Antagonistic Effect of Nanoemulsions of Some Essential Oils against Fusarium oxysporum and Root-Knot Nematode Meloidogyne javanica on Coleus Plants
Antagonistic Effect of Nanoemulsions of Some Essential Oils against Fusarium oxysporum and Root-Knot Nematode Meloidogyne javanica on Coleus Plants
Eman Alsayed Hammad1* and Mahmoud Mohamed Hassanin Hasanin2
1Agriculturl Research Center (ARC), Plant Pathology Research Institute, Nematode Diseases Research Department, Giza, Egypt; 2Ornamental, Medicinal and Aromatic Plant Disease Department, Plant Pathology Research Institute, Agricultural Research Center (ARC), Giza Egypt.
Abstract | Efficacy of nanoemulsions and emulsions of thyme and spearmint essential oils were examined as an alternative to chemical control for suppressing Meloidogyne javanica and Fusarium oxysporum on coleus plants, Coleus forskohlii in vitro and greenhouse conditions. Nanoemulsions of thyme (droplets size was in the range of 25.4–32.9 nm) and spearmint (droplets size was in the range of 5.91–9.77 nm) at concentrations 4000 and 5000ppm separately, recorded the best results in vitro investigations, completely prevented F. oxysporum growth at all concentrations, and increased M. javanica mortality by 100%, in comparison to the non-treated control. In the greenhouse, thyme and spearmint essential oils nanoemulsions and emulsions (at 5000 ppm) affected significantly on the final population (Pf) of M. javanica (671.8 and 1072.4), while emulsions of spearmint and thyme nanoemulsion completely infection prevented after 50 days from planting in infested soil with F. oxysporum, but thyme and spearmint essential oils nanoemulsions and emulsions recorded effect on Pf about 1193.6 and 1465.6, respectively, compared to Fenamiphose (328.4) and fungicide, Occidor 50% SC was completely infection prevented. A similar pattern was discovered in a greenhouse with a positive effect on increased shoot dry weight for coleus plants, where thyme and spearmint essential oil nanoemulsions at (5000ppm) achieved 3.3 and 3.9 g/plants for root-knot nematode infected plants, respectively, compared to 2.7 and 4.4 g/plants for F. oxysporum infected plants.
Received | January 23, 2022; Accepted | May 13, 2022; Published | June 10, 2022
*Correspondence | A. Eman Hammad, Agriculturl Research Center (ARC), Plant Pathology Research Institute, Nematode Diseases Research Department, Giza, Egypt; Email: dreman.hammad@yahoo.com
Citation | Hammad, A.E., and Hassanin, M.M.H., 2022. Antagonistic effect of nanoemulsions of some essential oils against Fusarium oxysporum and root-knot nematode Meloidogyne javanica on coleus plants. Pakistan Journal of Nematology, 40(1): 35-48.
DOI | https://dx.doi.org/10.17582/journal.pjn/2022/40.1.35.48
Keywords | Essential oil, Nanoemulsions, Fusarium oxysporum, Root-knot nematode Meloidogyne javanica, Thyme, spearmint, Coleus, Coleus forskohlii
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Coleus, Coleus forskohlii (Wild) Briq. Belonging to the family Lamiaceae is one of the commercial ornamental plants grown extensively in Egypt and cultivated primarily in India, Sri Lanka, Nepal, and Yunnan Province in China. It is used as a bedding plant for public gardens in Egypt. Coleus forskohlii is susceptible to many diseases, of which root rot and wilt disease caused by Fusarium oxysporum is a major constraint throughout the world (Miao et al., 2021; Halawa et al., 2018). Symptoms of the disease, which included wilting of the leaves, discolouration of the stem to brown and root rot (Zheng et al., 2012), However, root tubers are especially susceptible to the root-knot nematode Meloidogyne incognita Chitwood, which can reduce yields by up to 86 percent (Senthamarai et al., 2006a). Root-knot nematode also makes the root system vulnerable to infections by disease-causing fungi and bacteria. Meloidogyne incognita and M. arenaria have been shown to induce root-knot disease in C. forskohlii (Seenivasan and Devrajan, 2008) showed that M. incognita infestations cause yield reductions of up to 86 percent, whereas M. arenaria infestations produce severe loses in C. forskohli (Bhandari et al., 2007; Kulkarni et al., 2007) identified a collar rot the complex including C. forskohlii, F. chlaydosporum, and Rhizoctonia bataticola (Macrphomonia phaseolina). C. forskohlii has also been linked to a complex illness involving both fungal and nematode infections (Senthmarai et al., 2006b). Essential oils are compounds extracted from plants. The oils capture the plant’s scent and flavor. Essential oils are also known as volatile oils, ethereal oils. The antifungal activity of essential oils against phytopathogens have previously been reported. For instance, lemongrass and thyme oils exhibited complete inhibition against Fusarium oxysporum (Baioumy, 1997). Also, soil treating with essential oils of Eucalyptus citriodora Hook., E. globulus Labill., Pelargonium asperum Ehrh. ex. Spreng. and Ruta graveolens L. reduced Meloidogyne sp. (Kofoid and White) Chitwood multiplication and gall formation on tomato roots (Laquale et al., 2015). Since Meloidogyne sp. is an endoparasite, a translatable compound that could affect the nematode inside root plants is desirable. This compound could be found in the essential oils, once it contains several antimicrobial ingredients that work through various modes of action. Eugenol, a constituent of Ocimum sanctum L. and others, have shown effect on the viability of nematodes, in addition to a systemic the effect (Bala and Sukul, 1987; Li et al., 2013; Moreira et al., 2013). Using Nanotechnology is a tool to modify nano-scale material characteristics, to improve the properties of the essential oils (Huang et al., 2010). The difference between essential oil emulsion and essential oil nanoemulsion is in the size of the oil particles. The stability of the emulsion is significantly improved when the size of the oil particles becomes small. Surfactants are added to oil-water mixture to enhance the kinetic stability of such a system. A surfactant is an amphiphilic molecule that has a hydrophilic head group (polar region), which has a high affinity for water, and a lipophilic tail group (non-polar region), which has a high affinity for oil (Anton and Vandamme, 2011). Essential oils incorporated in nanoemulsions seem to penetrate faster in the microbial membranes due to the increased area per weight unit. This would allow reducing the concentration to achieve an equivalent or even greater microbial effect over conventional emulsions (Odriozola-Serrano et al., 2014). Therefore, the objective of this study was to assess the effects of emulsion and nanoemulsions of essential oils against Fusarium oxysporum and Meloidogyne javanica root-knot nematodes pathogenic infecting coleus plants to develop a management strategy for these pathogens.
