Amelioration of Thermo-Tolerance Ability in Spinacia oleracea L. Cultivars by Foliar Application of Chitosan
Muhammad Nazar1, Chaudhary Muhammad Ayyub2, Mujahid Ali3*, Malik Abdul Rehman4, Shoib ur Rehman5 and Saqib Ayyub2
1Department of Agriculture, Bhimber, Government of Azad Jammu and Kashmir; 2Institute of Horticultural Sciences, University of Agriculture, Faisalabad-38000, Pakistan; 3University of Agriculture, (Burewala sub-campus), Faisalabad, Pakistan; 4Citrus Research Institute, Sargodha, Pakistan; 5University of Agriculture, Sub-Campus Depalpur Okara, Pakistan.
Abstract | Heat stress causes deleterious effects on growth and production of spinach in tropical and subtropical areas. There are many techniques to cope with these stresses but foliar application of chitosan was a very significant tool. Three cultivars of spinach (All Pure Green, Desi Local, and Lahori Palak) were grown at normal temperature 18/20°C (day/night temperature) and then after four weeks after emergence 40/32°C (day/night temperature) was given by gradually enhancing 2°C per day. After one week of heat stress (40/32°C), four treatments (25, 50, 75, 100 ppm) of chitosan were applied as a foliar spray on spinach cultivars in comparison to control (non-treated with chitosan). The research findings evidenced that foliar application of chitosan significantly affected attributes under heat stress. All Pure Green was affected more followed by Desi Local, while Lahori Palak was least affected. Chitosan at 100 ppm revealed the optimized results. It was observed that maximum value (17.48 SPAD units) was found in Lahori Palak at 25 ppm of chitosan, followed (17.05 SPAD units) at 100 ppm in Lahori Palak for chlorophyll contents. The minimum EL (57.75%) was observed in Lahori Palak at 100 ppm, similarly chlorophyll content were also maximum (17.48 SPAD units) in Lahori Palak at 100 ppm, building Lahori Palak more responsive toward chitosan against heat stress. Chitosan application with optimum concentration may help to improve the production potential of spinach cultivars in areas hit by temperature stresses.
Received | August 14, 2020; Accepted | November 30, 2020; Published | December 13, 2020
*Correspondence | Mujahid Ali, University of Agriculture, (Burewala sub-campus), Faisalabad, Pakistan; Email: mujahidali2263@gmail.com
Citation | Nazar, M., Ayyub, C.M., Ali, M., Rehman, M.A., Rehman, S. and Ayyub, S., 2020. Amelioration of thermo-tolerance ability in Spinacia oleracea L. cultivars by foliar application of Chitosan Journal of Innovative Sciences, 6(2): 197-205.
DOI | http://dx.doi.org/10.17582/journal.jis/2020/6.2.197.205
Keywords | Chitosan, Heat stress, Spinach, Physiology, Growth
1. Introduction
Spinach (Spinacia oleracea L.) is a winter season vegetable, found in moderate climatic conditions and grows best at 15-18°C range of temperature. Pakistan ranked 8th in spinach production, contributing only 0.4% to overall global production. Pakistan produced about 109 thousand tons annually of spinach from the area of 8820 hectares (GOP, 2017-18). Due to climatic change at global level and in the region, temperature fluctuation is being observed which affects crop production. Abiotic stresses i.e. drought, heat and salinity have deleterious impact on growth and development of horticultural crops (Parmar et al., 2017). Heat stress is a leading climatic factor to food security, as it leads to reduction in productivity of crops and livestock population. Diverse species of vegetables showed different trend in response to heat stress, as data collected indicates 1°C rise in temperature cause 10-15% loss decline in crop production (Upadhyaya et al., 2011). Plants naturally compete stress condition and become agile in response to those hostile conditions (Wang et al., 2003). Depending on the severity caused by environmental abiotic factors; plant may adopt different functional, structural and anatomical changes (Potters et al., 2007). These fluctuations cause decline in growth, productivity, nutritional potential and metabolic profile of plants (Altman, 2003). Various genotypes of the vegetables vary in their thermos-tolerance ability (Ali et al., 2019). There are number of other ways to protect plants from heat stress viz. genetic approaches, ROS (reactive oxygen species) and various protectants against heat stress. These different approaches have their different pros and cons which make them suitable or inappropriate for plant protection against heat stress. In spinach at temperatures higher than 35°C, CO2 assimilation rate declined significantly which lead to lower yield (Tang et al., 2007).
