Analysis of Spatial and Temporal Composition and Sex Ratio of Decapod Crustaceans Catch from Southeast Sulawesi Waters of Indonesia
Analysis of Spatial and Temporal Composition and Sex Ratio of Decapod Crustaceans Catch from Southeast Sulawesi Waters of Indonesia
Oce Astuti1, La Sara2*, Muzuni3 and Safilu4
1Department of Aquaculture of Faculty of Fisheries and Marine Science, Halu Oleo University. Bumi Tridharma, Kendari 93232, Southeast Sulawesi, Indonesia
2Department of Aquatic Resources Management of Faculty of Fisheries and Marine Science, Halu Oleo University. Bumi Tridharma, Kendari 93232, Southeast Sulawesi, Indonesia
3Department of Biology of Faculty of Math and Natural Science, Halu Oleo University. Bumi Tridharma, Kendari 93232, Southeast Sulawesi, Indonesia
4Department of Biology of Faculty of Teaching and Education, Halu Oleo University. Bumi Tridharma, Kendari 93232, Southeast Sulawesi, Indonesia
ABSTRACT
The spatial and temporal decapod crustacean (DC) catch composition (CC) and its sex ratio (SR) taken from Tiworo strait were studied. Monthly samples from different habitat characteristics of intertidal zone (station A), river mouth (station B), sea grass (station C), and water depth of > 30 m (station D) using collapsible crab pots and gillnets were recorded, identified its species, sexed, counted its number, and analyzed its spatial and temporal CC and SR. The Chi-square test (α = 0.05) was used to test significant differences of expected 1: 1 SR. It was 12 DC species had been identified which BSCs were found at the entire stations and all years round. It had high CC at each station ranging 21.561–79.176% which was found the highest at station A, while CC in each month ranging 43.023–71.898% which was mostly found in March and April. Its CC reached 60.589% of total catch. The CC of other dominant species of C. anisodon, C. hellerii, and T. crenata had 5.721–50.186%, 0.458–1.550%, and 1.931–10.781%, respectively, while the rest species had CC of <3%. Spatial and temporal SR of females BSCs always preponderated over males, while C. anisodon, T. crenata, and T. danae were in contrary. Similar results were also showed for overall sex ratio of each species. The other species were mostly no patterns of SR. Chi-square test showed that those SRs were mainly significant different (P < 0.05). Data of CC implies that this waters constitutes main habitat for BSCs which prefer intertidal zone grown by mangroves (station A) particularly in March and April. Other species such as C. anisodon, T. crenata, and T. danae as bycatch which relatively low their CC may show that their population had been experiencing heavy pressure. Therefore, an action reducing bycatch should be implemented through for example providing fishing gears selective. The present BSC SR should be put in management policy in order to maintaining female adults are available all years round to produce eggs, hatch and grow to be juveniles. Therefore, BSC population stocks in this waters are continuously sustained.
Article Information
Received 12 November 2020
Revised 23 December 2020
Accepted 07 January 2021
Available online 28 April 2021
(early access)
Published 10 February 2022
Authors’ Contribution
OA and LS prepared equipment, collected data and identified crustacean samples. LS analysed data, drafted and finalized the article. Muzuni and Ssfilu collected monthly data and tabulated it.
Key words
Catch composition, Decapod crustacean species, Portunus pelagicus, Sex ratio, Intertidal zone
DOI: https://dx.doi.org/10.17582/journal.pjz/20201112121152
* Corresponding author: lasara_unhalu@yahoo.com
0030-9923/2022/0003-1017 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
Decapod crustaceans (DC) had been long known because of their high species numbers, not only in open waters but also in sea waters. Aside that their species also are very diverse due to differently to the varying environmental conditions (Shah and Pandit, 2013) whether biotic (Coleman, 2002) or abiotic (Rindi and Batelli, 2005; Charles et al., 2006), as a consequence diverse niche organizations come into being which consequently result in the evolution of diverse communities (Charles et al., 2006).
Those organisms in sea waters occupy wide range of areas from intertidal zone, estuary, mangrove forest, sea grass bed, coral reef up to deep sea waters. Those organisms are heavy exploited in the intertidal zone and are considered as the most relevant group in terms of their community dominance and biomass (Sheridan, 1992). Ecologically those DCs species are important particularly in the food web and in the structure of trophic level of the ecosystem such as in the tropical benthic communities (Cavalcante et al., 2012). Some species, including crabs have been recognized as regulators of the structure of estuarine communities (Dittel et al., 1995; Heck and Coen, 1995). Their biological outputs like feces of the crabs, which contain nitrogen, carbon, phosphorus and trace metals, form a rich source of food for other consumers (Kraeuter, 1976). Furthermore, in tropical countries DCs are very important resources and having huge role in social economic aspects for fisheries industries. Hendrickx (1995) explained that there are many of the larger and more abundant species represent important food resources for man. In Indonesia for instance, blue swimming crabs (BSCs), shrimps, and other DC species constitute a fish resources harvested by fishermen of small scale fisheries using mainly fishing gear of traps and bottom gillnet throughout the coastal areas since last three decades, while lobster caught in the coral reef ecosystem used hand picking aided with “small and short stick” to expel of the lobster from its hiding place (shelters). Initially local people knew that those DC species are only to fulfill their daily consumption as a source of protein in the main food consumed, but when demand of international people of US, Europe, and other Asian countries such as Japan, Singapore with premium price that it was caught unrestrained and intensive using unselective fishing gears of crab traps (cubic and rounded shapes) and bottom gillnets (La Sara et al., 2016a, 2019; Astuti et al., 2020a, 2020b). Exploitation of BSCs in particular and other commercial DC species had shown over exploitation (La Sara et al., 2017).
