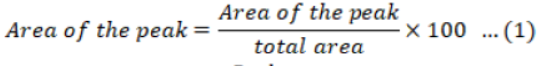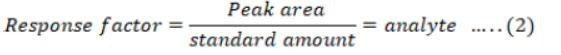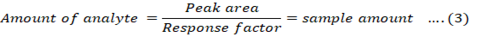Analysis of Pesticide Residues in Pollen and Nectar Samples from Various Agricultural Areas of Pakistan through High Performance Liquid Chromatography
Analysis of Pesticide Residues in Pollen and Nectar Samples from Various Agricultural Areas of Pakistan through High Performance Liquid Chromatography
Mahnoor Pervez and Farkhanda Manzoor*
Department of Zoology, Lahore College for Women University, Lahore, Pakistan
Abstract | The present study was devised with an aim to assess pesticide risk toward honeybees (Apis mellifera L.) by detecting pesticide residues in pollen and nectar samples collected from various agricultural areas of Pakistan through high performance liquid chromatography analysis. Pollen and nectar were collected from 2016 to 2017 from different agricultural areas of Pakistan during a field survey. Pollen was collected by installing traps and nectar was collected through capillary method allied with centrifugation. The HPLC technique was used for fast and simple detection of pesticide residues of commonly used pesticides (imidacloprid, deltamethrin, chlorpyrifos, fipronil, thiamethoxam, carbaryl, profenophos and bifenthrin). Among a total of 100 pollen samples, 47% were found to be positive. The most frequently found residues were imidacloprid (11%), thiamethoxam (7%) and carbaryl (6%). Among all 100 nectar samples, 36% samples were positive and most abundant pesticides in nectar were imidacloprid (8%), thiamethoxam (6%) and fipronil (5%). In pollen samples, the residual level of imidacloprid and thiamethoxam was detected at a level of 0.032 - 0.76 ng/g and 0.055 - 0.78 ng/g, respectively while in nectar samples imidacloprid and thiamethoxam were detected at a level of 0.009 - 0.53 ng/g and 0.045 -0.76 ng/g, respectively. The results obtained suggest careful monitoring of pesticide used in order to save honeybee population.
Received | February 02, 2019; Accepted | November 14, 2019; Published | January 10, 2020
*Correspondence | Farkhanda Manzoor, Department of Zoology, Lahore College for Women University, Lahore, Pakistan; Email: doc_farkhanda@yahoo.com
Citation | Pervez, M. and F. Manzoor. 2020. Analysis of pesticide residues in pollen and nectar samples from various agricultural areas of Pakistan through high performance liquid chromatography. Sarhad Journal of Agriculture, 36(1): 1-9.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.1.1.9
Keywords | Pollen, Nectar, Honeybees (A. mellifera), HPLC, Pesticide residues
Introduction
The long term security of insect pollination for maximum food crops is a major concern around the world (Aizen et al., 2008; Potts et al., 2010). Beekeepers have suffered major decline of honeybee population (Engelsdorp and Meixner, 2010; Engelsdorp et al., 2011). The decline in their population is attributed to extensive use of various pesticides (Williamson et al., 2013). Pesticides used on crops can drift by wind on surrounding areas (Porrini et al., 2003). Organochlorine pesticides persist in the environment and are bio-accumulated in pollens and plant tissues (Ruiz et al., 2018). Systematic pesticides are applied on seed coating. (Schmuck et al., 2001; Tapparo et al., 2011; Jeschke et al., 2011; Bonmatin et al., 2015). These pesticides travel through treated seeds to entire plant parts including flowers. Contaminated pollens and nectars taken up by foraging honeybees during foraging activities expose the hive bees to pesticides (Ornates et al., 2010). These residues are fed to the developing larvae and queen thus, possess greater risk to the colony and make it vulnerable to colony collapse (Tapparo et al., 2012). Exposure of honeybees to pesticides through contaminated pollen and nectar consumption impairs their natural foraging behavior. Lately, owing to climatic vulnerability, the honeybee population in Pakistan is at a sharp decline. This has alarmed all the stakeholders equally (Irshad and Stephen, 2014; Nafees et al., 2008). To the best of our knowledge, no work has been reported regarding assessment of pollen and nectar pesticide residues effects on honeybees.
