Allelopathic Effect of Moringa oleifera L. Aqueous Extract on the Germination and Seedling Growth of Okra (Abelmoschus esculentus L.)
Research Article
Allelopathic Effect of Moringa oleifera L. Aqueous Extract on the Germination and Seedling Growth of Okra (Abelmoschus esculentus L.)
Mahisba Waris1, Muhammad Azim Khan2*, Muhammad Fawad1*, Nabeela Jafer1, Rashid Hussain3 and Haseeb Ahmad3
1Government Frontier College for Women, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Weed Science and Botany, The University of Agriculture, Peshawar 25130, Pakistan; 3Directorate of Non-Timber Forest Products (NTFP), Forest Department, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | A lab study was carried out to determine the effect moringa leaves aqueous extract on germination of okra at Herbarium Lab, Department of Weed Science and Botany, The University of Agriculture, Peshawar. Dried leaves of Moringa were purchased from local market. The leaves were chopped by using a grinder and 50grams of dried leaves powder was added with 500 milliliters of distilled water in a beaker. Subsequently, the mixture was passed through muslin cloth and various concentration was prepared (25%, 50%, 75%, and 100%). Statistical analysis of data reveal that various concentration of moringa aqueous extract significantly affected germination indices of okra seeds with highest germination (93.3%), followed by germination energy (93.3%), germination rate index (22.7), and the mean germination rate (0.245) observed in the control. In addition, the mean germination time was short but exhibited a maximum control level of 4.1 days. At the 25% concentration, the germination was (80%), and germination energy (73.3%), being remarkably the lowest, while the germination rate index (18.2) and mean germination rate (0.24) are considerably lower in comparison to the control. It was further reveal that 50% concentrations resulted (76.7%) germination and (76.7%) germination energy, mean germination rate (0.227) and a mean germination time (4.4 days). Likewise, at 75% concentration, a substantial decline in the germination (43.3%) and germination energy (40%) was observed, with lowest mean germination rate (0.208) and highest mean germination time (4.8 days). Contrary concentration of 100% moringa aqueous extract caused maximum inhibition with (0%) germination, mean germination rate and shoot length. Seed vigor index and germination inhibition of the seeds ranged from 0 and -100%, respectively. Highest coefficient of velocity for both germination and shoot length was observed at 75% concentration, whereas, germination was completely inhibited at 100% concentration at 100% concentration. It is concluded that moringa extract at lower to moderate levels stimulate germination and seedling growth of okra, whereas at high concentration cause severe inhibition of okra seeds.
Received | August 03, 2024; Accepted | September 12, 2024; Published | September 27, 2024
*Correspondence | Muhammad Azim Khan and Muhammad Fawad, Department of Weed Science and Botany, The University of Agriculture, Peshawar 25130, Pakistan; Government Frontier College for Women, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: azim@aup.edu.pk, fawadagrarian@aup.edu.pk
Citation | Waris, M., Khan, M.A., Fawad, M., Jafer, N., Hussain, R. and Ahmad, H., 2024. Allelopathic effect of Moringa oleifera L. aqueous extract on the germination and seedling growth of Okra (Abelmoschus esculentus L.). Pakistan Journal of Weed Science Research, 30(3): 121-130.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2024/30.3.121.130
Keywords | Allelopathy, Aqueous extract, Moringa, Germination indices, Inhibition and stimulation
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Allelopathy is a natural process through which plants inhibit the growth of other plants of either the same or different species by releasing certain compounds (Ain et al., 2023). Certain plant species release chemicals that stimulate or depress the growth of other plants (De-Albuquerque et al., 2010). These positive and negative impacts are due to the active biomolecules referred to as allelochemicals. A large proportion of allelochemicals are secondary metabolites that arise from by-products during various plant physiological processes (Farooq et al., 2011). The allelopathic effects are concentration-dependent: They inhibit plant growth at high concentrations and promote plant growth at low concentrations (Einhellig, 2018; Ain et al., 2023). These, allelochemicals can be utilized effectively as natural herbicides at higher concentrations.