Materials and Methods
Extraction, preparation and analysis of essential oils
Fresh herb of spearmint (Mentha spicata L.) and thyme (Thymus vulgaris L.) were collected from El-Kanater El- Khairia Farm, Medicinal and Aromatic Plants Research Department, Horticulture Research Institute, Agricultural Research Center, Egypt. Samples from each plant were then shade dried and subjected to essential oil extraction. The essential oils were extracted by hydrodistillation using a Clevenger apparatus for 4 hours. Ten ml of each essential oil and 5 ml of non-ionic surfactant Tween 80 was added slowly with gentle stirring until a homogeneous mixture formed. Then, water (85 ml) was added to reach the final mixture of each oil to 100 ml, then stirred using a magnetic stirrer for 30 min. The mixture was sonicated using an Ultrasonicator (Bandelin Sonopuls HD 2200, Germany) for 30 min. at 700 W, all the treated essential oil was placed in an ice bath during the time of work. The particle size of 10% essential oils nanoemulsion for each amount was detected by Hydrodynamic light scattering analyzer (DLS) after 90 days of storage at room temperature (27˚C). Essential oils emulsions were prepared as mentioned above before without sonication. Essential oils were extracted from the nanoemulsions by redistillation. The Gas chromatography analysis of the essential oil samples was carried out using Ds Chrom 6200 Gas Chromatograph apparatus, fitted with capillary column BPX-5, 5 phenyls (equiv.) polysillphenylene-siloxane 30 x 0.25 mm ID x 0.25μ film. The temperature the program varied in the range of 70-200 °C, at a rate of 10 °C/min. Flow rates of gases were nitrogen at 1 ml/min, hydrogen at 30 ml/min and 330 ml/min for air. Detector and injector temperatures were 300 °C and 250 °C, respectively. This work was performed by Laboratories of Medicinal and Aromatic Plants Research Department, Horticulture Research Institute and Research Department of Ornamental, Medicinal and Aromatic Plants Diseases, Plant Pathology Research Institute, Agricultural Research Center, Egypt.
Measurement of nanoemulsion droplet size
Measurement of droplet size of nanoemulsions was performed by dynamic light scattering analyses using Zeta Nano ZS (Malvern Instruments, UK) at room temperature. Before measurement, 30 μl of each nanoemulsion was diluted with 3ml of water at 25 ˚C. Particle size data were expressed as the mean of the Z-average of 3 independent batches of the nanoemulsions. The droplet size and the poly disparity index (PDI) of the formulated nanoemulsion were measured. This work was performed by Nanotechnology Laboratory, Regional Center for Food and Feed, ARC, Giza, Egypt according to (Ghotbi et al., 2014).
Transmission electron microscopy (TEM)
Twenty micro liters of diluted samples were placed on a film-coated 200-mesh copper specimen grid for 10 min and the excess fluid was eliminated using a filter paper. The grid was then stained with one drop of 3 % phosphotungstic acid and allowed to dry for 3 min. The coated grid was dried and examined under the TEM microscope (Tecnai G20, Super twin, double tilt, FEI, The Netherlands), operating at 200 kV (Saloko et al., 2013).
Extraction of Meloidogyne javanica
Root-knot nematode eggs were isolated from the egg masses on the roots of the Coleus blumei plant using the Hussey and Barker (1973) method. The eggs were transferred to flasks and incubated at 28°C for three days, after which the eggs hatched in water and active juveniles (J2s) of M. javanica were collected, according to Coyne et al., (2007). A calculated suspension comprising viable juveniles was employed as an inoculum.
Source of F. oxysporum
The isolate of F. oxysporum used in this study was obtained from the fungal collection of Ornamental, Medicinal and Aromatic Plant Dis. Dept., Plant Pathol. Res. Inst. ARC, Giza, Egypt (Halawa et al., 2018).
Effect of emulsions and nanoemulsions of spearmint and thyme essential oils on inhibition of F. oxysporum and M. javanica in vitro
Nematode, M. javanica: The inhibitory impact of the usual particle size of spearmint and thyme essential oil nanoemulsion against second-stage juveniles (J2s) of M. javanica was determined using five rates (1000, 2000, 3000, 4000, and 5000ppm). Second stage juveniles were extracted, counted, and concentrated in suspension until they reached contented 1 ml of distilled water roughly 100 J2s, according to the methodology reported by Demeure et al. (1981). 1 ml of juvenile suspension was placed into screw-capped test tubes containing 5 ml of various rates of tested materials and incubated at 26±2°C for 3 days, with the number of active and inactive juveniles counted at 24, 48, and 72 hours using a nematode counting slide (Hussey, 1973). Each treatment was repeated five times. The distilled water serve as a control. The Schneider and Orelli’s formula was used to compute the revised mortality percentages (Schneider and Orelli, 1947).

Fusarium oxysporum: The efficacy of volatile substances in reducing fungal growth was tested. Essential oils of spearmint and thyme (emulsions and nanoemulsions) were added to sterilized PDA flasks before solidifying to obtain the proposed concentrations of 1000, 2000, 3000, 4000 and 5000 ppm (v/v). The bactericide (Chloramphenicol, 0.1mg/l) was added to the medium to avoid bacterial contamination. Three plates for each treatment were inoculated with discs (5-mm-diam.) of F. oxysporum. Petri dishes were incubated at 27±1°C is randomized complete design. Percentages of fungal growth inhibition was calculated when the fungal growth of the control plates filled the plates according to the formula as follows:
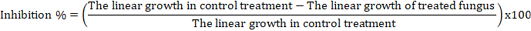
Greenhouse experiments
Essential oils emulsions and nanoemulsions of spearmint and thyme at the concentration (5 ml/L water) were tested for controlling C. forskohlii wilt and root rot diseases caused by a root-knot nematode, M. javanica and F. oxysporum fungus in pot experiments compared with treatments of nematicide, Fenamiphose 40 % EC. (Common name: Nemacur, Chemical composition: Ethyl-3methyl-4 (methyl thiophenyl)-methyl-ethyl) phposphoramidate. (6 ml/100 L water) and fungicide, Occidor 50% SC [Common name: Carbendazim, Chemical composition: Methyl 2-benzimidazole carbamate, Manufacture: Agriphar S.A., Belgium.] (2 g/L water) and untreated plants under greenhouse conditions during spring season 2021.
Three seedlings of coleus (20 days old) was planted in 25 cm plastic pot. Pots containing a mixture of clay and sandy soil (1:1 w/w). The coleus seedlings were infected with F. oxysporum at the rate of 1% (w/w), and 1000 newly hatched juveniles (J2s) of M. javanica for each one kg soil, so each pot contained 3000 hatched juveniles (J2s) of M. javanica plant after one week from seedlings. Each application was repeated five times, with the application of each spearmint and thyme essential oil nanoemulsion. As well as the same applications with nematicides Fenamephose (6 ml/100 L water) and fungicides Occidor 50% SC (2 g/L water) were replicated five times. All treatments were applied as a soil drench. Five inoculated by M. javanica and another separated inoculated by F. oxysporum pots were left without adding any materials as a negative control, in addition to another five replicated healthy seedlings without inoculation with nematodes and fungus as positive control. All pots were arranged in the greenhouse at 27±5˚C in randomized block design. After fifty days of inoculation with M. javanica and F. oxysporum, the plants were harvested. Percent dead plants were recorded (Booth, 1971) and juveniles of M. javanica were extracted from one kg. soil/pots by sieving and modified Baeman technique (Seinhorst, 1962). Roots were stained by acid fuchsin (Bybd et al., 1983) and examined under a stereomicroscope for counting developmental stages, females, galls, and egg masses. Root galling or egg masses were rated on a scale of 0-5 where 0= no galls or egg masses, 1= 1-2 galls or egg masses, 2= 3-10 galls or egg masses, 3= 11-30 galls or egg masses, 4= 31-100 galls or egg masses, 5= more than 100 galls or egg masses per root system (Taylor and Sasser, 1978). At the end of the experiment, shoot length, root length, shoot weight, root weight and shoot dry weight per plant were also recorded.