Chitosan is environment friendly natural polymer (Bakhshi et al., 2020), formerly used as a natural seed treatment but a study evidenced that its foliar application is more effective than seed treatment (Janmohahammadi et al., 2014). It (co-polymer of D-glucosamine and N-acetyl-d-glucosamine) has ability to mitigate heat stress in cucumber (Ali et al., 2020). It has been proved a vital chemical for sustainable crop production (Ahmed et al., 2020). In recent years, in search of biological approaches avoidance of chemicals in Agriculture has directed exploring the practice of bio-polymers based biomaterials. Among all the tested biomaterials, chitosan gave the best results (Pichyangkua and Chadchawan, 2015). Chitosan is obtainable in bulk masses from the de-acetylation of the chitin, it has numerous benefits: it is harmless, cheap and can be easily linked with the other compounds to accomplish to enhanced performance of this biomaterial. In recent years, this natural biomaterial with fascinating physiological prospective has been receiving more attention (Katiyar et al., 2015). It has been used as a natural seed treatment (Mohamed et al., 2020) its coating of fruits and vegetables enhanced the shelf life and quality (Cui et al., 2020; Shah and Hashmi, 2020) in abiotic stress resistance (Menazea et al., 2020).
Recent studies have revealed that chitosan influences mechanisms of the plants against numerous biotic factors i.e. bacteria, fungi and insects as well as against abiotic factors including cold, toxic metals, salinity, drought and heat stress to enhance the productivity of crops. This natural biomaterial with fascinating physiological prospective has been receiving more consideration and a noticeable interest in agriculture systems due to its tremendous properties particularly in horticultural crops. Chitosan ascertained essential to cope with heat stress in spinach plant. It is widely applicable in agricultural industry to overcome stresses especially heat stress as well as medicines and many other fields (Anusuya and Sathiyabama, 2016).
2. Material and Methods
The current experiment was conducted in growth room of Institute of Horticultural Sciences, University of Agriculture, Faisalabad, during 2018. Three cultivars of spinach were selected (All Pure Green, Desi Local, and Lahori Palak) were obtained from Ayyub Agriculture Research Institute (AARI), Faisalabad. Seeds were sown in plastic pot at (18°C/20°C day/night) in growth room having automated heating and cooling units. 14 hours’ day (lights remained turned on) 10 hours’ night (lights remained turned off). Sand was used as growing medium. Half strength Hoagland’s solution was used for nutrition. Growth room was lightened up by 10000 lux light intensity to provide suitable environment for photosynthesis with 65% humidity level. When seedlings were one-month old temperature was raised daily by 2°C to avoid osmotic shock, until highest temperature (32°C/40°C day/night) was achieved. Plants kept for one-week at 32°C/40°C day/night was considered heat stress. Chitosan was dissolved in water to make it more soluble 0.1 molar C2H4O2 (at room temperature, 12 hours with stirring) was added. One-week after application of high temperature (40/32°C with 14 hours’ day/10 hours’ night), various chitosan levels were sprayed on foliage of seedlings. While, four treatments of chitosan were applied on spinach cultivars i.e. 25, 50, 75, 100 ppm in comparison to control (non-treated with chitosan). Plants at control (without chitosan application) were just foliarly sprayed with distilled water. 1 week after the application of chitosan, data for different parameters viz. number of leaves, leaf area (cm2), along with root and shoot length (cm), root to shoot ratio, mass of fresh seedlings (g), mass of dry seedlings (g) and shoot length (cm) were collected.
2.1 Physiological attributes
2.1.1 Chlorophyll contents (SPAD value)
Chlorophyll content (SPAD value) of leaves of randomly selected 4 plants per pot were measured by the help of SPAD mater (CCM-200 plus Bio- Scientific USA).
2.2 Cell membrane thermo-stability
It was measured indirectly by determining electrolyte leakage (EL) (%). To calculate electrolyte leakage of leaf cells assessment of the cell membrane stability (CMS) was done by following method of Farkhondeh et al. (2012) with a few alterations. Leaf samples were taken, after washing 0.3 g of leaf samples with deionized water, these were placed in tubes which had 15 mL of deionized water and incubated for two hours at 25°C. After that electrical conductivity of the solution (L1) was determined. Samples were then autoclaved at 120°C for twenty minutes and the final conductivity (L2) was calculated after equilibration at 25°C. Leaf electrolyte leakage (EL) was measured by following formulae:
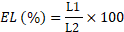
2.3 Statistical analysis
The experiment was conducted following 2 factor factorial (cultivars vs chitosan doses) under Completely Randomized Design (CRD). Collected data was evaluated statistically through ANOVA and significance was tested by means of LSD (least significance difference) test at probability level of 5%.