The present study was hence, devised with the main objective of assessing pesticide risk toward honeybees through detection of pesticide residues in their pollen and nectar samples collected from various agricultural areas of two provinces Punjab and Khyber Pakhtunkhwa, Pakistan using HPLC multi-residual analysis.
Materials and Methods
Study area
Pollen and nectar samples were collected from different agricultural areas of Pakistan viz. Khyber Pakhtunkhwa province (Peshawar and Swat Districts) and Punjab (Sargodha, Bhalwal, Sahiwal, Faislabad, Multan and Lahore Districts). In selected areas, different pesticides were used for the control of pests. In Punjab, the selected apiary sites had wheat (Triticum aestivum), corn (Zea mays), Sarsso (Brassica compestris) (crops), mango (Mangifera indica), orange (Citrus sinensis), ziziphus (Ziziphus jujube) and mulberry (Morus alba) orchards while in Khyber Pakhtonkhwa corn crops and orchards of apple (Malus domestica), almond (Prunus dulcis), cherries (Prunus avium) and peaches (Prunus persica) were present.
Pollen collection
At each studied site bee hives were placed. Pollen traps were installed in front of the hive. As foraging worker bees enter in the hive pollen grains dislodge from their body and fall in the trap draw. Pollen loads were gathered and stored in the glass vials at -20ºC until analysis.
Nectar collection
Nectar was collected from flowers through capillary action (Stoner and Eitzer, 2012) allied with centrifugation. Sepals and petals were removed to exposed flower bases where nectarines were present. Nectar drop was collected through capillary action of micropipette. In the same way, for small-sized flowers, nectar was collected through centrifugation. A small hole was made in the bottom of centrifuge tube with a needle. Flowers were placed in this tube and this tube was inserted into another centrifuge tube. Tubes were centrifuged at 12,000 rpm for 20 minutes until all nectar was collected at the bottom of the second tube. Nectar was collected with the help of micropipette and stored in to PTFE line tubes at -20ºC until analysis.
Extraction
Neonicotinoid pesticide residues were extracted by protocol of Chauzat et al. (2006). Pollen grains (10gram) were ground with water and few drops of acetonitrile were added to make it slurry. For imidacloprid and thiamethoxam analysis, samples were acidified by adding few drops of sulphuric acid (H2SO4). The slurry was centrifuged at 12,000×g for about 20 minutes. Supernatant was taken and filtered by passing through microfilter paper (0.45µm pore size). After filtration the sample was evaporated to dryness and dissolved dry sample residues in acetonitrile. They were re-filtered by passing through microfilters (0.45µm pore size). Resultant samples were stored in freezer at -20ºC until analysis. Acetonitrile was used as extracting solvent for neonicotinoid residues extraction.
Similarly, for detection of other pesticide residues, similar procedure was performed except that the samples were not acidified by H2SO4. For bifenthrin, deltamethrin and fipronil acetonitrile: water (76:24) was used as extractant solvent. Profenophos residues were detected by methanol: water (70:30) extractant. For chlorpyrifos and carbamate residue analysis, method of Manzoor et al. (2012) was used. For chlorpyrifos and carbamate, 10 grams of pollens were taken and slurry was made by adding water and acetone. Acetone: Water (70: 30) was used as extracting solvent for chlorpyrifos and carbaryl. Slurry was added to Erlenmeyer flasks (250ml) along with 5ml of acetone: water (70:30 v/v). All flasks were placed into the horizontal shaker incubator set at 170rpm and 28 ºC for 2 hours for agitation. The extractant solvent (acetone: water) was evaporated by placing samples in rotary evaporator. To remove fat content and other organic debris, extracts were cleaned by passing through the separatory funnel. Aqueous phase eluted from separatory funnel collected and stored in PTFE lined screw cap glass vials after filtration at -5 ºC (to avoid degradation) until analysis.