The main target of DCs caught by fishermen using bottom gillnet and traps is for BSCs, but there are other DC species as bycatch of those fishing gears which ranged 60–70% of total catch per trip. It is well known that several fishing grounds of BSCs particularly in Southeast Sulawesi waters have indicated over exploitation. We are not aware that this phenomenon of over exploitation actually have also figured out the population of other DC species in several fishing grounds due to bycatch of the fishing gears is higher than those of main catch. In previous studies elucidated that over the past three decades, it has been recognized that bycatch and discard is one of the most significant issues affecting fisheries management (Saila, 1983; Alverson et al., 1994).
Those DC populations much more suppressed due to their habitats have also been experiencing affected by anthropogenic impacts. For examples, mangrove forest as DC juveniles habitat has been cut off for human settlements, river mouth and estuary as many kinds of mature crustacean have been altered for jetty and sea port, and intertidal zone and sea grass bed area for feeding ground have been affected by sedimentation come from land during flooding of rain. However, it has been still far away from proper attention, responsibility and documentation and hence the human effects on them are difficult to be quantified or assessed.
Up to the present there is no study has evaluated or designed the shape, dimension and mesh size of fishing gears used by fishermen to get optimal DCs main catch and minimum bycatch. It has been designed a rectangular collapsible crab pot equipped with escape vent of 5.0 cm x 3.5 cm attached in left and right sides for catching BSCs with carapace width (CW) of > 10 cm (La Sara et al., 2016b). The smaller CW of BSCs and other crustacean crabs than CW of 10 cm are systematically return to the sea in good condition and preferably as close as possible to their home waters to grow to attain bigger size and have opportunity to breed to essentially maintain their populations. The aims of the present study which focus on identification of DC species caught by traps and bottom gillnet in the different fishing ground characteristics are to analyze spatial and temporal of DCs catch composition and its SR caught. It is the first study conducted around Southeast Sulawesi waters in particular dan Indonesia waters in general.
MATERIALS AND METHODS
Design of study location
Tiworo strait waters is a well known BSC fishing ground in Southeast Sulawesi and in Indonesia in general. This fishing ground is surrounded by main land of Muna Island, Southeast Sulawesi peninsula (South Konawe and Bombana). Between main land and peninsula, there are scattered small islands of Tiworo inhabited by fishermen. The strait waters constitute a dynamics waters system affected by hidrooceanographic. The different of waters characteristics is showed by different ecosystems such as mangrove, sea grass, coral reef, and estuary ecosystems. It is also affected by sedimentation and fresh water flow through rivers and tributaries. Almost of strait waters constitute fishing ground of BSCs (as main target) using traps and bottom gillnets. Those gears generally each trip of fishing catch several DC species as bycatch. The location of study was purposively chosen at 4 different fishing ground characteristics namely coastal waters grown by mangroves (intertidal zone) (station A), river mouth (station B), coastal area with coarse sand substrate grown sea grass (station C), and water depth of > 30 m (station D) (Fig. 1). The DCs sampling were carried out from March to September 2020.
Sampling procedure
The DCs sampling at each station was taken monthly. Fishing gears used at station A-C were collapsible rectangular collapsible crab pot (length= 54 cm, width = 36 cm, and height = 19 cm) covered with nylon net of ± 0.5 cm mesh size (Fig. 2), while at station D was bottom gillnet. As many 150 units of crab pots were deployed at each station. Each crab pot was tied at main nylon polypropylene (0=0.5 mm) using small nylon polypropylene (0=0.25 mm). The distance between crab pots at main nylon polypropylene was ±10 cm. Each crab pot was put fresh fish bait with size relatively the same at each crab pot. All crab pots tied in the main nylon polypropylene were deployed during flood tide then hauled during ebb tide. The DCs sampling at station D used a bottom gillnet of ± 1 km length, 1 m height, and 4 inch mesh size (Fig. 2). Each sample caught at each station (spatial) and month (temporal) was recorded, identified, sexed according to abdomen morphometric characteristics (male crustacean has V-shape abdomen, while female has relatively broad abdomen or rounded (Van Engel, 1958; Ingles and Braum, 1989; Potter and de Lestang, 2000), and then each sex counted its number (La Sara et al., 2016a, 2017).
Data analysis
The DC species catch composition (CC) taken from each station (spatial) and month (temporal) was analyzed using a formula as follows:
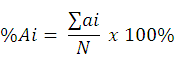
where Ai = species percentage of i (i = 1, 2, 3, … n); ai = species individual number of i = i = 1, 2, 3, … n); and N = total number of all species
The males and females DC taken from each station (spatial) and month (temporal) were counted and its sex ratio (SR) was analyzed using a formula as follows:
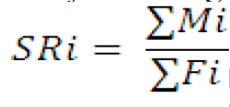
where SRi = sex ratio of species of i (i = 1, 2, 3, … n); Mi = number of male species of i (i = 1, 2, 3, … n); Fi = number of female species of i (i = 1, 2, 3, … n)
The Chi-square test (α = 0.05) was used to test significant differences of expected 1: 1 SR of each species (Gomez and Gomez, 1976) as follows:
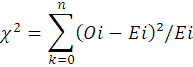
where xi2= Chi-square, Oi = frequency number of observed male and female each species of i (i = 1, 2, 3, … n), E = frequency number of expected male and female each species.