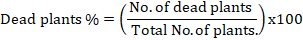
Data analysis
The data were subjected to analysis of variance (ANOVA) (Gomez and Gomez, 1984) and Duncan’s multiple range tests in the form of probability (P ≤0.05) (Duncan, 1955) using Costat software was used to separate means.
Results and Discussion
Chemical composition of essential oils emulsions and nanoemulsions
Chemical composition of investigated essential oils emulsions and nanoemulsions analyzed by gas chromatography is presented in Table 1. Carvone (60.03%) and Limonene (16.63%) were identified as major constituents of spearmint essential oil emulsion. Thymole (23.62%) and ρ-cymene (52.36%) were identified as the major constituents of thyme essential oil emulsion. Changes in the essential oil components were observed in the nanoemulsions compared with the corresponding original essential oils emulsions. Data presented in Table 1 show the main component content of spearmint oil nanoemulsions Carvone and ρ-cymene (66.34% and 7.36%, respectively) were increased, also, in thyme oil nanoemulsions was Borneol increased (9.03%), while, ρ-cymene and Thymol (29.18% and 14.40%, respectively) were decreased compared with essential oil emulsion.
Effect of ultrasonication on nanoemulsion droplet size
The effect of ultrasonication on the droplet size of essential oils of spearmint and thyme nanoemulsions was determined. Figure 1A shows the stable essential oil nanoemulsions prepared by ultrasonication method for 30 min. at 700 W after 3 months of storage under room temperature. Tween 80 was used as the surfactant for its high HLB value that favors the formulation of oil in water emulsion. Also, small molecule surfactants like Tween 80 gets rapidly adsorbed onto emulsion droplet surface and hence they are more effective in decreasing droplet diameter than polymeric surfactants (Ghosh et al., 2014). Spearmint nanoemulsion droplets were tiny (around 6.4 nm) (Figure 1). Thyme nanoemulsion droplets were tiny (around 48.1 nm) (Figure 2A) (Sampathi et al., 2015; Hassanin et al., 2017).
Transmission electron microscopy (TEM)
Transmission electron microscopy characterization of spearmint and thyme essential oil nanoemulsions gives the actual size and shape, the droplets in the nanoemulsion appears dark. The TEM micrograph showed that the essential oils nanoemulsions were spherical and moderately mono or di-dispersed. Spearmint nanoemulsion droplets were in the range of 5.91–9.77 nm (Figure 1B). Thyme nanoemulsion droplets were in the range of 25.4 – 32.9 nm (Figure 2B). The droplet size was correlated well with the results obtained from droplet size analysis using the dynamic light scattering (Abd-Elsalam and Khokhlov, 2015; Hassanin et al., 2017).
Table 1: Chemical composition of essential oils emulsions and nanoemulsions.
|
Components (%) |
Essential oils of |
|||
|
Spearmint (Mentha spicata L.) |
Thyme (Thymus vulgaris L.) |
|||
|
Emulsion |
Nanoemulsion |
Emulsion |
Nanoemulsion |
|
|
α-thujene |
- |
- |
- |
- |
|
α-pinene |
1.07 |
0.72 |
1.76 |
1.63 |
|
Camphene |
- |
- |
1.83 |
1.76 |
|
Sabinene |
- |
- |
- |
|
|
β-pinene |
- |
- |
1.87 |
1.76 |
|
β-myrcene |
2.49 |
1.18 |
1.75 |
7.25 |
|
α-terpinene |
- |
- |
- |
- |
|
ρ-cymene |
1.27 |
7.36 |
52.36 |
29.18 |
|
Limonene |
16.63 |
4.96 |
0.96 |
6.74 |
|
1,8-cineol |
6.54 |
2.04 |
- |
- |
|
β-ocimene |
1.48 |
1.58 |
1.10 |
9.45 |
|
γ-terpinene |
- |
- |
- |
- |
|
α –terpinolene |
- |
- |
2.09 |
3.98 |
|
Linalool |
- |
- |
- |
- |
|
Menthone |
- |
- |
- |
- |
|
Iso menthone |
- |
- |
- |
- |
|
Menthofuran |
- |
- |
- |
- |
|
Borneol |
- |
- |
5.80 |
9.03 |
|
Menthol |
- |
- |
- |
- |
|
Terpinene-4-ol |
- |
- |
- |
- |
|
γ-terpineol |
1.12 |
1.47 |
- |
- |
|
Dihydrocarveol |
3.32 |
3.83 |
- |
- |
|
Dihydrocarveon |
0.29 |
2.41 |
- |
- |
|
Methyl chavicol |
- |
- |
- |
- |
|
Carvone |
60.03 |
66.34 |
- |
- |
|
Menthyl acetate |
- |
- |
- |
- |
|
Thymol |
- |
- |
23.62 |
14.40 |
|
Eugenol |
- |
- |
- |
- |
|
β-caryophyllene |
- |
0.95 |
2.65 |
0.79 |
|
Caryophyllene oxide |
- |
2.59 |
- |
- |
In vitro efficacy of essential oils emulsions and nanoemulsions on two bioagantis
Mycelial growth of F. oxysporum: Data in Tables 2 and 3, represented the effect of spearmint and thyme essential oil emulsions and essential oil nanoemulsion solutions at five concentrations of 1000, 2000, 3000, 4000 and 5000 ppm (v/v) on juveniles mortality of M. javanica and mycelial growth of F. oxysporum. Data in Table 2, indicated that increasing concentration of essential oil emulsions and nanoemulsions can be correlated with a decrease in linear fungal growth. Nanoemulsion of thyme oil completely inhibited growth of F. oxysporum at the 2000 ppm concentration. While, spearmint essential oil nanoemulsion completely inhibited the growth of F. oxysporum at the 4000 ppm concentration. Nanoemulsions were always the most active against fungal growth at all concentrations. The biggest difference between emulsion and nanoemulsion is in the size of the nanoemulsion particles.
The mortality percentage of M. javanica
The effect of spearmint and thyme essential oil emulsions and essential oil nanoemulsion solutions at five concentrations of 1000, 2000, 3000, 4000, and 5000 ppm (v/v) on M. javanica juvenile mortality was reflected in Table 3 and Figure 1. When compared to the non-inoculated control, all of the tested therapies were found to generate a significant increase in juvenile M. javanica death %. In general, the effect of the essential oil emulsions and nanoemulsions investigated varies depending on the concentrations and exposure time. Whereas, in terms of the effect of the relationship between concentration and exposure time as a steady relationship, all treatments were defined by a stable line, as this offered a stable perception of the effective value, especially at higher concentrations. According to the findings, the higher rates (5000 ppm) of thyme and spearmint essential oil nanoemulsions resulted in the highest mortality increase percentages (100%) at the end of the 24-hour test. When evaluating the middle effect of the lowest rates (2000 and 3000ppm) of thyme and spearmint essential oil emulsions (1000 ppm), which was the polar opposite of what was expected as a biomaterial, a reduced percentage of the morality of juvenile to M. javanica juvenile was reached, respectively, of 61.8 and 37.5%.