HPLC analysis
All samples and mobile phase were filtered by passing through filtration assembly (microfilters 0.45 µm pore size) apparatus. Samples were sonicated at 31°C for15 minutes. All extracted pollen and nectar samples were analyzed separately using HPLC (Varian 9012 pump) system. Validate HPLC instrument by using different combination of HPLC graded acetonitrile and water solvents. Acetonitrile/water (70:30 v/v) showed best extraction efficiency, giving straight base line without noise peaks. Finally, acetonitrile/water (70:30 v/v) was used as mobile phase for validation of HPLC instrument as well as pesticide residues in unknown samples. A reverse phase C18 column with 250 × 4.6mm internal diameter and 5µm particle size was used for pesticide analysis. Analysis was carried out under isocratic conditions at a flow rate of 1.0 ml/min, temperature 28°C and high pressure about 20Mpa. Injection volume for each sample was 10µl. The running time for each standard was 20 minutes. Peak analysis was carried out by using UV detector set at 270nm and 204nm.
Standard preparation
Technical grade of powder form of fipronil (Termadore from BASF), bifenthrin and deltamethrin (FMC Corporation, Pakistan), imidacloprid, thiamethoxam (Sigma-Aldrich), carbaryl, prophenophos, chlorpyrifos with (99.9% purity) were purchased from Ali Akbar Group of Industry. Standard stock solutions were prepared by dissolving standard in acetonitrile (0.5, 1, 1.5, 2 ng/ml).
Statistical analysis
Data collection and peak analysis were performed using Breeza chromatography workstation connected to a computer. Standard curve was plotted between absorbance and known concentrations (0.5, 1, 1.5 and 2 ng/ml) of pesticide standards as shown in Figure 1. Standard curve values were used as reference values to calculate the unknown pesticide concentrations in collected pollen and nectar samples. Percentages of contaminated pollen and nectar samples were calculated by dividing the number of positive samples by the total number of samples. Descriptive statistic was used to calculate minimum and maximum residual level in particular samples by using Graph pad prism (version 4). Limit of detection (LOD) and limit of quantification (LOQ) values were calculated for all pesticides by using following formulas.
LOD= 3.3 (STD/slope) and LOQ= 10 (STD/ slope)
Amount of the pesticides was calculated from the peak area by using Equation 1, 2 and 3.
Table 1: Percentage frequency of pesticide residues detection in pollen grains and nectar samples.
|
Pesticide detected
|
Pollen samples N= 100 |
Nectar samples N= 100 |
| Fipronil | 3.0% | 5.0% |
| Deltamethrin | 2.0% | 3.0 % |
| Chlorpyrifos | 2.0% | 4.0 % |
| Imidacloprid | 11.0% | 8.0% |
| Thiamethoxam | 7.0% | 6.0% |
| Profenophos | 3.0% | ND |
| Carbaryl | 6.0% | 4.0% |
| Bifenthrine | 2.0% | 4.0% |
| Carbaryl + Thiamethoxam | 4.0% | ND |
| Bifenthrin+ Imidacloprid | 3.0% | ND |
| Bifenthrin+ Fipronil | 2.0% | ND |
| Carbaryl+ Imidacloprid | 2.0% | 2.0% |
| Total | 47% | 36% |
ND*: Not detected.