RESULTS
The DC species CC
The DC samples taken from each station (spatial) in Tiworo Strait waters from March to September 2020 (temporal) were identified and it was found 12 crustacean species (Fig. 3 and 4). Of 12 species of those crustacean, BSCs (P. pelagicus) was a species which much frequent found and having higher catch composition at each station (spatial) ranging 21.561–79.176% and each month (temporal) ranging 43.023–71.898%. The CC of BSCs taken from all stations (spatial) or during the course of study (temporal) reached 60.589%. It was followed by CC of other DC species of C. anisodon, C. hellerii, and T. crenata ranged 5.721–50.186%, 0.458–1.550%, and 1.931–10.781%, respectively. The combined CC from all stations (spatial) or during the course of study (temporal) of aside those 3 DC species was 19.624%, 6.214%, and 3.025%, respectively was Artorigus sp of 5.560%. The catch composition of other crustacean species was relatively low of < 3%.
The DC species SR
The DC samples taken from 4 stations of different habitat characteristics (spatial) of sampling sites of Tiworo Strait during the study in March–September 2020 (temporal) were identified and classified into 12 species (Tables I and II). Among those crustacean, BSCs (P. pelagicus), C. anisodon, C. hellerii, Thalamita crenata were dominant and were always found in the all stations, while other species occupied certain stations (Table I). Similar trend of those species (except C. hellerii) were also found during the period of sampling (temporal) (Table II). T. danae, T. cerasma, and T. prymna were among crustacean species found in several stations (Table I) and several months (Table II), while other species such as M. rumphi, A. lunaris, Artorigus s.p, P. sanguinolentus, and S. serrata were found very few in certain stations and months.
The spatial and temporal SR of male and female crustacean caught showed that females P. pelagicus always preponderated over males (female > male) in all stations, while C. anisodon, T. crenata, and T. danae were in contrary (male > female) (Tables I and II). Similar results were also showed for overall SR of each species. The other species were mostly no patterns of SR due to SR in a species of certain stations (spatial) and months (temporal) sometime males preponderated over females, but in the other stations (spatial) and months (temporal) was in contrary. Chi-square test (α= 0.05) showed that those SR of male and female crustacean were mainly significant different (α < 0.05).
DISCUSSION
The DC species CC
Identification of DC species in the present study aimed to better understanding their characteristics biology, habitats and optimal management of sustainable exploitation of crustacean stocks. In the present study, the BSCs were found at all stations (spatial) and all months (temporal) and had CC of 60.589%, while other crustacean species were less than 40%. Of 12 species of those crustaceans, BSC was a species which was much frequent found, having higher CC at each station (spatial) ranging 21.561–79.176% (spatial) and each month ranging 43.023–71.898% (temporal) (Figs. 3 and 4; Tables I and II) and to be main target species of fishermen. It was followed by CC of other crustacean species of C. anisodon, C. hellerii, and T. crenata ranged 5.721–50.186%, 0.458–1.550%, and 1.931–10.781%, respectively. The combined CC from all stations (spatial) or during the course of study (temporal) of aside those 3 crustacean species mentioned was 19.624%, 6.214%, and 3.025%, respectively was Artorigus sp. of 5.560%. The CC of others than those mentioned crustacean species were relatively low of < 3%, namely P. sanguinolentus, Artorigus sp., and S. serrata (< 1%). Other crustacean species than BSC, P. sanguinolentus and S. serrata included bycatch species.
According to Fazrul et al. (2015) abundance of bycatch in Pattani Bay was influenced by habitat, season and interaction between habitat and season, while number of species per sampling was affected only seasonal variation, but in the present study season and location affect number of crustacean species (Figs. 3 and 4). Previous studies revealed that fishing gears and various fishing methods may affect the amount of bycatch (Eayrs, 2007; Raeisi et al., 2012). The more unselective fishing gears is the higher amounts of bycatch composition produced, such as trawl nets (Harrington et al., 2006; Paighambari and Daliri, 2012; Hosseini et al., 2012; Raeisi et al., 2012; Eighani and Paighambari, 2013). Bycatch refers to an incidental catch causing mortality and injuries (Kelleher, 2005).
Table I. The spatial SR of male and female DC in Tiworo Strait Waters of Southwest Sulawesi, Indonesia.
|
Station |
Sex ratio of male and female crustacean |
|||||||||||
|
BSC |
Ca |
Ch |
Tcr |
Td |
Tce |
Tp |
Mr |
Al |
As |
Ps |
Ss |
|
|
1 |
1 : 1.35* |
2.57 : 1* |
2 : 0 |
4.20 : 1* |
1.33 : 1* |
|
13 : 0 |
|
3 : 0 |
0 : 1 |
|
|
|
2 |
1 : 1.41* |
2.80 : 1* |
1 : 3* |
1.67 : 1* |
1.50 : 1* |
|
13 : 1* |
|
1 : 0 |
|
0 : 1 |
|
|
3 |
1 : 1.31* |
1.30 : 1* |
1 : 2* |
0.75 : 1* |
2.50 : 1* |
|
1.20 : 1* |
|
|
|
|
|
|
4 |
1 : 1.43* |
2.16 : 1* |
2 : 1* |
1.80 : 1* |
|
1.19 : 1 |
|
1 : 1.5 |
|
1 : 0 |
|
0 : 1 |
|
Overall sex ratio |
1 : 1.33* |
2.24 : 1* |
1 : 1ns |
2.17 : 1* |
1.63 : 1* |
1.18 : 1* |
2 : 1* |
1 : 1.5* |
4 : 0 |
1 : 1ns |
0 : 1 |
0 : 1 |
BSC, P. pelagicus; Tcr, T. crenata; Mr, Menippe rumphi; Al, Ashtoret lunaris; Ca, Charybdis anisodon; Tce, T. cerasma; Ch, C. hellerii; As, Artorigus sp; Ps, P. sanguinolentus; Td, T. danae; Tp, T. prymna; Ss, Scylla serrata; *, significant different at P < 0.05; ns, no significant different at P < 0.05
Table II. The temporal SR of male and female DC in Tiworo Strait Waters of Southeast Sulawesi, Indonesia.