Table 2: Effect of essential oils emulsions and nanoemulsions at different concentrations on mycelial growth of F. oxysporum
|
Mean |
Linear growth (cm) of mycelial growth at different concentrations (ppm) |
Treatments |
|||||
|
5000 |
4000 |
3000 |
2000 |
1000 |
Control |
||
|
5.5 |
0.0 i |
3.0fg |
5.1d |
7.5 b |
8.3 ab |
9.0 a |
Spearmint emulsion |
|
4.6 |
0.0 i |
0.0 i |
4.6 e |
6.0 c |
7.9 b |
9.0 a |
Spearmint nanoemulsion |
|
2.4 |
0.0 i |
0.0 i |
0.0 i |
1.5 h |
3.9 f |
9.0 a |
Thyme emulsion |
|
2.0 |
0.0 i |
0.0 i |
0.0 i |
0.0 i |
2.7 g |
9.0 a |
Thyme nanoemulsion |
|
- |
Treatments (T)= 0.5 Concentrations (C) = 0.1 T X C = 0.2 |
L.S.D. at 5% : |
|||||
Each value is the mean of five replicates. Means in each column followed by the same letter(s) are not significantly different (P≤0.05) by Duncan’ s multiple range test.
Greenhouse experiments
Evaluation of spearmint and thyme essential oil emulsions and essential oil nanoemulsion solutions against Fusarium oxysporum wilts of coleus plants and root-knot nematodes in a greenhouse setting (M. javanica). This investigation looked at the effects of two essential oil emulsions and two essential oil nanoemulsion solutions on wilt disease and root-knot incidence and severity % on coleus plants inoculated with F. oxysporum and M. javanica in greenhouse settings.
Table 3: Evaluation of thyme and spearmint essential oil nanoemulsion solutions on the mortality percentage of second stage juveniles of M. javanica at different exposure periods in vitro (25±2 °C).
|
Treatments |
concentrations (ppm) V/V |
Mortality % |
||
|
24h. |
48h. |
72h. |
||
|
Spearmint emulsion |
1000 |
35.7 |
52.6 |
70.1 |
|
2000 |
49.7 |
68.9 |
84.3 |
|
|
3000 |
66.5 |
81.2 |
97.2 |
|
|
4000 |
78.9 |
96.6 |
100 |
|
|
5000 |
100 |
100 |
100 |
|
|
Spearmint nanoemulsion |
1000 |
62.1 |
76.2f |
82.3 |
|
2000 |
73.6 |
82.0 |
89.7 |
|
|
3000 |
85.1 |
90.1 |
99.9 |
|
|
4000 |
96.3 |
100 |
100 |
|
|
5000 |
100 |
100 |
100 |
|
|
Thyme emaulsion |
1000 |
47.8 |
63. |
86.7 |
|
2000 |
61.8 |
79.8.6 |
96.0 |
|
|
3000 |
77.8 |
88.9 |
98.7 |
|
|
4000 |
87.2 |
96.7 |
100 |
|
|
5000 |
100 |
100 |
100 |
|
|
Thyme nanoemaulsion |
1000 |
61.8 |
75.3 |
91.2 |
|
2000 |
75.1 |
90.2 |
99.1 |
|
|
3000 |
82.6 |
91.3 |
100 |
|
|
4000 |
94.3 |
100 |
100 |
|
|
5000 |
100 |
100 |
100 |
|
|
Control |
- |
1.4 |
2.9 |
3.8 |
Each value presented the mean of five replicates.
Evaluation of spearmint and thyme essential oil emulsions and essential oil nanoemulsion solutions against F. oxysporum: Drenching soil with various control treatments resulted in an increase in coleus resistance against infection with the tested fungi and nematode (Table 4). Data in Table 4 indicate that all treatments reduce disease incidence. Spearmint emulsion, thyme emulsion, thyme nanoemulsion and fungicide, Occidor 50% SC completely infection prevented after 50 days from planting in infected soil with F. oxysporum compared with the control. Whereas spearmint emulsion and spearmint nanoemulsion completely infection prevented after 50 days from planting in infected soil with nematodes compared with the control. The contrast, spearmint nanoemulsion was the least effective treatment in decreasing disease incidence (%) in infected soil with F. oxysporum, while thyme emulsion was the least effective treatments in decreasing disease incidence (%) in infected soil with the nematode.
Table 4: Effect of various control treatments as soil drenching on incidence (%) of root rot and wilt disease of coleus plants 50 days after planting in soil infected with pathogenic root-knot nematode, M. javanica and F. oxysporum fungus, under greenhouse conditions (27±3 °C).
|
Treatment |
% Dead plants after 50 days |
% Plant survival |
|||
|
Fungi |
Fungi+ Nematode |
Fungi |
Fungi+ Nematode |
||
|
Spearmint emulsion |
0.0 c |
0.0 d |
100 a |
100 a |
|
|
Spearmint nanoemulsion |
13.3 b |
0.0 d |
86.7 b |
100 a |
|
|
Thyme emaulsion |
0.0 c |
20.0 b |
100 a |
80 b |
|
|
Thyme nanoemaulsion |
0.0 c |
6.7 c |
100 a |
93.3 a |
|
|
Occidor 50% SC |
0.0 c |
0.0 d |
100 a |
100 a |
|
|
Untreated plant(inoculated) |
40.0 a |
53.3 a |
60 c |
46.7 c |
|
|
Untreated plant(uninoculated) |
0.0 c |
0.0 d |
100 a |
100 a |
|
|
LSD at 0.05 |
1.5 |
2.1 |
12.4 |
8.6 |
|
Each value is the mean of five replicates. Means in each column followed by the same letter(s) are not significantly different (P≤0.05) by Duncan’ s multiple range test.
Table 5: Effect of spearmint and thyme essential oils emulsions and nanoemulsions solutions on plant length of coleus infected with, M. javanica and F. oxysporum under greenhouse conditions (27±3°C).
|
Treatment |
Shoot length |
Root length |
|||||
|
Nematode |
Fungi |
Nematode + Fungi |
Nematode |
Fungi |
Nematode + Fungi |
||
|
Spearmint emulsion |
30.7d |
36.7d |
32.4d |
6.0b |
7.1cd |
6.4d |
|
|
Spearmint nanoemulsion |
37.7c |
43.5c |
40.0c |
9.0ab |
9.7bc |
7.4bc |
|
|
Thyme emaulsion |
49.4ab |
52.7b |
41.4bc |
12.4a |
6.4de |
9.7b |
|
|
Thyme nanoemaulsion |
52.7a |
61.7a |
43.4bc |
6.7b |
16.7a |
9.4b |
|
|
Fenamephose and/or Occidor 50% SC |
47.1b |
45.2c |
45.9b |
8.4ab |
5.4 de |
9.5b |
|
|
Untreated plant (inoculated) |
27.8d |
30.3e |
27.2e |
6.7b |
4.4e |
3.2c |
|
|
Untreated plant (uninoculated) |
52.7a |
52.7b |
52.7a |
12.4a |
12.4b |
12.4a |
|
|
L.S.D. at 5%: |
4.49 |
4.26 |
4.91 |
4.27 |
3.02 |
2.36 |
|
Each value is the mean of five replicates. Means in each column followed by the same letter(s) are not significantly different (P≤0.05) by Duncan’ s multiple range test.