Results and Discussion
Total 100 pollen and 100 nectar samples were analyzed. Acetonitrile/water (70:30) was used as mobile phase for validation of HPLC instrument as well as pesticide residues in unknown samples. Chromatogram of technical grade standards obtained at optimized conditions were shown in Figure 2A and 2B. Among a total of 100 pollen samples, 47% were found to be positive. The most frequent residues were imidacloprid (11.0%), thiamethoxam (7.0%) and carbaryl (6.0%) as shown in Table 1. About 11% Pollen samples polluted with multiple pesticides residues. Mean level of pesticide residues in pollen samples is shown in Table 2. Pollen sample chromatograms were shown in Figure 3A and 3B. Retention time (RT) of unknown pollen sample peaks was compared with the RT of standard peaks. Chromatogram peak of unknown pollen samples appeared near to standard peak retention time (RT) were consider as respective
pesticide residue. HPLC analysis showed that as carbaryl standard run, a peak appeared at 2.796 min retention time (RT). Any peak in unknown pollen and nectar samples appeared near to it was considered as carbaryl peak. Based on pollen HPLC analysis, carbaryl was detected at a level 0.032 - 0.60 ng/g. For imidacloprid and thiamethoxam, standard peaks appeared at 5.46 min and 6.58 min RT respectively. Residual level of imidacloprid and thiamethoxam was detected at a level 0.032 - 0.76 ng/g and 0.055 - 0.78 ng/g, respectively. Similarly, for bifenthrin standard peak appeared at 4.057 min RT and deltamethrin standard peak seemed at 3.394 min RT. Analysis showed that bifenthrin and deltamethrin residues were detected at a level 0.001-0.20 ng/g and 0.007-0.54 ng/g, respectively. In the same way, fipronil standard run a sharp peak appeared at 8.621min RT. The fipronil residue detected at a level 0.026-0.65 ng/g. Chlorpyrifos showed peak at 19.56 min and profenophos standard showed peak at 15.25 min RT. Analysis showed that chlorpyrifos and profenophos residues were detected at a level 0.025 - 0.56 ng/g and 0.02 – 0.40 ng/g respectively. LOD and LOQ values of insecticides residues detected in pollens were shown in Table 2. Box plot shows graphical representation of pesticide residues in pollen samples (Figure 5).
About 100 nectar samples were collected and analyzed. Among all nectars, 36% samples were positive. The most abundant pesticide in nectar was imidacloprid (8.0%), thiamethoxam (6.0%) and fipronil (5.0%). Mean LOD and LOQ values of pesticides detected in nectar samples were shown in Table 3. For each pesticide residue analysis in nectar pesticide standard were run. Chromatogram peaks of unknown nectar samples were shown in Figure 4A and 4B. Retention time (RT) of standard peaks compared with RT of unknown sample peak. Peak of unknown nectar samples appeared near to standard peak retention time (RT) were consider as respective pesticide residue. HPLC results showed that in nectar samples carbaryl residue was detected at a level (0.032-0.035 ng/g), imidacloprid (0.009-0.53 ng/g), deltamethrin (0.008-0.32ng/g), thiamethoxam (0.045-0.76 ng/g), bifenthrin (0.038-0.19 ng/g) and fipronil (0.056-0.19 ng/g). Box plot shows graphical representation of pesticide residues in nectar samples (Figure 6). Systematic pesticides (thiamethoxam and imidacloprid) were detected at maximum level in both type samples. While deltamethrin and bifenthrin were also detected at considerable amount.
Pesticide usage for insect management has come a long way. Pakistan is an agricultural area. Various chemicals are used to manage pests and insects. Presence of pesticide residues in pollen and nectar samples is a serious concern as they directly affect pollinator. Pesticide residues in pollen and nectar are
Table 2: Mean, limit of detection (LOD) and limit of quantification (LOQ) values of pesticides residues detected in pollen samples.
| pesticides detected | Mean (ng/g) | Min-max (ng/g) | Sum | Std. Error mean | Std. Deviation | LOD (ng/g) | LOQ (ng/g) |
| Carbaryl | 0.668 | 0.032-0.60 | 4.161 | 0.070 | 0.252 | 0.0026 | 0.0020 |
| Profenophos | 0.377 | 0.02-0.40 | 0.723 | 0.085 | 0.170 | 0.0013 | 0.0020 |
| Imidacloprid | 0.751 | 0.032-0.76 | 3.876 | 0.075 | 0.261 | 0.0051 | 0.0016 |
| Deltamethrin | 0.933 | 0.007-0.54 | 2.091 | 0.135 | 0.359 | 0.0011 | 0.0013 |
| Thiamethoxam | 0.632 | 0.055-0.78 | 1.915 | 0.156 | 0.313 | 0.0018 | 0.0022 |
| Bifenthrin | 0.638 | 0.001-0.20 | 0.903 | 0.1005 | 0.2463 | 0.0012 | 0.0023 |
| Fipronil | 0.174 | 0.026-0.65 | 0.509 | 0.029 | 0.870 | 0.0015 | 0.0001 |
| Chlorpyrifos | 0.735 | 0.025-0.56 | 1.12 | 0.1709 | 0.3919 | 0.0018 | 0.0011 |
Table 3: Mean, limit of detection (LOD) and limit of quantification (LOQ) values of pesticides residues detected in nectar samples.