3. Results and Discussion
3.1 Agronomic traits
Under heat stress (32°C/40°C day/night), it was observed that maximum value (4.10 cm) was found in Desi Local at 75 ppm of chitosan, followed (3.65 cm) at 100 ppm in Lahori Palak under heat stress. However, it was seen that minimum value (2.40 cm) of shoot length was observed in Lahori Palak at 75 ppm, followed by (2.70 cm) at control in All Pure Green under heat stress. On overall basis, it was observed that Desi Local spinach have more shoot length as compared with other cultivars under heat stress regimes (Table 1).
Among cultivars, All Pure Green got maximum value (6.10 cm) at 100 ppm of chitosan, followed by Lahori Palak (5.20 cm) at 75 ppm regarding root length at 40°C. However, the minimum value (4.18 cm) of root length was observed in Lahori Palak at control, followed by All Pure Green (4.28 cm) at control at 32°C/40°C (day/night). On the basis of average results, it was noticed that 100 ppm was best dose regarding root length. It was also found that with increasing the concentration of chitosan overall the value of root length was increased in all cultivars of spinach under heat stress. At highest temperature, it was seen that All Pure Green got highest position regarding root length (Table 2).
Table 1: Effect of chitosan on shoot length (cm) of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
2.70 cd |
3.58 a-d |
3.45 a-d |
3.24 A |
|
25 ppm |
3.00 a-d |
3.50 a-d |
3.13 a-d |
3.21 A |
|
50 ppm |
3.43 a-d |
4.02 ab |
2.40 d |
3.28 A |
|
75 ppm |
2.88 bcd |
4.10 a |
2.68 cd |
3.22 A |
|
100 ppm |
3.43 a-d |
2.90 bcd |
3.65 abc |
3.33 A |
|
Mean |
3.09 B |
3.62 A |
3.06 B |
3.38 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results. CHT; chitosan.
Table 2: Effect of chitosan on root length (cm) of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
4.33 ab |
4.45 ab |
4.18 b |
4.32 A |
|
25 ppm |
4.28 ab |
4.35 ab |
4.73 ab |
4.45 A |
|
50 ppm |
5.10 ab |
5.10 ab |
4.73 ab |
4.98 A |
|
75 ppm |
4.63 ab |
4.58 ab |
5.20 ab |
4.80 A |
|
100 ppm |
6.10 a |
4.63 ab |
5.23 ab |
5.32 A |
|
Mean |
4.89 A |
4.62 A |
4.81 A |
4.77 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results.
On overall basis, it was seen that Desi Local cultivar of spinach showed maximum number of leaves. The maximum value (6.00) was gained by Desi Local at 100 ppm of chitosan, followed by Desi Local (5.50) at 25 ppm regarding number of leaves under heat stress. However, it was seen that minimum value (3.00) of number of leaves was observed in Lahori Palak at 75 ppm, followed by in 25 ppm in Lahori Palak (3.25) under heat stress. It was noticed that on the basis of average results 100 ppm was best dose regarding number of leaves. It was also found that with increasing the concentration of chitosan overall numbers of leaves were increased in all cultivars of spinach under heat stress (Table 3).
Table 3: Effect of high temperature on number of leaves of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
4.75 abc |
4.25 b-e |
4.25 b-e |
4.42 A |
|
25 ppm |
4.25 b-e |
5.50 ab |
3.25 de |
4.33 A |
|
50 ppm |
4.25 b-e |
5.00 abc |
4.50 bcd |
4.58 A |
|
75 ppm |
4.50 bcd |
4.25 b-e |
3.00 e |
3.92 A |
|
100 ppm |
4.25 b-e |
6.00 a |
4.00 cde |
4.75 A |
|
Mean |
4.40 AB |
5.00 A |
3.80 B |
4.40 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results.
However, maximum value of mass of fresh seedling (0.772 g) was found in Lahori Palak at 75 ppm of chitosan, followed (0.690 g) at control in Lahori Palak for fresh weight under heat stress (40°C). It was seen that minimum value (0.304 g) of fresh mass was observed in All Pure Green at control, followed by (0.341 g) at 25 ppm in Desi Local at 32°C/40°C. On the basis of average result, it was noticed that 75 ppm was best dose regarding the fresh mass (g). It was also found that with increasing the concentration of chitosan overall fresh mass increased in all cultivars of spinach under heat stress. Lahori Palak gained highest position regarding fresh mass at 32°C/40°C day/night (Table 4).