Okra technically known as Abelmoschus esculentus L., is an important vegetable crop grown particularly in the tropical, subtropical, and mild temperate climates of almost all continents of the world. It is the only important vegetable-growing member of the Malvaceae family and is primarily grown in the Indo-Pak peninsula. The origin of okra is from Ethiopia, Sudan, and North-Eastern African countries, although it is very popular in India. Medicinal plants like okra are natural wealth for healthy and disease-free living. The herbal products have rapidly surged into usage in affluent countries and the Western world within the last few decades. India is a land of immense diversity regarding medicine and culture, and its medicinal herbs have been in continuous use since time unknown. Okra is particularly mentioned as having medicinal value in almost all the classical traditional Indian systems of medicine, such as Ayurveda, Siddha, and Unani (Das et al., 2019).
Okra has been used medicinally to replenish plasma or increase blood volume. It is also helpful in treating chronic dysentery, spermatorrhoea, and genito-urinary problems. The benefits of reviving Okra cultivation in commercial production have been highlighted by the crop’s fruits. Okra immature fruits, which are eaten as vegetables, can be used in salads, soups, and stews, either fresh or dried, fried or cooked. It is utilized to enlarge blood volume or replace plasma (Adetuyi et al., 2008; Kumar et al., 2010). Because it contains iodine, it is used as a goiter treatment. In addition, it is a treatment for a wide range of illnesses, including diabetes ulcers, genito-urinary problems, and chronic dysentery. Okra mucilage has medicinal applications when used as a plasma replacement or blood volume expander (Sayyad et al., 2024).
Regardless of its nutritional value, many components of this plants are widely utilized in traditional medicine (antidiabetic, antipyretic, diuretic, antispasmodic, etc.) all over the world. Okra polysaccharides and fiber are also recognized to be sources of energy and bio therapeutics that may be used to create vital medications. Okra has the potential to replace the majority of medications in the treatment of numerous ailments. It also offers the possibility of Okra going through the many stages of the clinical features. Okra plants are considered herbaceous plants. Okra can grow up to seven feet tall. Okra flowers are symmetrical and bisexual. The five petals of an okra flower are golden in color. The base of the petal is purple in color. Okra, a summer crop, contains a variety of components, including proteins, minerals, vitamins, and iodine. Although roasted seeds can be used in place of coffee grounds, the young pod is eaten raw. Because of its antioxidant, antidiabetic, antineoplastic, anticancer, antibacterial, and antiulcerogenic properties, okra mucilage extract has been widely employed in medicine (Basnet et al., 2023).
Moringa is a highly valued plant, distributed in many countries of the tropics and subtropics. Growing wild in tropical regions of Africa, Hawaii, India, Pakistan, and the Philippines, among other countries. It yields more each year than most other secondary veggies. Moringa, a medicinal tree in the Moringaceae family, is described by Shahzad et al. (2013). It has numerous uses in agriculture. Crops such as wheat, maize, and tomatoes have benefited from the use of Moringa leaf extracts. Moringa leaf extract is widely available, safe for the environment, and used in medicinal treatments. Furthermore, Moringa extracts contain antibacterial characteristics, which make them effective against disease-causing microorganisms such as soil-borne disorders (Ali et al., 2004). It significantly improved sickness treatment and enhanced yield by 20-30%.
M. oleifera L. is significant not only for its high nutritional content and potent water purifying properties, but also for its therapeutic properties. This plant has many different parts that are used in traditional medicine, especially in South Asia, to treat a variety of illnesses. The leaves, roots, seeds, bark, fruit, flowers, and immature pods all stimulate the heart and circulatory system and have antitumor, antipyretic, antiepileptic, anti-inflammatory, antiulcer, antispasmodic, diuretic, antihypertensive, cholesterol-lowering, antioxidant, hepato protective, antibacterial, and antifungal properties (Dixit et al., 2016). Moringa contain numerous beneficial nutrients (i.e., macro and micro-nutrient) and amino acids needed for human body, so this plant is also known as a “Miracle tree” (Koul and Chase, 2015). Depending on the cultivar and source, the chemical makeup of the various portions of the Moringa tree may differ. Moringa leaves, seeds, and flowers are used in food in a variety of ways. In Pakistan, Moringa grows well in semi-arid conditions and blossoms in the summer (Yasmeen, 2011). In order to ensure strong plant growth in late spring and summer before the arrival of the winter cold, Moringa is typically sown in the early spring. Moringa is a member of the rapidly expanding Moringaceae family of trees (Wei et al., 2021).