| Pesticides detected | Mean (ng/g) | Min-Max (ng/g) | Sum | Std. Error mean | Std. Deviation | LOD (ng/g) | LOQ (ng/g) |
| Carbaryl | 0.31 | 0.032-0.35 | 1.047 | 0.042 | 0.111 | 0.0012 | 0.0023 |
| Imidacloprid | 0.521 | 0.009-0.53 | 1.819 | 0.079 | 0.1941 | 0.0013 | 0.0023 |
| Deltamethrin | 0.321 | 0.008-0.32 | 1.210 | 0.067 | 0.231 | 0.0011 | 0.0027 |
| Thiamethoxam | 0.725 | 0.045-0.76 | 2.525 | 0.1259 | 0.2816 | 0.0045 | 0.0012 |
| Bifenthrin | 0.152 | 0.038-0.19 | 0.444 | 0.0376 | 0.0752 | 0.0030 | 0.0009 |
| Fipronil | 0.425 | 0.056-0.19 | 0.465 | 0.064 | 0.153 | 0.0048 | 0.0015 |
taken by forager bees to their colonies and remain in the hive food for quite some time. These residues are then fed to the larvae and queen which are affected in similar ways as the forager bees (Sanchez and Goka, 2014). This seems evident through the results harvested from the present study.
In the present study, systematic pesticide residues were detected at a highest level. Pollen and nectar samples collected from areas of Punjab province contained more pesticide residues as compared to those from KPK province. In Punjab cotton and wheat are major crops, on which various chemicals are used to control pests at different growing stages, while in KPK mostly selected areas had fruit orchards which were sprayed before flower blooming. Previous study also supported these results. Hayat et al. (2018) stated that higher proportion of pesticides being used in Punjab Province (88.3%) followed by Sindh (8.2%), Khyber Pakhtunkhwa (2.8%) and Balochistan (0.76%). These results correlated with those of Blacquiere et al. (2012). They demonstrated that bees exposed to neonicotinoid pesticides toxicity via contaminated pollen and nectar ranged between 0.9-3.1 ng/g. Similarly, in another study, imidaclopid residues ranging from 2 to 5 ng/g in pollen and >1.5 ng/g in nectar of treated seed of corn, sunflower and rape (Maus et al., 2003; Bonmatin et al., 2005) poses sublethal and lethal effects toward forging honeybees. In present study imidacloprid (0.751 ng/g) and thiamethoxam (0.632 ng/g) residue level in pollen level were close to reported values and showed potential threat of sublethal toxicity toward foraging and nurse bees. In another study Pohorecka et al. (2012) studied sublethal toxicity of thiamethoxam treated seeds toward bees. Consumption of thiamethoxam pesticide residues intake (0.4- 3.3 ng/bee/day) were lead to disruption of learning and memory. Similarly, in another study residue of chlorpyrifos at a level of 0.072 µg were meet oral LD50 (Sanchez and Goka, 2014). Similarly, consumption of fipronil active ingredient in the range of 0.07- 0.15 ng were had deleterious effects toward honeybee learning performance. Literature showed that consumption of 33 grams of contaminated pollen by one individual would be needed to meet the oral LD50 (Chauzat et al., 2006). Brood and adult bees fed with pollen or bee bread and are directly and indirectly exposed to pesticide residues. Different researchers have been focused on quantifying the amount of pollen needed to rear a larvae. It had been shown that worker honeybee larvae required average 86 mg of maize pollen for complete development (Babendreier et al., 2004). So, bees consumed contaminated pollen and nectar in such quantity would be enhanced possible risk of pesticide poisoning in forging, nurse and larvae honeybees. Similarly, Chauzat et al. (2006) conducted pesticide residue analysis in pesticides. They were conducted a field survey to monitor honeybee (A. mellifera) population decline in France, multiple residual analysis was done on collected pollen grains. About 19 pesticide residues were found in pollen samples. Boily et al. (2013) evaluated that in Quebe rapid decline of honeybee (A. mellifera) population attributed to pesticide exposure. Another study conducted by Bonmatin et al. (2005) for the quantification