Table 4: Effect of chitosan on fresh weight (g) of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
0.304 e |
0.384 cde |
0.690 ab |
0.459 A |
|
25 ppm |
0.447 b-e |
0.341 de |
0.672 abc |
0.487 A |
|
50 ppm |
0.522 a-e |
0.502 a-e |
0.569 a-e |
0.531 A |
|
75 ppm |
0.578 a-e |
0.360 de |
0.772 a |
0.570 A |
|
100 ppm |
0.629 a-d |
0.389 b-e |
0.591 a-e |
0.536 A |
|
Mean |
0.496 B |
0.395 B |
0.659 A |
0.517 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results.
The maximum value (0.088 g) was found in Lahori Palak at 100 ppm of chitosan, followed by Lahori Palak (0.081 g) at 75 ppm regarding dry mass under heat stress. However, it was seen that minimum value (0.033 g) of dry mass was observed in All Pure Green at control, followed by (0.039 g) at 25 ppm in Desi Local under heat stress. On the basis of average results, it was noticed that 100 ppm was best dose regarding seedling dry mass (g). It was also found that with increasing the concentration of chitosan, overall the dry mass was increased in all cultivars of spinach under heat stress. Overall, Lahori Palak cultivar of spinach got highest position regarding dry mass under heat stress (Table 5).
Table 5: Effect of chitosan on dry weight (g) of spinach cultivars at 40°C
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
0.033 e |
0.039 de |
0.060 a-e |
0.044 A |
|
25 ppm |
0.051 b-e |
0.039 de |
0.071 a-d |
0.054 A |
|
50 ppm |
0.051 b-e |
0.055a-e |
0.059 a-e |
0.055 AB |
|
75 ppm |
0.061 a-e |
0.045 cde |
0.081 ab |
0.062 AB |
|
100 ppm |
0.078 abc |
0.057 a-e |
0.088 a |
0.074 A |
|
Mean |
0.055 B |
0.047 B |
0.072 A |
0.058 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results.
Table 6: Effect of chitosan on root to shoot ratio of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
1.61 abc |
1.29 bc |
1.32 bc |
1.41 A |
|
25 ppm |
1.47 abc |
1.28 bc |
1.62 abc |
1.45 A |
|
50 ppm |
1.46 abc |
1.30 bc |
2.28 a |
1.68 A |
|
75 ppm |
1.59 abc |
1.14 c |
2.10 ab |
1.61 A |
|
100 ppm |
1.80 abc |
1.86 abc |
1.39 bc |
1.68 A |
|
Mean |
1.58 A |
1.37 A |
1.74 A |
1.57 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant.
It was observed that maximum value (2.28) was found in Lahori Palak at 50 ppm of chitosan, followed by (2.10) at 75 ppm in Lahori Palak root to shoot ratio under heat stress. It was seen that minimum value (1.14) root to shoot ratio was observed in Desi Local at 75 ppm, followed by (1.28) at 25 ppm in Desi Local under heat stress. However, on the basis of average result it was noticed that 100 ppm was best dose at par with 50 ppm regarding root to shoot ratio. It was also found that with increasing the concentration of chitosan overall root to shoot ratio increased in all cultivars of spinach under heat stress. On average basis, Lahori Palak got highest position regarding root to shoot ratio under heat stress (Table 6).
Cultivar and treatment comparison was significantly observed (Table 8). It was observed that maximum value (11.98 cm2) was found in All Pure Green at 100 ppm of chitosan, followed by Lahori Palak (10.8 cm2) at control regarding leaf area under heat stress. It was seen that minimum value (3.65 cm2) leaf area was observed in Desi Local at 25 ppm, followed by at 3.75 cm2 in Desi Local under heat stress. On the basis of average result 100 ppm was best dose regarding leaf area. It was also found that with increasing the concentration of chitosan overall leaf area increased in all spinach cultivars under heat stress. During heat stress, Lahori Palak got highest position regarding the leaf area (Table 7).
Table 7: Effect of chitosan on leaf area (cm2) of spinach cultivars at 40°C.
|
Treatments |
All pure green |
Desi local |
Lahori palak |
Mean |
|
No CHT |
5.40 def |
4.25 f |
10.78 ab |
6.81 A |
|
25 ppm |
5.90 def |
3.65 f |
10.33 abc |
6.63 A |
|
50 ppm |
7.18 c-f |
4.63 ef |
6.25 def |
6.02 A |
|
75 ppm |
8.23 bcd |
4.18 f |
8.45 a-d |
6.95 A |
|
100 ppm |
11.98 a |
3.75 f |
8.10 b-e |
7.94 A |
|
Mean |
7.74 A |
4.09 B |
8.78 A |
6.87 |
Different letters in each column and row indicate significant difference among means, while same letters in each column and row showed non-significant results.