|
Month |
Sex ratio of male and female crustacean |
|||||||||||
|
BSC |
Ca |
Ch |
Tcr |
Td |
Tce |
Tp |
Mr |
Al |
As |
Ps |
Ss |
|
|
March |
1 : 1.38* |
2.29 : 1* |
1 : 1ns |
1 : 0 |
1 : 0 |
|||||||
|
April |
2.08 : 1* |
1.24 : 1* |
1.67 : 1* |
1 : 0 |
0 : 1 |
0 : 1 |
||||||
|
May |
1 : 1.43 |
2.60 : 1* |
2.5 : 1* |
1.25 : 1* |
1 : 1.09ns |
3 : 1* |
1 : 0 |
|||||
|
June |
1 : 1.21* |
9.5 : 1* |
3 : 1* |
2 : 0 |
2.14 : 1* |
1.6 : 1* |
3 : 0 |
2 : 0 |
||||
|
July |
1 : 1.57* |
3.5 : 1* |
1 : 1.67* |
3.33 : 1* |
1 : 1.14* |
1 : 0 |
15 : 0 |
1 : 0 |
||||
|
August |
1 : 1.50* |
1.62 : 1* |
2 : 1* |
2 : 1* |
5 : 1* |
1 : 3* |
||||||
|
September |
1 : 1.24* |
1.89 : 1* |
1 : 1ns |
0 : 1 |
||||||||
|
Overall sex ratio |
1 : 1.33* |
2.24 : 1* |
1 : 1 |
2.17 : 1* |
1.62 : 1* |
1.18 : 1 |
8 : 1 |
1: 1.15 |
4 : 0 |
1 : 1 |
0 : 1 |
0 : 1 |
* = significant different at P < 0.05; ns = no significant different at P < 0.05. See Table I for abbreviations.
Using unselective crab pots and bottom gillnet in the present study were not only catching main target of BSCs but also catching other organisms as bycatch (non-target species) (Figs. 3 and 4) which are thrown away (discarded) overboard, or dumped at sea for a variety of reasons. Those bycatch organisms generally are due to low or less value of the catch in market. Those crustacean species bycatch may include as threatened, endangered or protected species or may be economically worthless but play an important role in the marine life cycle. Therefore, those should be maintained and guaranteed that sustainable harvesting has to be kept in fishing management. In Indonesia, there are already the BSC regulations (the Regulation of Ministry of Marine Affairs and Fisheries No. 12/PERMEN-KP/2020) to control BSCs exploitation, nevertheless the species is presently overfished in some regions of Indonesia.
High proportion of CC of BSCs as main target species and bycatch (i.e. other crustacean, fish, mollusca, etc.) will result unstable of tropic level structure of the organisms such as in the tropical benthic communities (Cavalcante et al., 2012), contribute to biological overfishing, food web disturbance, changing the structure of marine communities and/or ecosystems such as the structure of estuarine communities (Dittel et al., 1995; Heck and Coen, 1995), certain species dominant and low diversity of organisms with serious implications for marine populations and sustainability of ecosystems (FAO, 1997; Rebecca et al., 2004). Identification of the crustacean species characteristics and its variability in aquatic ecosystems and the interaction between species and ecosystems lead to better understanding of the stock population management.
It is an issue affecting the ecosystem and survival of marine population (Read, 2013). When the aquatic ecosystem is unstable that aquatic productivity is to be low and as a consequence there is no any organisms could be harvested by fishermen to be used for protein need and income for their households. There is no comprehensive data on catch composition of crab pot available to be referred in regulation of fishing gears operation due to no study had been done and are not taken into account for assessment and management programs (Dıaz-Uribe et al., 2007). Low CC percentage of other crustacean (Figs. 3 and 4) in this waters could indicate that those organisms had decreased their population due to high exploitation intensity of BSCs using unselective crab pots and bottom gillnets which in the present already reaches “over exploited status”. This phenomenon has to be solved among other strategies i.e. using selective crab pot to reduce small size of main target species of BSCs retained and to avoid bycatch organisms. It is in line with FAO (2014) which develops international guideline on bycatch management and reduction of discards.
In Thailand was reported that crab gill net has not much effect to non-target species or bycatch because it is a highly selective methodology of operation (Fazrul et al., 2015). Compare with large-scale fisheries, this fishing gear is not to be major risk to marine ecosystem due to a lower and more selective fishing capacity (Dıaz-Uribe et al. (2007). However, scientists in fisheries management agree that bycatch production in commercial fishing is one of the serious threats to fish stocks and includes about 40.4% of the total marine catch which is poured into the sea as discard fish (Worm et al., 2006; Davies et al., 2009; Queirolo et al., 2011). In recent year, a very serious apprehensive due to approximately 30% of world landings were fish trash or non-target species and 40% of them caught by artisanal fishing gears (FAO, 2014).
Data CC (Figs. 3 and 4) in the present study showed that Tiworo strait constitutes the BSCs main habitats and to be among fishing ground in Indonesia aside Java Sea (Ernawati, 2013; Ernawati et al., 2017), East Lampung waters (Zairion et al., 2015). All those BSC fishing grounds had been heavy exploited since last 2 decades using crab pots and gill nets, when global consumers demand increased sharply such as United States of 71%, Japan of 9%, and Malaysia of 7%. In 2016 total volume export of BSCs reached 19,837 tons and decreased in 2018 to be 16,845 tons. It leads to BSC population decrease dramatically and in danger condition as shown its exploitation rate (E) of > 0.5. Previous study showed in this waters had showed over exploited status of male BSCs (E = 0.67), while female BSCs was still under exploited (E = 0.03) (La Sara et al., 2017). Other studies on DC species conducted in different regions also showed data of over exploited due to intensive exploitation using unselective fishing gears as shown its exploitation rate (E) of > 0.5 (Table III). Over exploitation of BSC population and even in some places already disappeared are mainly caused by fishermen using unselective fishing gears of pots without escape vents, bottom gillnet with small mesh size, and some mini trawls (La Sara et al., 2016a, b, 2017, 2019). The first two gears are stationary gears which are preferred used by fishermen (La Sara et al., 2016a). It is indicated that the rate of fishing activities are higher than those of BSC population recovery rates produced by adult stages.