Effect of experimental spearmint and thyme essential oil emulsions and nanoemulsion solutions on growth parameters of coleus plants which afflicted with pathogenic M. javanica and F. oxysporum under greenhouse conditions: Data presented in Table 5 show that all treatments tested gave significant increases in shoot length compared with the controls (without treatment) in soil infected with each of pathogenic root-knot nematode, M. javanica and F. oxysporum fungus, except spearmint emulsion with soil infested with the nematode, however, thyme nanoemulsion was the superior treatments in increasing shoot length compared with the other treatments in cases of nematode and fungi followed by thyme emulsion treatment. Whereas (Fenamephose + Occidor 50% SC) was the superior treatment in increasing shoot length compared with the other treatments in cases of (nematode + fungi). In this respect, spearmint emulsion was the least effective treatment in increasing shoot length with the nematode, fungi and (nematode + fungi) tested. On the other hand, thyme emulsion was the superior treatments in increasing root length compared with the other treatments in cases of nematode and (nematode + fungi), while thyme nanoemulsion was the superior treatments in increasing root length compared with the other treatments in cases of fungi.
Also, data in Table 6 show that spearmint nanoemulsion was the superior treatment in increasing shoot weight, root weight and shoot dry weight compared with the other treatments in cases of nematode and/or fungi, except root weight and shoot dry weight in case of (nematode + fungi), while thyme nanoemulsion treatment was the superior treatments in increasing root weight compared with the other treatments in cases of (nematode + fungi), whereas (Fenamephose + Occidor 50% SC) was the superior treatments in increasing shoot dry weight compared with the other treatments in cases of (nematode + fungi).
Efficacy of tested spearmint and thyme essential oils emulsions and nanoemulsions solutions against M. javanica: The purpose of this study was to see how they tested materials (spearmint and thyme essential oil emulsions and essential oil nanoemulsion solutions 5000 ppm. and Fenamiphose) affected the population of M. javanica infected coleus plants during the regular growing season (2021). In general, the current study found that all tested treatments reduced root-knot nematode parameters, such as the number of root galls, build-up, and nematode reduction, to varying degrees, as compared to control and chemical nematicide. All treatments resulted in a significant reduction in the total number of root-knot juvenile nematodes in the soil and on the roots of coleus plants, as shown in Figure 3 and Tables 7, with nematode build-up rates ranging from 0.35 percent for infected only with M. javanica to 0.5 percent for infected only with M. javanica and F. oxysporum for Fenemiphose to 3.46 and 2.89 percent Fenemiphose provided the greatest reduction in the nematode population, root galls index, and egg masses for M. javanica witch infested coleus plants only (328.4, 13.9, and 9.2), while in complex infection by M. javanica and F. oxysporum, Fenemiphose provided the the greatest reduction in the nematode population, root galls index, and egg masses for M. javanica witch infested coleus plants only (328).
Following that, Thyme nanoemulsion solutions 5000 ppm resulted in significant reductions in nematode population, root galls index, and egg masses (671.8, 26.7, and 17.7) for M. javanica alone infested coleus plants, while mixed infection yielded (695.9, 29.5, and 19.6).
In both cases, infection to coleus plants, Spearmint nanoemulsion, and Thyme emulsion solutions 5000 ppm caused a medium reduction in nematode population, root galls index, and egg masses for root-knot nematode. Infected only by M. javanica 1465.6 and 1582.3 for infected with root-knot nematode and F. oxysporum, respectively, Spearmint emulsion, solutions 5000 ppm, had the lowest nematode population levels, with infected only by M. javanica 1465.6 and 1582.3 for infected with root-knot nematode and F. oxysporum, respectively. Root gall index and egg masses of M. javanica infested coleus plants or F. oxysporum revealed a similar pattern of treatments (Table 7).
Changes in the essential oil components were observed in the nanoemulsions compared with the corresponding original essential oils emulsions. Carvone and Limonene were identified as major
Table 6: Impact of spearmint and thyme essential oils emulsions and nanoemulsions solutions on fresh and dry weight of coleus plant infected with M. javanica and F. oxysporum under greenhouse conditions(27±3°C).
|
Treatments |
Shoot weight |
Root weight |
Shoot dry weight |
||||||||
|
Nema-tode |
Fungi |
Nematode + Fungi |
Nema-tode |
Fungi |
Nematode + Fungi |
Nema-tode |
Fungi |
Nematode + Fungi |
|||
|
Spearmint emulsion |
20.7cd |
20.8c |
17.7c |
5.83d |
4.7b |
2.8d |
3.4a |
3.1bc |
2.6b |
||
|
Spearmint nanoemulsion |
26.8a |
27.0a |
20.1ab |
7.47a |
5.63a |
5.7b |
3.9a |
4.4a |
2.6b |
||
|
Thyme emaulsion |
23.2bc |
26.3a |
19.5b |
3.73g |
5.57a |
5.8b |
1.8bc |
3.7ab |
2.7b |
||
|
Thyme nanoemaulsion |
24.9ab |
23.3b |
17.1cd |
6.46b |
3.37d |
6.2a |
3.3a |
2.7c |
1.4c |
||
|
Fenamephose and/or Occidor 50% SC |
24.1ab |
20.8c |
16.2e |
4.13f |
4.03c |
2.9d |
2.9ab |
4.1a |
4.1a |
||
|
Untreated plant (inoculated) |
18.1d |
20.8c |
16.2de |
6.1c |
3.53d |
5.6b |
1.5c |
1.2d |
0.9c |
||
|
Untreated plant (uninoculated) |
20.1cd |
20.8c |
21.4a |
4.83e |
4.83b |
4.8c |
3.8a |
3.8ab |
3.8a |
||
|
L.S.D. at 5%: |
2.9 |
2.5 |
0.99 |
0.175 |
0.23 |
0.27 |
1.26 |
0.96 |
0.95 |
||
Each value is the mean of five replicates. Means in each column followed by the same letter(s) are not significantly different (P≤0.05) by Duncan’ s multiple range test.
Table 7: Efficiency of essential oil emulsions and essential oil nanoemulsion solutions on population density of M. javanica alone or interacted with Fusarium wilt disease under greenhouse condition(27±3°C).
|
Treatment |
Nematode final population |
Galls (RGI) |
Egg masses (GI) |
|||||
|
Nematode |
Nematode+ Fungi |
Nematode |
Nematode+ Fungi |
Nematode |
Nematode+ Fungi |
|||
|
Spearmint emulsion |
1465.6b |
1582.3b |
60.8b |
67.1b |
40.2b |
44.6b |
||
|
Spearmint nanoemulsion |
1072.4d |
1171.8c |
44.9c |
49.7cd |
29.1c |
32.9b |
||
|
Thyme emaulsion |
1193.6c |
1221.6c |
46.8c |
51.8c |
31.1c |
36.5b |
||
|
Thyme nanoemaulsion |
671.8e |
695.9d |
26.7d |
29.5d |
17.7d |
19.6c |
||
|
Fenamiphose |
328.4f |
361.9e |
13.9e |
15.4e |
9.2d |
12c |
||
|
Control |
3490.6a |
3645.8a |
139.7a |
154.7a |
92.7a |
102.8a |
||
|
L.S.D. at 5% |
50.9 |
233.4 |
11.41 |
13.5 |
10.2 |
12.3 |
||
Each value is the mean of five replicates. Means in each column followed by the same letter(s) are not significantly different (P≤0.05) by Duncan’ s multiple range test. Nematode final population= No. of nematode in one Kg. soil + one root for plant.
constituents of spearmint essential oil emulsion. Thymole and ρ-cymene were identified as the major constituents of thyme essential oil emulsion.