3.2 Physiological traits
It was observed that maximum value (17.48 SPAD units) was found in Lahori Palak at 25 ppm of chitosan, followed (17.05 SPAD units) at 100 ppm in Lahori Palak for chlorophyll contents under heat stress. It was seen that minimum value (8.33 SPAD units) chlorophyll was observed in Desi Local at control, followed by (9.08 SPAD units) at 100 ppm in Desi Local under heat stress. Based on average results it was noticed that 100 ppm was best dose regarding chlorophyll contents. It was also found that with increasing the concentration of chitosan overall chlorophyll contents increased in all cultivars of spinach under heat stress. On the overall basis the Lahori Palak got highest Position regarding chlorophyll contents (SPAD value) under heat stress (Figure 1).
It was found that various concentrations of chitosan significantly affect electrolyte leakage of spinach cultivars over control (Table 8). It was observed that maximum value (79.50%) was found in All Pure Green at control, followed by Lahori Palak (77.50%) at 25 ppm regarding electrolyte leakage under heat stress. It was seen that minimum value (57.75%) electrolyte leakage was observed in Lahori Palak at 100 ppm, followed by All Pure Green (63.25%) at 100 ppm of chitosan under heat stress. Based on average results it was noticed that 100 ppm was best does regarding this parameter It was also found that with increasing he concentration of chitosan overall electrolyte leakage %age increased in all spinach cultivars under heat stress. Under heat stress, on the overall basis Desi Local cultivar of spinach got highest position regarding to electrolyte leakage (Figure 2).
To maintain water relation is the key for proper functioning of metabolism. So the use of antitranspirants have been proved an effective technique to mitigate effect of heat stress in horticultural crops recently (Dash et al., 2020; Tonhati et al., 2020).
Table 8: Analysis of variance table for influence of chitosan on different spinach cultivars under heat stress.
|
Parameters |
Source of variation |
P value |
Parameters |
Source of variation |
P value |
|
Shoot length |
Cultivar (G) |
0.0657NS |
Root length |
Cultivar (G) |
0.8046NS |
|
Treatment (T) |
0.9966NS |
Treatment (T) |
0.3503NS |
||
|
V x T |
0.1134NS |
G x T |
0.9079NS |
||
|
No of leaves |
Cultivar (G) |
0.0026* |
Fresh Weight |
Cultivar (G) |
0.0013** |
|
Treatment (T) |
0.3533NS |
Treatment (T) |
0.7310NS |
||
|
G x T |
0.197NS |
G x T |
0.5009NS |
||
|
Dry Weight |
Cultivar (G) |
0.0061** |
Plant height |
Cultivar (G) |
0.0036** |
|
Treatment (T) |
0.0385** |
Treatment (T) |
0.3153NS |
||
|
G x T |
0.8856NS |
G x T |
0.2952NS |
||
|
Root to shoot ratio |
Cultivar (G) |
0.0000** |
Leaf area |
Cultivar (G) |
0.0000** |
|
Treatment (T) |
0.8233NS |
Treatment (T) |
0.4545NS |
||
|
G x T |
0.1652NS |
G x T |
0.0131* |
||
|
EL |
Cultivar (G) |
0.2940NS |
|||
|
Treatment (T) |
0.0000** |
||||
|
G x T |
0.0248* |
When P >0.05=Non-significant NS; P ≤0.05=Significant*; P ≤0.01=Highly significant**
Chitosan has played vital role in alleviation of heat stress in plants (Sharif et al., 2018). In present study various levels of chitosan (0, 25, 50, 75, 100 ppm) were utilized to optimize its level to gain maximum alleviation from drastic effects of heat stress because optimization of growth stimulants, regulators and promotors is very vital step in alleviation of abiotic stress in vegetables. But foliar application oat 100 ppm it was seen that it gives the maximum potential to reduces the stress condition giving release. Cultivars varied in their capacity to tolerate heat stress (Hussain et al., 2016; Ali et al., 2020). Chlorophyll luminescence is considered a key parameter for heat stress injury (Bilger et al., 1990). High temperature above threshold temperature weakened the cell membrane, becomes a cause to produce ROS that mainly attacks photosystem II and respiratory pathways (Goraya et al., 2017).