Using all those fishing gears catch not only BSCs, but also other DC species as bycatch which their population also threatened to disappeared such as Artorigus sp, P. sanguinolentus, and other species of very low CC species, albeit there is opportunity reason that this waters does not constitute main habitat of those species. This condition has risen an apprehensive of biologists, fish managers, miniplant owners, and exporters.
The DC species SR
Information on SR is important for understanding the relationship between individuals, the environment and the state of the population (Vicentini and Araújo, 2003). Data of spatial and temporal of SR ratio of male and female (M: F) of 12 crustacean species found in this waters were generally significant different (α < 0.05) (Tables I and II). The BSCs as dominant species found spatially and temporally in this waters all females preponderated over males, while in contrary happened in other crustacean species of C. anisodon, C. hellerii, Thalamita crenata, and T. danae namely all males preponderated over males. Data in Tables I and II indicated that this waters constituted habitat of all stages BSCs, particularly for juvenile and mature stages due to their abundance were high enough both males and females, then at the end of mature stage and enter to adult stage that female BSCs particularly those who were after copulation and berried were gradually move to the deep sea waters for ripen and to extrude their eggs up to hatch to be zoea larvae.
Similar BSC population behavior had been found in Tiworo strait waters in 2016 (La Sara et al., 2016a). The recent studies showed that SR of female BSCs preponderated over males in Tiworo strait waters (Astuti et al., 2020a), P. pelagicus taken from Java Sea of 1:1.45 (Rohmayani et al., 2018), P. pelagicus population in Mayangan waters of Indonesia of 1: 1.22 (Hermanto et al., 2019), P. pelagicus in Tiworo strait waters of 1: 1.032 (La Sara et al., 2016a), P. segnis population in Boushehr coast (Persian Gulf) (Hosseini et al., 2014), P. pelagicus population in Khuzestan coasts, Iran (Jazayeri et al., 2011), and Bantayan waters of Philippines (Ingles, 1996). Sukumaran (1997) revealed that sex distribution in Portunids in relation to size indicated that females were more pronounced in the smaller sizes, whereas males dominated in the larger sizes.
The SR at present study was different with several previous studies conducted in different regions which showing male BSCs was outnumbered female BSCs (Table IV). Xiao and Kumar (2004) reported that fishermen in southern Australia waters proportionally caught male BSCs much higher than those of female BSCs, and male carapace width also much bigger than those of female BSCs. The authors also explained that the differences of SR related with time of fishing exploitation condition where dead male BSCs were higher proportion than those live BSCs. There was a phenomenon that male BSCs increased with increasing water depth from January to September and then decreasing from October to December. However, those SR differences of males and females were not caused by single parameter or single factor, but there were complex factors interacted each other. It is always also found that SR may vary from the expected 1:1 from species to species, or even in the same population at different times (Oliviera et al., 2012). It is influenced by several factors such as adaptation of the population, reproductive behavior, food availability, environmental conditions, and fishing gears used. For example, Ingles (1996) revealed that SR differences may be due to gillnet catchability different. Other factor is caused by differences of each sex behavior when attain maturity stage. Xiao and Kumar (2004) detected yearly variation of male BSCs caught in southern Australia waters due to season condition which influence environmental condition such as temperature. Normally, reproductive success of crustacean species is mainly related to access to resources and the environmental conditions which females usually migrate to oceanic condition to extrude their eggs, while males sometimes still occupy intertidal zone or adjacent sea grass ecosystem. It leads to an unbalance in the number of individuals of each sex in the population.
The effect season situation, migration and changes of the weather on BSCs can affect the SR in the population (Smith and Sumpton, 1989). Seasonal effects on BSCs or other Portunids abundance and distribution may be due to different climate conditions such as rainfall and temperature fluctuations. For example, juvenile BSCs occupied intertidal zones closed to or around mangrove forests with sandy muddy substrates in all year rounds (Astuti et al., 2020b), attained mature stage with carapace width of 7–9 cm at 8–10 months (La Sara et al., 2016a, 2017), and further females grow to be berried at ± 1 year old and then leave inshore to extrude their eggs on pleopods in offshore or saline waters (Potter and de Lestang, 2000; de Lestang et al., 2003), while juvenile BSCs still occupy shallow waters of < 5 m depth. Such those factors cause differences in BSC SR. Other previous study on Callinectes sapidus showed that salinity fluctuation affect their distribution according to their carapace width sizes (Archambault et al., 1990; Ault et al., 1995), while temperature affect their behavior and activities for searching food which may affect crabs caught in traps which rely on baits.