Data presented show that main component content of spearmint oil nanoemulsions Carvone and ρ-cymene were increased, also, in thyme oil nanoemulsions was Borneol increased, while, ρ-cymene and Thymol were decreased compared with essential oil emulsion.
The components of essential oils include different groups of distinct biosynthetically origin. The main group is composed of terpenoids, phenylpropanoids, and short-chain aliphatic hydrocarbon derivatives, which are all characterized by low molecular weight. Terpenes are made from combinations of several 5- carbon-base (C5) units called isoprene and form structurally and functionally different classes. The biosynthesis of the terpenes consists of synthesis of the isopentenyl diphosphate (IPP) precursor, repetitive addition of IPPs to form the prenyldiphosphate precursor of the various classes of terpenes, modification of the allylic prenyldiphosphate by terpene specific synthetases to form the terpene skeleton, and, finally, secondary enzymatic modification (redox reaction) of the skeleton to attribute functional properties to the different terpenes (Bilia et al., 2014). It is suggested that, maybe there was a breakdown of ultrasonic chemical bonds during the manufacture of the nanoemulsion. Once again the atoms are redistributed through the donor atom, which carries a positive charges such as the hydrogen atom and the recipient the atom with a negative charge such as the oxygen atom.
The compounds are then formed within aromatic oils again but at different concentrations. In vitro and in vivo investigations, the results showed that all treatments, including thyme and spearmint essential oil nanoemulsions, solutions, have a positive effect on F. oxysporum, and M. javanica. At 4000 and 5000 ppm concentrations from two essential oil nanoemulsion were used to completely stop F. oxysporum growth at all concentrations. Nanoemulsions have been working efficiently for six months, compared to ordinary oil emulsions. The most notable difference between emulsion and nanoemulsion is the size of the nanoemulsion particles.
According to the data, the highest concentrations (5000 ppm) of thyme and spearmint essential oil nanoemulsions resulted in the highest mortality reduction percentages (100%) for M. javanica at the end of the 24-hour test. In vivo experiments, the results observed in decreasing diseases incidence (%) in infested soil with F. oxysporum, while and a greater reduction in the final population M. javanica juveniles in the soil. The most notable difference between emulsion and nanoemulsion is the size of the nanoemulsion particles. According to the data, the highest concentrations (5000 ppm) of thyme and spearmint essential oil nanoemulsions resulted in the highest mortality percentages (100%) at the end of the 24-hour test.
The main finding was that essential oil nanoemulsion solutions were more effective as a natural nematicide on M. javanica and a fungicide on F. oxysporum. The size of the nanoemulsion particles is the difference between emulsion and nanoemulsion.
The emulsion’s stability improves considerably when the size of the oil particles is lowered (Anton and Vandamme, 2011). Nanomaterials (tiny particles) are a type of material (Zedan et al., 1994). Because essential oils (which have low water solubility) are larger than nanoemulsion particles, they cannot easily interact with cell membranes. However, nanoemulsion particles can deliver essential oils to the surface of nematode cell membranes, which could be related to the ability of smaller particles to kill or hinder the nematode at any stage of its life cycle (Pérez et al., 2003; Barbosa et al., 2010).
Nanoemulsions are generated from a specific the concentration of oil phase, surfactant, and water, with no phase separation, and are kinetically stable for more than six months at room temperature (Abd-Elsalam and Khokhlov, 2015; Hassanin et al., 2018). The ability and performance of surfactants may influence the size reduction of droplets. In oil in water emulsion, stirring is known to diminish droplet size (Sajjadi et al., 2002). Dai et al. (1997) investigated the synthesis of nanoemulsions with smaller droplet sizes in the presence of double bonds in the nonpolar chain of non-ionic surfactants. The findings were consistent with previous findings (Shahavi et al., 2015).
Essential oils as nanoemulsions or natural nematicides, on the other hand, have a variety of efficiency mechanisms (Park et al., 2005; Laquale et al., 2015). The chitin penetration of the cell wall destroys the lipoprotein cytoplasmic membrane, enabling cytoplasm to escape, resulting in antifungal action. Or, as Mendes et al. (2018) suggested, the nanoemulsion’s antipictide activity was boosted while cytotoxicity was lowered. In this study, a nanoemulsion containing mint essential oil extract and chitosan has nematicidal activity against root-knot nematodes with low cytotoxicity in a human cell line (Kumar et al., 2019).
Because the optimal sample contained chitosan-containing mint essential oil nanoemulsion extract, it is reasonable to speculate that the presence of chitosan can govern the nanoemulsion’s size, nucleation, and nematicidal activity. Essential oil emulsion and nanoemulsion components may adversely affect nematodes nervous system. Another possibility is that essential oils disrupt the cell membrane of the nematode and change its permeability.
This mechanism has also been suggested to explain the fungicidal activity of essential oils. Aldehydes of essential oil components may cause irreversible changes to protein structures, especially those located on the nematode surface, like formaldehyde and other aldehydes. Interestingly, benzaldehyde and furfural (D2-furaldehyde) have been found to attract C. elegans at low concentrations (Bargmann et al., 1993). Also, the antifungal activity of essential oils emulsion and nanoemulsion against F. oxysporum was reported by (Hassanin et al., 2017). The essential oil can penetrate and disrupt the fungal cell wall and cytoplasmic membranes, permeabilize them and finally damage mitochondrial membranes.
The changes in electron flow through the electron transport system inside the mitochondria damage the lipids, proteins, and nucleic acid contents of the fungal cells (Arnal-Schnebelen et al., 2004). The nematicidal action of benzaldehyde in conjunction with thymol on M. javanica J2 has been extensively studied (Serratosa et al., 1995). In soil infested with root-knot nematodes, a combination of chitin and benzaldehyde increased tomato plant growth and health (Kokalis-Burelle et al., 1999). Citral, an aliphatic aldehyde found in essential oils like Cymbopogon grasses, and perillaldehyde have also been reported to exhibit nematicidal properties (Sangwan et al., 1985; Tsao and Yu, 2000). In addition, sabinene, myrcene, and trans-caryophyllene concentrations in Thyme essential oil nanoemulsions, which is a group of terpenoid compounds, sabinene plays a role in nematicidal activity (Santana et al., 2014; Bahmani et al., 2020; Sarkar, 2020).
According to the chemical makeup of thyme essential oil nanoemulsion, the primary components are sabinene, myrcene, and trans-caryophyllene, which may be responsible for its anti-nematode capabilities (Sangwan et al., 1990; Oka et al., 2000). The gains in plant growth metrics might be attributable to biochemical changes in the stem base tissues, or they could be owing to their effectiveness in partially or preventing disease infection and development. Peroxidase enzyme activity, growth hormones, and phenol chemicals all increase as a result of this shift. Zedan et al. (2011) and Hassanin (2013) observed somewhat comparable results on several crops in naturally or artificially contaminated soil.