During optimization, it was comprehended that spinach cultivars sustainably reduced transpiration rate at each level as it is an effective anti-transpiring chemical. These results confirmed the past research in pepper plants, where 26-43% reduction of water loss upon treatment with chitosan was estimated without changing in biomass production under controlled growth room conditions in pepper plant (Bittelli et al., 2001). It was observed that by chitosan treatment, stem length of plants, number of leaves per plant and mass of fresh and dry leaves was increased (Shehata et al., 2012). This fact also indicated that chitosan was applied as foliar spray on basil plants enhanced height, inflorescence, number of branches, leaf area index, fresh and dry mass of seedling roots and shoots (Pirabalouti et al., 2017). Leaf area was also enhanced as was described earlier by Salachna et al. (2014). It is proved that chitosan is plant growth enhancer and act similar to a plant promoter (Uthairatankij et al., 2007), enhanced stem length, number of leaves, relative growth rate and yield of okra (Pichyangkura and Chadchawan, 2015). In one of study, it was seen that cultivars with less surface temperature and more chlorophyll contents revealed better performance in photosynthetic rate. Such conclusions were reinforced by Shaheen et al. (2016) who observed that that if a cultivar has low temperature of leaf surface and higher lead SPAD value would have more photosynthetic rate. During examination it was observed that heat tolerant cultivars have high chlorophyll contents (SPAD value) than susceptible cultivars in all sowing dates, which is also confirmed (Hussain et al., 2016). Charoenwattana (2013) explored the response of chitosan polymer and lotus extracts (an herbal) to on morphological attributes of orchid. Orchid plantlets were treated with various chitosan levels (10, 30, 50 and 100 ppm) and various concentrations of lotus extract. Highest value for number of leaves and maximum and other growth parameters were observed in plants spray with 100 ppm chitosan. Heat stress produced more electrolyte leakage as compared to normal conditions in recent findings which might be due to damage to cell membrane damage as a result of lipid peroxidation which are indicator of heat stress (Yoshida, 2012) this lipid peoxidation lead to oxidative stress (Kipp and Boyle, 2013) and in response plant develop defensive mechanism (Gommers, 2020). Previous finding of Nguyen et al. (2020) revealed that nano-chitosan mixed with calcium chloride improved antioxidant in strawberry and reduced the MDA content by adopting defensive mechanism. Current findings revealed that chitosan maintained the cell membrane thermos-stability and chlorophyll contents of spinach cultivars under heat stress which are in line with the outcomes of Ali et al. (2020).
Conclusions and Recommendations
It was stated that different cultivars have different heat tolerance ability, but chitosan at 100 ppm enhanced the ability to respond against high stress at an optimized level. Moreover, chitosan improved heat resistance capacity under elevated temperature (40°C) prominently more in Lahori Palak. Furthermore, cultivars having a comparatively high capacity to tolerate high temperature could be used in the breeding programs to ensure gene transfer in high yield varieties for the economical yield potential in spinach.
Acknowledgements
Authors acknowledge Australian Centre for International Agricultural Research (ACIAR) and University of Sydney Australia for construction of the plant growth room with automatic heating system along with providing supplementary instruments.
Novelty Statement
The study explored characterization of spinach cultivars, All Pure Green, Desi Local, and Lahori Palak under heat stress regime. These cultivars would not only boost cucumber production during hot summer in Punjab, Pakistan but also in hot/tropical areas of the world by the application of chitosan which has been proven to maintain turgor potential during excessive water loss in plant.
Muhammad Nazar conducted research trial and Chaudhary Muhammad Ayyub supervised it. Mujahid Ali and Malik Abdul Rehman reviewed the article. Shoib ur Rehman guided about treatments. Saqib Ayyub corrected grammar and spelling mistakes.
Conflict of interest
The authors have declared no conflict of interest.
References
Ahmed, K.B.M., Khan, M.M.A., Siddiqui, H. and Jahan, A., 2020. Chitosan and its oligosaccharides, a promising option for sustainable crop production, a review. Carbohydrate Polymer, 227: 115331. https://doi.org/10.1016/j.carbpol.2019.115331
Ali, M., Ayyub, C.M. Hussain, Z. Hussain R. and Rashid, S., 2020. Optimization of chitosan level to alleviate the drastic effects of heat stress in cucumber (Cucumis sativus L.). Journal of Pure and Applied Agriculture, 5(1): 30-38.