CONCLUSIONS
It is concluded that among 12 DC species found in Tiworo strait waters, BSCs are the most abundance and main catch of crab pots and gillnets compare to others species as shown its CC of > 60% of total catch. The rest DC species are mostly discarded, except P. sanguinolentus and S. serrata which its CC is few. The BSCs species is found at all fishing grounds and all years round which implies that this waters constitutes its main habitat, however, it prefers habitat in intertidal zone with sandy substrate closed to mangrove forest and most abundance in this habitat in March and April. Among those species, spatial and temporal SR of females BSCs always preponderated over males, while C. anisodon, T. crenata, and T. danae were in contrary, while other species has no pattern. The number of female BSCs is higher than those of males could benefit for sustaining BSC population due to they have opportunity to mate, produce eggs, then extrude and hatch them in oceanic condition and grow to be juveniles. Therefore, BSC population stock in this waters could be continuously sustained.
Table III. Exploitation rate of BSCs (P. pelagicus) in different regions.
|
No. |
Location |
Exploitation rate (E) |
Sources |
|
1. |
Cirebon, West Java |
0.73 |
|
|
2. |
East Lampung, Indonesia |
0.72 |
|
|
3. |
Pati, Central Java |
0.78 |
|
|
4. |
Bone Bay, Indonesia |
0.73 |
Table IV. The sex ratio of portunids from different regions.
|
No. |
Location |
Species |
Sex ratio |
References |
|
1. |
Ragay Gulf, Philippines |
P. pelagicus |
23 : 1 |
|
|
2. |
Karnataka waters, India |
P. sanguinolentus |
1.13 : 1 |
|
|
3. |
Tiworo strait waters, Indonesia |
Juvenile P. pelagicus |
1.44 : 1 |
ACKNOWLEDGEMENTS
This manuscript is a part of a research project entitled “The DC Spatial Diversity in Tiworo Strait Waters of Southeast Sulawesi, Indonesia” headed by the second author. The study was funded by “Badan Layanan Umum” through schema of Internal Basic Research of Halu Oleo University 2020 No.1542a/UN29.20/PG/2020, which is gratefully acknowledged. The authors would like to thank to Head of Research and Services Institution of Halu Oleo University who provided grant for this research. We also thanked to our students and alumni who involved during monthly sampling in Tiworo Strait.
Statement of conflict interests
The authors have declared no conflict of interest.
REFERENCES
Alverson, D.L., Freeberg, M.H., Pope, J.G., Murawski, S.A., 1994. A global assessment of fisheries bycatch and discard. FAO Fisheries Technical Paper. No.339. Rome. pp. 233.
Archambault, J.A., Wenner, E.L. and Whitaker, J.D., 1990. Life history and abundance of blue crab, Callinectes sapidus rathbun, at charleston harbor, South Carolina. Bull. mar. Sci., 46: 145-158.
Astuti, O., Alimina, N., Safilu, Emiyarti, La Sara, Wa Nurgayah and Rahmadani, I., 2020a. Temporal sex ratio, growth patterns and condition factor of the blue swimming crab (Portunus pelagicus) in Northern of Tiworo Strait waters, Southeast Sulawesi, Indonesia. Aceh J. Anim. Sci., 5: 104-111. https://doi.org/10.21608/ejabf.2020.107583
Astuti, O., Alimina, N., La Sara, Emiyarti and Rahmadani. 2020b. Spatial and temporal size structure abundance of the blue swimming crab (Portunus pelagicus) in Tiworo Strait, Southeast Sulawesi, Indonesia. Egypt. J. aquat. Biol. Fish., 24: 375–392. https://doi.org/10.21608/ejabf.2020.107583
Ault, J.S., Patrick, E.V. and Rothschild, B.J., 1995. Physical factors affecting recruitment and abundance of the Chesapeake Bay blue crab stock. Bull. mar. Sci., 57: 917.
Cavalcante, D.V., da Silva, B.B. and Martinelli-Lemos, J.M., 2012. Biodiversity of decapod crustaceans in the estuarine floodplain around the city of Belém (Pará) in Brazilian Amazonia. Zoologia, 29: 203–209. https://doi.org/10.1590/S1984-46702012000300003
Charles, D.F., Acker, F.W., Hart, D.D., Reimer, C.W. and Cotter, P.B., 2006. Large-scale regional variation in diatom-water chemistry relationships: Rivers of the eastern United States. Hydrobiologia, 561: 27–57. https://doi.org/10.1007/s10750-005-1603-5
Coleman, M.A., 2002. Small-scale spatial variability in intertidal and subtidal turfing algal assemblages and the temporal generality of these patterns. J. Exp. mar. biol. Ecol., 267: 53-74. https://doi.org/10.1016/S0022-0981(01)00358-6
Davies, R.W.D., Cripps, S.J., Nickson, A. and Porter, G., 2009. Defining and estimating global marine fisheries bycatch. Mar. Pol., 33: 661-672. https://doi.org/10.1016/j.marpol.2009.01.003
de Lestang S., Hall, N.G. and Potter, I.C., 2003. Do the age compositions and growth of the crab Portunus pelagicus in marine embayments and estuaries differ? J. Mar. biol. Assoc. U.K., 83: 1–8. https://doi.org/10.1017/S0025315403008166h
Dıaz-Uribe, J.G., Arreguın-Sanchez, F. and Cisneros-Mata. M.A., 2007. Multispecies perspective for small-scale fisheries management: A trophic analysis of La Paz Bay in the Gulf of California, Mexico. Ecol. Model., 201: 205–222. https://doi.org/10.1016/j.ecolmodel.2006.09.015
Dineshbabu, A.P., Shridhara, B., Muniyappa, Y., 2008. Biology and exploitation of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758), from South Karnataka Coast, India. Indian J. Fish., 55: 215.
Dittel, A.I., Hines, A.H., Ruiz, G.M. and Ruffin, K.K., 1995. Effect of shallow water refuge on behavior and density dependent mortality of juvenile blue crabs in Chesapeake Bay. Bull. mar. Sci., 57: 902-916.