Conclusions and Recommendations
The efficacy of treatments with thyme and Spearmint essential oil nanoemulsions compared to Nematicide and fungicide resulted in higher coleus development, indicating a viable use as an eco-friendly nematode and fungal control technique.
Novelty Statement
Developing effective methods for using natural extracts in the soil to reduce nematode populations and boost plant productivity.
Author’s Contribution
Both Authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References
Abd-Elsalam, K.A. and Khokhlov, A.R., 2015. Eugenol oil nanoemulsion: antifungal activity against Fusarium oxysporum f. sp. vasinfectum and phytotoxicity on cotton seeds. Appl. Nanosci., 5: 255–265. https://doi.org/10.1007/s13204-014-0398-y
Arnal-Schnebelen, Hadji-Minaglou, F., Peroteau, J.F., Ribeyre, F. and Billerbeck, V.G.D., 2004. Essential oils in infectious gynaecological disease; a statistical study of 658 cases. Int. J. Aromather., 14(4): 192–197. https://doi.org/10.1016/j.ijat.2004.09.003
Anton, N. and Vandamme, T.F., 2011. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm. Res., 28: 978–985. https://doi.org/10.1007/s11095-010-0309-1
Bahmani, M., Jalilian, A., Somayeh, I., Shahsavari, S. and Abbasi, N., 2020. Phytochemical screening of two ilam native plants ziziphus nummularia (Burm. f.) Wight and Arn. and Ziziphus Spina-Christi (Mill.) Georgi Using HS-SPME and GC-MS Spectroscopy. Plant Sci. Today, 7(2): 275–280. https://doi.org/10.14719/pst.2020.7.2.714
Baiuomy, M.A.M., 1997. Studies on the inhibitory activity of different plants against some casual pathogens. Ph.D. thesis, Fac. Agric. Al-Azhar Univ., pp. 145.
Bala, S.K. and Sukul, N.C., 1987. Systemic nematicidal effect of eugenol. Nematropica, 17(2): 219–222.
Barbosa, P., Lima, A.S.P., Vieira, L.S, Dias, M.T., Tinoco, J.G., Barroso, L.G., Pedro, A.C., Figueiredo, and Manuel, M., 2010. Nematicidal activity of essential oils and volatiles derived from portuguese aromatic flora against the pinewood nematode, Bursaphelenchus Xylophilus. J. Nematol., 42(1): 8.
Bargman, C.I., Hartwieg, E. and Horvitz, H.R., 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell, 74: 515-527. https://doi.org/10.1016/0092-8674(93)80053-H
Bhandari, S., Harsh, N.S.K. and Singh P., 2007. First report on Meloidogyne arenaria on coleus forskohlii in India. Ind. Forester, 133: 1709-1710.
Bilia, A.R., Guccione, C., Isacchi, B, Righeschi, C., Firenzuoli, F., and Bergonzi, M.C., 2014. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med., Article ID 651593, 14 pages. https://doi.org/10.1155/2014/651593
Booth, C., 1971. The genus Fusarium. Commonwealth Mycol. Inst., Kew, Surrey, UK, pp. 235.
Bybd, J.R., Kirkpatrick, D.W.T., and Barker, K.R., 1983. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol., 15(1): 142.
Coyne, D., Nicol, J., and Claudius-Cole, A., 2007. Practical plant nematology: Field and laboratory guide. II TA, Ibadan, Nigeria, pp. 28.
Demeure, Y.V., Diana, E.S., and Freckman, W., 1981. Recent advances in the study of anhydrobiotic nematodes. Plant Parasitic Nematodes, 3: 205–226. https://doi.org/10.1016/B978-0-12-782203-7.50014-5
Dai, L., Li, W., and Hou, X., 1997. Effect of the molecular structure of mixed nonionic surfactants on the temperature of miniemulsion formation. Colloids and Surf. A-physicochemical and Eng. Aspects, 125: 27-32. https://doi.org/10.1016/S0927-7757(96)03859-9
Duncan, D.B., 1955. Multiple range and multiple. F-test Biometrics, 11(1): 1-42. https://doi.org/10.2307/3001478
Ghotbi, R.S., Khatibzadeh, M. and Kordbacheh, S., 2014. Preparation of neem seed oil nanoemulsion. Proceedings of the 5th International Conference on Nanotechnology: Fundamentals and Applications Prague, Czech Republic, August 11-13, Paper No. 150-1.
Ghosh, V., Mukherjee, A. and Chandrasekaran, N., 2014. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces, 114: 392–397. https://doi.org/10.1016/j.colsurfb.2013.10.034
Gomez, K.A. and Gomez, A.A., 1984. Statistical procedures for agricultural research. John Wiley and Sons.
Halawa, A.E.A., Ali, A.A.M. and Hassanin, M.M.H., 2018. Efficiency of some organic acids as safe control mean against root and stem rot disease of Coleus forskohlii. J. Phytopathol. Pest Manag., 5(2): 48-62.
Hassanin, M.M.H., 2013. Pathological studies on root rot and wilt of black cumin (Nigella sativa) and their management in Egypt. Ph. D. thesis, Fac. Agric., Al-Azhar Univ. (Egypt), pp. 137.
Hassanin, M.M.H., Abd-El-Sayed, M.A. and Abdallah, M.A., 2017. Antifungal activity of some essential oil emulsions and nanoemulsions against Fusarium oxysporum pathogen affecting cumin and geranium plants. Sci. J. Flowers Ornament. Plants, 4(3): 245-258. https://doi.org/10.21608/sjfop.2017.11326
Hassanin, M.M.H., Mohamed, N.T. and Abd-El-Sayed, M.A., 2018. Fungicidal activity of nanoemulsified essential oils against Botrytis Leaf Blight of poinsettia (Euphorbia pulcherrima) in Egypt. Egypt. J. Agric. Res., 96(4): 1259-1273. https://doi.org/10.21608/ejar.2018.141420
Huang, Q., Yu, H. and Ru, Q., 2010. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci., 75(1): R50-R57. https://doi.org/10.1111/j.1750-3841.2009.01457.x
Hussey, R.S., 1973. A comparison of methods of collecting inocula of Meloidogyne Spp., including a new technique. Plant Dis. Rep., 57: 1025–1028.
Hussey, R.S. and Barker, K.R., 1973. A comparison on methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep., 57: 1925-1928.
Kokalis-burelle, N., Rodriguez-KabaNa, R. and Klop-Per, J.W., 1999. Organic amendments and natural chemicals as components of transplant mixes for control of root knot nematode. Phytopathology, 89(6 Sup): S41.
Kulkarni, M.S., Ramprasad, S., Hedge, Y., Laxminarayan, H. and Hedge, N.K., 2007. Management of collar rot complex disease of Coleus forskohlii (Wild) Briq. Using bioagents, organic amendments and chemicals. Biomedicine, 2: 37-40.