Ali, M., Ayyub, C.M., Amjad, M. and Ahmad, R., 2019. Evaluation of thermo-tolerance potential in cucumber genotypes under heat stress. Pakistan Journal of Agricultural Science, 56(1): 53-61.
Altman, A., 2003. From plant tissue culture to biotechnology: scientific revolutions, abiotic stress tolerance and forestry. In vitro cell division. Biological Plant, 39: 75–84. https://doi.org/10.1079/IVP2002379
Anusuya, S. and Sathiyabma, M., 2016. Effect of chitosan on growth and curcumin content in turmeric under field condition. Biocatalysis and Agricultural Biotechnology, 6: 102-106. https://doi.org/10.1016/j.bcab.2016.03.002
Bakshi, P.S., Selvakumar, D., Kadirvelu, K. and Kumar, N.S., 2020. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. International Journal of Biological Macromolecules, 150: 1072-1083. https://doi.org/10.1016/j.ijbiomac.2019.10.113
Bilger, W. and Schreiber, U., 1990. Chlorophyll luminescence as an indicator of stress-induced damage to the photosynthetic apparatus. Effects of heat-stress in isolated chloroplasts. Photosynthetic Research, 25(3): 161-171. https://doi.org/10.1007/BF00033158
Bittelli, M., Flury, M. Campbell, G.S. and Nichola, E.J. 2001. Reduction of transpiration drought foliar application of chitosan. Agricultural and Forest Meteorology, 107: 167-175. https://doi.org/10.1016/S0168-1923(00)00242-2
Charoenwattana, P., 2013. Effects of chitosan and lotus extracts as growth promotor in dendrobium orchid. International Journal of Environmental and Rural Development, 42: 113-137.
Cui, K., Shu, C., Zhao, H., Fan, X., Cao, J. and Jiang, W., 2020. Preharvest chitosan oligochitosan and salicylic acid treatments enhance phenol metabolism and maintain the postharvest quality of apricots (Prunus armeniaca L.). Scientia Horticulture, 267: 109334. https://doi.org/10.1016/j.scienta.2020.109334
Dash, P.K., Chase, C.A., Agehara, S. and Zotarelli, L., 2020. Heat stress mitigation effects of kaolin and s-abscisic acid during the establishment of strawberry plug transplants. Scientia Horticulture, 267: 109276. https://doi.org/10.1016/j.scienta.2020.109276
Farkhondeh, R., Nabizadeh, E. and Jalilnezhad, N., 2012. Effect of salinity stress on proline content, membrane stability and water relations in two sugar beet cultivars. International Journal of Agricultural Science. 2: 385-392.
Gommers, C., 2020. Keep cool and open up: Temperature-induced stomatal opening. Plant Physiology, 181: 1188-1189. https://doi.org/10.1104/pp.20.00158
GOP (Government of Pakistan). 2017-18. MNSFR (Ministry of national food security and research economic). Fruit, vegetables and condiments statistics of Pakistan.
Goraya, G.K., Kaur, B., Asthir, B., Bala, S., Kaur, G. and Farooq, M., 2017. Rapid injuries of high temperature in plants. Journal of Plant Biology, 60: 298-305. https://doi.org/10.1007/s12374-016-0365-0
Hussain, R., Ayyub, C.M. Amjad, M., Waraich, E.A., Shaheen, M.R., Mustafa, Z., Shah, S.Z.H., Adnan, A., Haider, M.W., Faiz, H. and Raza, A. 2016. Evaluation of heat tolerance potential of okra genotypes in field conditions at different sowing dates. Transylvanian Review, 12: 3416-3427.
Janmohahammadi, M., Mostafavi, H., Kazemi, H., Mahadavinia G.R. and Sabaghnia. N., 2014. Effect of chirosan application on the performance of lentil genotypes under rain fed conditions. Acta Technologica Agriculturae, 17: 86-90. https://doi.org/10.2478/ata-2014-0020
Katiyar, D., Hemantaranjan, A. and Singh, B., 2015. Chitosan as a promising natural compound to enhance potential physiological responses in plant a review. Indian Journal of Plant Physiology, 201-209. https://doi.org/10.1007/s40502-015-0139-6
Kipp, E. and Boyle, M., 2013. The effects of heat stress on reactive oxygen species production and chlorophyll concentration in Arabidopsis Thaliana. Research in Plant Sciences, 1(2): 20-23.