Eayrs, S., 2007. A guide to bycatch reduction in tropical shrimp-trawl fisheries. Revised Edition. Food and Agricultural Organisation, Rome. pp. 108.
Eighani, M. and Paighambari, S.Y., 2013. Shrimp, bycatch and discard composition of fish caught by small-scale shrimp trawlers in the Hormuzgan coast of Iran in the Persian Gulf. Philipp. Agric. Sci., 96: 314-319.
Ernawati, T., 2013. Population dynamics and stock assessment of blue swimmer crab (Portunus pelagicus Linnaeus) resources in Pati and adjacent waters. Master thesis. Bogor Agricultural University, Bogor, Indonesia. pp.80. (in Indonesian).
Ernawati, T., Sumiono, B. and Madduppa, H., 2017. Reproductive biology, spawning potential, and breeding season of blue swimming crab (Portunidae: Portunus pelagicus) in Java Sea, Indonesia. Biodiversitas, 18: 1705-1713. https://doi.org/10.13057/biodiv/d180450
FAO (Food and Agriculture Organization of the United Nations), 1997. Review of the state of world fishery resources: marine fisheries. 2. Northeast Atlantic FAO Statistical Area No.27. FAO Fish. Cir. Rome, 920: 1-9.
FAO (Food and Agriculture Organization of the United Nations), 2014. The state of world fisheries and aquaculture 2014. FAO Fish. Tech. Pap. Rome, pp. 223.
Fazrul, H., Hajisamae, S., Ikhwanuddin, M. and Pradit, S., 2015. Assessing impact of crab gill net fishery to bycatch population in the lower Gulf of Thailand. Tur. J. Fish. aquat. Sci., 15: 761-771. https://doi.org/10.4194/1303-2712-v15_3_21
Gomez, K.A. and Gomez, A.A., 1976. Statistical procedures for agricultural research. The International Rice Institute. Los Banos, Laguna, Philippines. pp. 294.
Harrington, J.M., Myers, R.A. and Rosenberg, A.A., 2006. Wasted fishery resources: Discarded by-catch in the USA. Fish Fish., 6: 350-361. https://doi.org/10.1111/j.1467-2979.2005.00201.x
Heck, K.L. and Coen, L.D., 1995. Predation and the abundance of juvenile blue crabs: A comparison of selected East and Gulf Coast (USA) studies. Bull. mar. Sci., 57: 877-883.
Hendrickx, M.E., 1995. Checklist of brachyuran crabs (Crustacea: Decapod) from the eastern tropical Pacific. Bull. Inst. R. Sci. Nat. Bel. Biol., 65: 125-150.
Hermanto, D.T., Sulistiono, Riani, E., 2019. Study on some reproductive aspects of blue swimming crab (Portunus pelagicus) in Mayangan Waters, Subang, West Java. Biospecies, 12: 1–10.
Hosseini, M, Pazooki, J. and Safaei, M., 2014. Size at maturity, sex ratio and variant morphometrics of blue swimming crab Portunus segnis (Forskal, 1775) from Boushehr Coast (Persian Gulf). J. mar. Sci. Res. Dev., 4: 149-153.
Hosseini, S.A., Raeisi, H. and Paighambari, S.Y., 2012. Temporal and spatial variations of finfish bycatch of cutlass fish trawl in Bushehr and Hormozgan marine waters, the Northern Persian Gulf. J. Persian Gulf (Mar. Sci.), 3: 1-8.
Ingles, J.A. and Braum, E., 1989. Reproduction and larval ecology of the blue swimming crab, Portunus pelagicus, in Ragay Gulf, Philippines. Int. Rev. Ges. Hydrobiol. Hydrog., 74: 471-490. https://doi.org/10.1002/iroh.19890740503
Ingles, J.A., 1996. The crab fisheries off Bantayan, Cebu, Philippines. IMFO-CF, University of the Philippines, PCMARD, Philippines, pp. 88.
Jazayeri, A., Papan, F., Savari, A. and Sakinejad, T., 2011. Biological investigation of Persian Gulf blue swimmer crab (Portunus pelagicus) in Khuzestan coasts, Iran. J. Am. Sci., 7: 7-13.
Kelleher, K., 2005. Discards in the world’s marine fisheries an update. FAO Fish. Tech. Pap., No.470. FAO, Rome, pp. 131.
Kembaren, D.D., Ernawati, T. and Suprapto, 2012. Biology and population parameters of blue swimming (Portunus pelagicus) in Bone waters and its adjacent. J. Indonesia Fish. Res., 18: 273-281. (in Indonesia).
Kraeuter, J.N., 1976. Biodeposition by salt marsh invertebrates. Mar. Biol., 35: 215-223. https://doi.org/10.1007/BF00396870
La Sara, Astuti, O. and Muzuni, Safilu, 2019. Status of blue swimming crab (Portunus pelagicus) population in Southeast Sulawesi waters, Indonesia. AACL Bioflux, 12: 1909-1917.
La Sara, Muskita, W.H, Astuti, O. and Safilu, 2016a. The reproductive biology of blue swimming crab Portunus pelagicus in Southeast Sulawesi waters, Indonesia. AACL Bioflux, 9: 1101-1112.
La Sara, Halili, Mustafa, A. and Bahtiar, 2016b. Appropriate escape vent sizes on collapsible crab pot for blue swimming crab (Portunus pelagicus) fishery in Southeast Sulawesi waters, Indonesia. J. Fish. aquat. Sci., 11: 402-410. https://doi.org/10.3923/jfas.2016.402.410
La Sara, Muskita, W.H., Astuti, O. and Safilu, 2017. Some population parameters of blue swimming crab (Portunus pelagicus) in Southeast Sulawesi waters, Indonesia. AACL Bioflux, 10: 587-601.