of pesticide residues in maize crops. It was concluded that maize crops and flowers contained pesticide residues at a level sufficient to cause bee mortality. Pesticide used for treatment of seeds can be transported throughout growing plants and contaminate the nectars and pollens. The presence of imidaclopride residues (3µg/kg) in pollen grains has been reported in Gaucho seed dresses sunflower (Bonmatin et al., 2003). Similarly, a survey of pesticide residues in pollen loads was conducted in France. Survey report was showing the presence of imidacloprid and its metabolites nicotic acid (49% of 81 analyzed samples), Fipronil and its metabolites fipronil sulfone and fipronil disulfinile (12% of 81 analyzed samples) (Chauzat and Faucon, 2007). In present study level of pesticide residues in nectar samples less compared to pollens, as depending upon the treatment regimen and type of crop (Kyriakopoulou et al., 2017). Pollen grains were collected from corn, wheat and brassica crops, as these crops have large proportion of pollen compared to nectar and frequently sprayed at different developmental stages. While nectar collected from ornamental and fruit flowers which were less likely sprayed with pesticides after blooming. These results correlated with Dively and Kamel (2012). They measured neonicotinoid residues in pollen and nectar from pumpkin crops. Results showed that in nectar pesticide residues were 73.5 to 88.8% less than pollen residues. In present study deltamethrin and bifenthrin detected in considerable amount. These pesticides are lipophilic in nature and easily absorbed in plant body. The presence of pesticide residues in pollen and nectar not only caused mortality but also lead to sublethal toxicity such as disrupt normal flight, learning and memory (Decourtye et al., 2005; Aliouane et al., 2009). Exposure of bees to low levels of pesticide residues can elicit sublethal toxicity, not killing them outright but affecting their behavior and immune system (Desneux et al., 2007). So, it can be infer from present study pesticides which were detected even at low level can induce sublethal toxicity toward bees.
Conclusions and Recommendations
In conclusion, this study has been demonstrated the presence of wide range of pesticides in nectar and pollen grains collected by honeybees. These pesticides were found at various concentrations and provide possible route of exposure. Systematic insecticides were detected at highest residual level and imidacloprid was most persistent pesticide in all collected samples. Imidacloprid and thiamethoxam residues level in pollen and nectars were close to previously known sublethal values. Other pesticide residues were also detected in considerable amount. Thus, represent a serious concern in agriculture sector of Pakistan as they possess greater risk to honeybee colony development.
Acknowledgements
The paper is based on original research conducted at Entomology lab of Lahore College for Women University, Lahore. The data presented in said article has neither been submitted elsewhere and nor is under consideration of publication at any other journal. All the authors are well-conversant with the material of the article and have willingly presented their consent for its consideration at Sarhad Journal of Agriculture.
Novelty Statement
This is a novel approach to monitor the level of pesticide residues in pollen and nectar because contaminated nectars and pollen grains are used as food source by hive bees, therefor have deleterious effects on brood and colony development. The developed analytical procedure (HPLC) was suitable for the detection of pesticides in pollen and nectar.
Author’s Contribution
Mahnoor Pervez: Collected the samples, designed methodology, analysed the data and wrote the article.
Farkhanda Manzoor: Supervised the research, facilitated in sample collection, helped in article writing.