Menazea, A.A., Eid, M.M. and Ahmed, M.K., 2020. Synthesis, characterization, and evaluation of antimicrobial activity of novel Chitosan/Tigecycline composite. International Journal of Biological Macromolecules, 147: 194-199. https://doi.org/10.1016/j.ijbiomac.2020.01.041
Mohamed, C., Etienne, T.V. and Yannick, K.N.G., 2020. Use of bioactive chitosan and Lippia multiflora essential oil as coatings for maize and sorghum seeds protection. EurAsian Journal of BioSciences, 14(1): 27-34.
Nguyen, V.T., Nguyen, D.H. and Nguyen, H.V., 2020. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biology and Technology, 162: 111103. https://doi.org/10.1016/j.postharvbio.2019.111103
Parmar, N., Singh, K.H., Sharma, D., Singh, L., Kumar, P., Nanjundan, J., Khan, Y.J., Chauhan, D.K., and Thakur, A.K., 2017. Genetic engineering strategies for biotic and abiotic stress tolerance and quality enhancement in horticultural crops: a comprehensive review. 3. Biotechnology, 7(4): 239. https://doi.org/10.1007/s13205-017-0870-y
Pichyangkura, R. and Chadchawan, S., 2015. Biostimulant activity of chitosan in horticulture. Scientia Horticulture, 196: 49-65. https://doi.org/10.1016/j.scienta.2015.09.031
Pirabalouti, A., Malekpoor, G.F., Salimi, A. and Golparvar, A., 2017. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Scientia Horticulture, 217: 114-122. https://doi.org/10.1016/j.scienta.2017.01.031
Potters, G.T.P. Pasternak, Guisez, Y., Palme K.J., and Janse, M.A.K., 2007. Stress induced morphogenic responses growing out of trouble. Trends in Plant Science, 12: 98–105. https://doi.org/10.1016/j.tplants.2007.01.004
Salachna, P., Wilas, J. and Zawadzińska, A., 2014. The effect of chitosan coating of bulbs on the growth and flowering of Ornithogalum saundersiae. In: XXIX international horticultural congress on horticulture: Sustaining lives, livelihoods and landscapes (IHC2014): 1104, pp. 115-118. https://doi.org/10.17660/ActaHortic.2015.1104.18
Shah, S. and Hashmi, M.S., 2020. Chitosan aloe vera gel coating delays postharvest decay of mango fruit. Horticulture, Environment, and Biotechnology, 61: 279–289. https://doi.org/10.1007/s13580-019-00224-7
Shaheen, M.R., Ayyub, C.M., Amjad, M. and Waraich, E.A., 2016. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. Journal of the Science of Food and Agriculture, 96: 2698-2704. https://doi.org/10.1002/jsfa.7388
Sharif, R., M. Mujtaba, M. Ur Rahman, A. Shalmani, H. Ahmad, T. Anwar, D. Tianchan and X. Wang. 2018. The multifunctional role of chitosan in horticultural crops. A review. Molicules, 23(4): 872. https://doi.org/10.3390/molecules23040872
Shehata, S.A., Fawzy, Z.F. and El-Ramady, H.R., 2012. Response of cucumber plants to foliar allication of chitosan and yeast under greenhouse conditions. Australian Journal of Basic and Applied Sciences, 64: 63-71.
Tang, Y., Wen, X., Lu, Q., Yang, Z., Cheng, Z. and Lu, C., 2007. Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiology, 143(2): 629-638. https://doi.org/10.1104/pp.106.090712
Tonhati, R., Mello, S.C., Momesso, P. and Pedroso, R.M. 2020. L-proline alleviates heat stress of tomato plants grown under protected environment. Scientia Horticulture, 268: 109370. https://doi.org/10.1016/j.scienta.2020.109370
Upadhyaya, H.D., Dronavalli, N., Gowda, C.L.L. and Singh, S., 2011. Identification and evaluation of chick pea germplasm for tolerance of heat stress. Crop Science, 51: 2079-2094. https://doi.org/10.2135/cropsci2011.01.0018
Uthairatankij, A., da-Silva, J.A.T. and Obsuwan, K., 2007. Chitosan improving orchid production and quality. Orchid Science and Biotechnology, 1: 1-5.
Wang, W., Vinocur, B. and Altman, B., 2003. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 218: 1-14. https://doi.org/10.1007/s00425-003-1105-5
Yoshida, Y., Umeno, A. and Shichiri, M., 2012. Lipid peroxidation biomarkers for evaluating oxidative stress and assessing antioxidant capacity in vivo. Journal of Clinical Biochemistry and Nutrition, 52: 9-16. https://doi.org/10.3164/jcbn.12-112
To share on other social networks, click on any share button. What are these?