Oliveira, M.R, Costa, E.F.S., Araújo, A.S., Pessoa, E.K.R., Carvalho, M.M., Cavalcante, L.F.M. and Chellappa, S., 2012. Sex ratio and length-weight relationship for five marine fish species from Brazil. J. mar. Biol. Oceanog., 1: 2-4. https://doi.org/10.4172/2324-8661.1000103
Paighambari, S.Y. and Daliri, M., 2012. The bycatch composition of shrimp trawl fisheries in Bushehr coastal waters, northern Persian Gulf. J. Persian Gulf (Mar. Sci.), 3: 27-36.
Permen, K.P., 2020. Peraturan menteri kelautan dan perikanan. Republik Indonesia No. 12/PERMEN-KP/2020 Tentang Pengelolaan Lobster (Panulirus spp.), Kepiting (Scylla spp.), dan Rajungan (Portunus spp.) di Wilayah Negara Republik Indonesia.
Potter, I.C. and de Lestang, S., 2000. Biology of the blue swimmer crab Portunus pelagicus in Leschenault Estuary and Koombana Bay, South-western Australia. J. R. Soc. West. Aust., 83: 443-458.
Queirolo, D., Erzini, K., Hurtado, C.F., Gaete, E. and Soriguer, M.C., 2011. Species composition and bycatches of a new crustacean trawl in Chile. Fish Res. 110: 149-159. https://doi.org/10.1016/j.fishres.2011.04.001
Raeisi, H., Hosseini., S.A., Paighambari, S.Y., Shabani, M.J. and Kiaalvandi, S., 2012. Study of natural and fishing mortality and exploitation rate of Largehead hairtail, Trichiurus lepturus (Linnaeus, 1758) from the Northern Persian Gulf, Iranian waters. Caspian J. appl. Sci. Res., 1: 22-27.
Read, A.J., 2013. Development of conservation strategies used to mitigate the bycatch of harbor porpoises in the Gulf of Maine. Endang. Sp. Res., 20: 235-250. https://doi.org/10.3354/esr00488
Rebecca, L.L., Crowder, L.B., Read, A.J. and Freeman, S.A., 2004. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol., 19: 598-604. https://doi.org/10.1016/j.tree.2004.09.004
Rindi, F. and Battelli, C., 2005. Spatio-temporal variability of intertidal algal assemblages of the Slovenian coast (Gulf of Trieste, northern Adriatic Sea). Bot. Mar., 48: 96–105. https://doi.org/10.1515/BOT.2005.022
Rohmayani, V., Pahlevi, M.R., Irawan, B. and Soegianto, A., 2018. Length-weight relationship, sex ratio and condition factor of blue swimming crab (Portunus pelagicus Linnaeus, 1758) from Java Sea Indonesia. AIP Conf. Proc. 2002. 020071. pp. 1–5. https://doi.org/10.1063/1.5050167
Saila, S.B., 1983. Importance and assessment of discard in commercial fisheries. FAO Fish. Cir., No.765. Rome. pp. 62.
Shah, J.A. and Pandit, A.K., 2013. Relation between physico–chemical limnology and crustacean community in Wular lake of Kashmir Himalaya. Pak. J. biol. Sci., 16: 976-983. https://doi.org/10.3923/pjbs.2013.976.983
Sheridan, P.F., 1992. Comparative habitat utilization by estuarine macrofauna within the mangrove ecosystem of Rookery Bay, Florida. Bull. mar. Sci., 50: 21-39.
Smith, S.S. and Sumpton, W.D., 1989. Behavior of the commercial sand crab Portunus pelagicus (L.) at trap entrances. Asian Fish. Sci., 3: 101-113.
Sukumaran, K.K., 1997. Sex ratio, fecundity and reproductive potential in two marine portunid crabs, Portunus (Portunus) sanguinolentus (Herbst) and Portunus (Portunus) pelagicus (Linnaeus) along the Karnataka coast. Indian J. mar. Sci., 26: 43-48.
Van Engel, W.A., 1958. The blue swimmer crab and its fishery in Chesapeake Bay, Part 1: Reproduction, early development, growth and migration. Commer. Fish. Rev., 20: 6 –17.
Vicentini, R.N. and Araújo, F.G., 2003. Sex ratio and size structure of Micropogonias furnieri (Desmarest, 1823) (Perciformes, Sciaenidae) in Sepetiba Bay, Rio de Janeiro, Brazil. Braz. J. Biol., 63: 559-566. https://doi.org/10.1590/S1519-69842003000400003
Worm, B., Barbier, E.B., Beaumont, N., Duffy, J.E., Folke, C., Halpern, B.S., Jackson, J.B.C., Lotze, H.K., Micheli, F., Palumbi, S.R., Sala, E., Selkoe, K.A., Stachowicz, J.J. and Watson, R., 2006. Impacts of biodiversity loss on ocean ecosystem services. Science, 314: 787-790. https://doi.org/10.1126/science.1132294
Xiao, Y. and Kumar, M., 2004. Sex ratio, and probability of sexual maturity of females at size, of the blue swimmer crab, Portunus pelagicus Linneaus, off southern Australia. Fish. Res., 68: 271–282. https://doi.org/10.1016/j.fishres.2003.11.012
Zairion, Wardiatno, Y., Boer, M. and Fahrudin, A., 2015. Reproductive biology of the blue swimming crab Portunus pelagicus (Brachyura: Portunidae) in East Lampung waters, Indonesia: Fecundity and reproductive potential. Trop. Life Sci. Res., 26: 67–85. https://doi.org/10.17485/ijst/2015/v8i6/69368
To share on other social networks, click on any share button. What are these?