References
Aizen, M.A., L.A. Garibaldi, S.A. Cunningham and A.M. Klein. 2008. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 18: 1572 - 1575. https://doi.org/10.1016/j.cub.2008.08.066
Aliouane, Y., K. Adessalam, A.K. El Hassani, V. Gray, C. Armengaud, M. Lambin and M. Gauthier. 2009. Subchronic exposure of honeybees to sublethal doses of pesticides: effect on behavior. Environ. Toxicol. Chem. 28: 113-122. https://doi.org/10.1897/08-110.1
Babendreier, D., N. Kalberer., J. Romeis., P. Fluri and F. Bigler. 2004. Pollen consumption in honeybee larvae: A step forward in the risk assessment of transgenic plants. Apidiol. 35: 293-300. https://doi.org/10.1051/apido:2004016
Blacquiere T., G. Smagghe, C.A. Gestel and V. Mommaerts. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxico. 21(4): 973-992. https://doi.org/10.1007/s10646-012-0863-x
Bonmatin, J., M.I. Moineau, R. Charvet, C. Fleche, M.E. Colin and E.R. Bengsch. 2003. A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants and in pollens. Analyt. Chem. 75(9): 2027 - 2033. https://doi.org/10.1021/ac020600b
Bonmatin, J., M.G. Giorio, V. Girolami, D. Goulson, D.P. Kreutzweiser and C. Krupke. 2015. Environmental fate and exposure; neonicotenoids and fipronil. Environ. Sci. Poll. Res. 22 (1): 35 - 67. https://doi.org/10.1007/s11356-014-3332-7
Chauzat, M.P and J.P. Faucon. 2007. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Manage. Sci. 63(11):1100 - 1106. https://doi.org/10.1002/ps.1451
Chauzat, M.P., J.P. Faucon, A.C. Martel, J. Lachaize, N. Cougoule and M.A. Aubert. 2006. Survey of pesticide residues in pollen loads collected by honeybees (A. mellifera) in France. J. Econ. Entomol. 99 (2): 253 -262. https://doi.org/10.1093/jee/99.2.253
Decourtye, A., J. Devillers, E. Genecque, K. Menac, H. Budzinski, S. Cluzeau and P.M.H. Delegue. 2005. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch. Environ. Contam. Toxicol. 48: 242-250. https://doi.org/10.1007/s00244-003-0262-7
Desneux, N., A. Decourtye and J.M.M. Delpuech. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52:81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Dively, G.P and A. Kamel. 2012. Insecticide residues analysis in pollen and nectar of cucurbit crop and their potential exposure to pollinators. J. Agric. Food Chem. 60 (18): 4449 - 4456. https://doi.org/10.1021/jf205393x
Engelsdorp, D. and M.D. Meixner. 2010. A historical review of managed honey bee populations in Europe and the United States and factors that may affect them. J. Invertebr. Pathol. 103: 80-95. https://doi.org/10.1016/j.jip.2009.06.011
Engelsdorp, V.D., J. Hayes, R.M Underwood, D. Caron and J. Pettis. 2011. A survey of managed honeybee colony losses in the USA, fall 2009 to winter 2010. J. Apic. Res. 50(1): 1-10. https://doi.org/10.3896/IBRA.1.50.1.01
Hayat, K., M. Afzal., M.A. Aqueel., S. Ali., M.F. Saeed., Q.M. Khan., M. Ashfaq and C.A. Damalas. 2018. Insecticide exposure affects DNA and antioxidant enzymes activity in honeybee species Apis florea and A. dorsata: Evidence from Punjab, Pakistan. Sci. Tota. Environ. 635: 1292-1301. https://doi.org/10.1016/j.scitotenv.2018.04.221
Irshad, M. and E. Stephen. 2014. Review: Pollination, Pollinated and Pollinators interaction in Pakistan. J. Biores. Manage. 1(1): 19-25. https://doi.org/10.35691/JBM.4102.0003
Jeschke, P., R. Nauen, M. Schindle and A. Elbert. 2011. Overview of the status and global strategy for neonicotinoids. J. Agric. Food. Chem. 59: 2897 - 2908. https://doi.org/10.1021/jf101303g
Kyriakopoulou, K., I. Kandris, I. Pachiti, M. K. Kasiotis, A. Spyropoulous, A. Santourian, S. Kitromillidou, G. Pappa and M. Glossioti. 2017. Collection and analysis of pesticide residue data for pollen and nectar. Final report. Eur. Food Safe. Authority. https://doi.org/10.2903/sp.efsa.2017.EN-1303
Maus, C., G, Cure and R. Schmuck. 2003. Safety of imidacloprid seed dressings to honey bees: a comprehensive overview and compilation of the current state of knowledge. Bull. Insectol. 56: 51-57.
