Zafar-ul-Ahsan, Q. and Imran, S., 2016. In-vitro antitumor activity and metabolic finger printing of the actinomycetes isolated from various ecological niches in Pakistan. Pakistan J. Zool., 48: 1291-1305.
Yamada, T., Ikeno, T., Ohtsuka, Y., Kezuka, S., Sato, M. and Iwakura, I., 2006. Synthesis and insecticidal activity of benzoheterocyclic analogues of N′-benzoyl-N-(tert-butyl)benzohydrazide: Part 3. Modification of N-tert-butylhydrazine moiety. Sci. Technol. Adv. Mater., 7: 184-196. https://doi.org/10.1016/j.stam.2005.11.023
To share on other social networks, click on any share button. What are these?