Kumar, S., Monika N., Dilbaghi, N., Giovanna, M., Hassan, A.A., and Ki-Hyun, K., 2019. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Controlled Release, 294: 131–153. https://doi.org/10.1016/j.jconrel.2018.12.012
Laquale, S., Candido, V., Avato, P., Argentieri, M.P. and Addabbo, T.D., 2015. Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Annals Appl. Biol., 167(2): 217–224. https://doi.org/10.1111/aab.12221
Li, H.Q., Liu, Q.Z., Liu, Z.L., Du, S.S. and Deng, Z.W., 2013. Chemical composition and nematicidal activity of essential oil of agastache rugosa against Meloidogyne incognita. Molecules, 18(4): 4170–4180. https://doi.org/10.3390/molecules18044170
Mendes, J.F., Martins, H.H.A., Otoni, C.G., Santana, N.A., Silva, R.C.S., Da Silva, A.G., Silva, M.V., Correia, M.T., Machado, S.G. and Pinheiro, A.C.M., 2018. Chemical composition and antibacterial activity of eugenia brejoensis essential oil nanoemulsions against pseudomonas fluorescens. LWT, 93: 659–564. https://doi.org/10.1016/j.lwt.2018.04.015
Miao, Y.H., Chen, Q.H., Wang, Y.H. and Liu, D.H., 2021. First report of Fusarium wilt of Coleus forskohlii caused by Fusarium oxysporum in China. J. Plant Dis., 105: 1559. https://doi.org/10.1094/PDIS-11-20-2489-PDN
Moreira, L.C.B., Vieira, B.S., Mota Júnior, C.V. da, Lopes, E.A. and Canedo, E.J., 2013. Ação nematicida do eugenol em tomateiro. Pesquisa Agropecuária Tropical, 43(3): 286– 291. https://doi.org/10.1590/S1983-40632013000300011
Odriozola-Serrano, I., Oms-Oliu, G. and Martin-Belloso, O., 2014. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front. Nutr., 1: https://doi.org/10.3389/fnut.2014.00024
Oka, Y., Nacar, A., Putievsky, E., Ravid, U., and Spiegel, Y., 2000. Nematicidal Activity of Essential Oils and Their Components against the Root-Knot nematode. Phytopathology. Vol. 90: 711-715. https://doi.org/10.1094/PHYTO.2000.90.7.710
Park, Il-Kwon, Ju-Yong, P., Kyung-Hee, K., Kwang-Sik, C., In-Ho, C., Chul-Su, K., and Sang-Chul, S., 2005. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology, 7(5): 767–774. https://doi.org/10.1163/156854105775142946
Pérez, M.P., Juan, A., Navas-Cortés, Pascual-Villalobos, M.J. and Pablo, C., 2003. Nematicidal activity of essential oils and organic amendments from asteraceae against root-knot nematodes. Plant Pathol. 52(3): 395–401. https://doi.org/10.1046/j.1365-3059.2003.00859.x
Sajjadi, S., Zerfa, M. and Brooks, W.B., 2002. Dynamic behavior of drops in oil/ water/ oil dispersions. Chem. Eng. Sci., 57: 663-675. https://doi.org/10.1016/S0009-2509(01)00415-8
Sarkar, S., 2020. Incidental finding of root knot symptoms in lavandula angustifolia mill: First report from India. J. Med. Plants, 8(4): 292–299.
Saloko, S., Darmadji, P., Setiaji, B., Pranoto, Y. and Anal, A.K., 2013. Encapsulation of coconut shell liquid smoke in chitosan-maltodextrin based nanoparticles. Int. Food Res. J., 20: 1269-1276.
Sampathi, S., Mankala, S.K., Ankar, W., and Dodoala, J.S., 2015. Nanoemulsion based hydrogel of itraconazole for transdermal drug delivery. J. Sci. Ind. Res., 74: 88-92.
Sangwan, N.K., Verma, K.K., Verma, B.S., Malik, M.S. and Dhindsa, K.S., 1990. Nematicidal activity of some essential plant oils. Pest Manag. Sci., 28(3): 331-335. https://doi.org/10.1002/ps.2780280311
Sangwan, N.K., Verma, K.K., Verma, B.S., Malik, M.S. and Dhindsa, K.S., 1985. Nematicidal activity of essential oils of Cymbopogon grasses. Nematologica, 31: 93-99. https://doi.org/10.1163/187529285X00120
Santana, O., Andres, M.F., Sanz, J., Errahmani, N., Abdeslam, L. and Coloma, A.G., 2014. Valorization of essential oils from moroccan aromatic plants. Nat. Prod. Commun., https://doi.org/10.1177/1934578X1400900812
Schneider, P. and Orelli, O., 1947. Entomologisches praktikum (Entomological internship). Verlag. H. R. Sauerländer Co., Aarau, Switzerland. pp. 237.
Seenivasan, N. and Devrajan, K., 2008. Management of Meloidogyne incognita on medical Coleus by commercial biocontrol formulations. Nematol. Medit., 36: 61-67.
Senthamarai, M., Poornima, K. and Subramanian, S., 2006a. Pathogenicity of Meloidogyne incognita on Coleus forskohlii Briq. Indian J. Nematol., 36: 123- 125.
Senthamarai, M., Poornima, K., and Subramanian, S., 2006b. Bio-management of root knot nematode, Meloidogyne incognita on Coleus forskhlii Briq. Indian J. Nematol., 36: 206-208.
Seinhorst, J.W., 1962. Modifications of the elutriation method for extracting nematodes from soil. Nematologica, 8(2): 117–128. https://doi.org/10.1163/187529262X00332
Shahavi, M.H., Hosseini, M., Jahanshahi, M., Meyer, R.L. and Darzi, G.N., 2015. Clove oil nanoemulsion as an effective antibacterial agent: Taguchi optimization method. Desalination and water treatment, pp. 1-12. https://doi.org/10.1080/19443994.2015.1092893
Serratosa, A.S., Kokalis, N.B., Rodriguez, R.K., Weaver, C.F., and King, P.S., 1995. Allelochemicals for control of plant-parasitic nematodes. 1. In vivo nematicidal efficacy of thymol and thymol/ benzaldehyde combinations. Nematropica, 26: 57-71.
Tsao, R. and Yu, Q., 2000. Nematicidal activity of monoterpenoid compounds against economically important nematodes in agriculture. J. Essential Oil Res., 12: 350-354. https://doi.org/10.1080/10412905.2000.9699533
Taylor, A.L. and Sasser, J.N., 1978. Biology, identification and control of root-knot nematodes. North Carolina State University Graphics, pp. 111.
Zheng, L., Liu, J., Liu, T. and Jiang, D., 2012. Fusarium wilt of Coleus forskohlii caused by Fusarium oxysporum in China. Can. J. Plant Pathol., 34: 310-314. https://doi.org/10.1080/07060661.2012.688771
Zedan, A.M., E1-Toony, A.M. and Awad, N.G., 1994. Comparative study on antifungal activity of certain plant extracts, essential oils and fungicides on tomato wilt pathogens. A1-Azhar J. Agric. Res. (Egypt.), 20: 217-236.
Zedan, A.M., Arab, Y.A., El-Morsy, S.A. and Hassanin, M.M.H., 2011. Pathological studies on root rot and wilt of black cumin (Nigella sativa) and their management in Egypt. Egypt. J. Appl. Sci., 26(4): 89-108.
To share on other social networks, click on any share button. What are these?