Nafees, M., M.R. Jan and H. Khan. 2008. Pesticide use in Swat valley, Pakistan. Mount. Res. Dev. 28 (3): 201-205. https://doi.org/10.1659/mrd.1042
Ornates, B.F.J., A.G. Pajuelo, M.M. Megias and P.C.T. Fernandez. 2010. Pesticide residues in beewax and beebread samples collected from honeybee colonies (A. mellifera) in Spain. Possible implication for bee loses. J. Apic. Res. 48 (1): 243-250. https://doi.org/10.3896/IBRA.1.49.3.03
Pohorecka, K., P. Skubida, A. Miszczak, P. Semkiw, P. Sikorski, K.I. Zagibajlo, D. Teper, Z. Koltowoski, M. Skubida, D. Zdanska and A. Bober. 2012. Residues of neonicotinoid insecticides in bee collected plant materials from oilseed rape crops and their effects on bee colonies. J. Apic. Sci. 56 (2): 116-133. https://doi.org/10.2478/v10289-012-0029-3
Potts, S.G., J.C. Biesmeijer, C. Kremen, P. Neumann and O. Schweiger. 2010. Global pollinator declines: trends, impacts and drivers. Trends. Ecol. Evol. 25: 345-353. https://doi.org/10.1016/j.tree.2010.01.007
Ruiz, T.J., R. Vandame., C.A.R. Chan, P.P.R. Navarro, J. Gomez and D. Sanchez. 2018. Organochlorine pesticides in honey and pollen samples from managed colonies of the honeybee Apis mellifera Linnaeus and the stingless bee Scaptotrigona mexicana Guerin from Southern, Mexico. Insects. 9 (54): 1-18. https://doi.org/10.3390/insects9020054
Sanchez, B.F and K. Goka. 2014. Pesticide residues and bees – A risk assessment. PLoS One. 9(4): e94482. https://doi.org/10.1371/journal.pone.0094482
Schmuck, R., R. Schöning, A. Stork and O. Schramel. 2001. Risk posed to honeybees (Apis mellifera L., Hymenoptera) by an imidacloprid seed dressing of sunflowers. Pest. Manage. Sci. 57: 225 - 238. https://doi.org/10.1002/ps.270
Stoner, A.K. and B.D. Eitzer. 2012. Movement of soil applied imidacloprid and thimethoxam in to nectar and pollen of Squash (Cucurbita pepo). PLoS One, 7 (6): e39114. https://doi.org/10.1371/journal.pone.0039114
Tapparo, A., C. Giorio, M. Marzaro, D. Marton, L. Solda and V. Girolami. 2011. Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. J. Environ. Monit. 13: 1564-1568. https://doi.org/10.1039/c1em10085h
Tapparo, A., D. Marton and C. Giorio. 2012. Assessment of the environmental exposure of honeybees to particulate matter containing neonicotinoid insecticides coming from corn coated seeds. Environ. Sci. Tech. 46 (5): 2592-2599. https://doi.org/10.1021/es2035152
Williamson, S.M., C. Moffat, A.E.M. Gomersall, N. Saranzewa, C.N. Connolly and A.G. Wright. 2013. Exposure to Acetylcholinesterase inhibitors alters the physiology and motor function of honeybees. Front. Physiol. 2 (1): 4-13. https://doi.org/10.3389/fphys.2013.00013
To share on other social networks, click on any share button. What are these?