Moringa can be spread by cuttings or seeds, and the rate of germination is extremely high (Gadzirayi et al., 2013). It can withstand drought and requires very little maintenance. Moringa grows quickly and has a light crown. Its delicate, deciduous leaflets may readily separate, break down, and release nutrients for the crops growing beneath the canopy. Growing Moringa trees has significant multifunctional and enormous benefits in the cropland agroforestry system above flood level, where wheat is also a key cereal crop (Balakumbahan et al., 2024). Moringa extract promotes growth by containing plant hormones like cytokinins and gibberellins. It also improves root development and nutrient up take. Moringa extract improves soil nutrition by providing vitamins, minerals, and amino acids, boosting plant health. It improves plant resilience to environmental stressors including drought and high temperatures through antioxidant activity. Moringa extract has been demonstrated in studies to boost crop yields. Cytokinin help prolong photosynthesis by increasing cell division and delaying leaf senescence. Antioxidants improve overall plant health by reducing oxidative stress (Mashamaite et al., 2022).
Numerous studies have documented that extracts from Moringa leaves significantly enhanced seed germination as well as crop growth and production. It enhances plant development, water balance, cell membrane stability, and antioxidant level, all of which improve crop productivity (Yasmeen et al., 2012, 2013). It is evident that Moringa leaf extract can enhance many crops vegetative and reproductive growth. As a result, the goal of the current study is to determine how Moringa leaf extract affects the germination and growth of Okra.
Objectives
- To determine the effect of Moringa aqueous extract on the germination indices of Okra seeds.
- To evaluate the overall seedling vigor in response to different concentrations of the extract.
- To find the inhibitory or stimulatory effect of moringa aqueous extract on germination percentage and shoot length of okra seed.
Materials and Methods
Apparatus
The following materials were used during the experiment; petri plates, beakers, flasks, strainer, filter paper, scissors/paper, permanent marker, dropper, grinder, weight-balance, measuring tape, moringa dried powdered leaves, okra seeds, distilled water and aluminium foil.
Experimental description
The present study investigated the allelopathic effect of Moringa leaf extract (Moringa oleifera L.) on okra seed germination and development. The experiment was performed in the Herbarium Lab, Department of Weed Science and Botany, The University of Agriculture, Peshawar-Pakistan.
Preparation of moringa aqueous extract
The Okra (Abelmoschus esculentus L.) seeds were selected based on their complete consistency in size and color. Mature, fresh Moringa leaves were gathered and meticulously cleaned. 50 grams of Moringa leaves were crushed in 500 milliliters of distilled water using a mixer grinder. After that, the mixture was passed through muslin cloth to create distinct solutions at the required 25%, 50%, 75%, and 100% concentrations. In order to do this, the stock solution was appropriately diluted with distilled water (Fouad et al., 2019). For instance, to make 25% solution, we combined 25% stock solution with 75% distilled water. Similarly, to get 100% concentration, we combined 50% stock solution with 50% distilled water and 75% stock solution with 25% distilled water to make a 100% concentration solutions in all beaker.
Procedure
This study looks into the allelopathic effects of Moringa aqueous extract on the germination of Okra seeds and the growth of seedlings. It is believed that Moringa influences the physiological processes of nearby plants, such as seed germination and early growth phases, because of its bioactive compounds. The plant’s aqueous extract validates the outcome. The study aims to conduct a scientific evaluation of the effects of varying doses of Moringa extract on the percentage of germination of seeds and the growth characteristics of seedlings, such as shoot length, among other things, with respect to the control treatments. A statistical analysis was performed in order to ascertain the significance of the discrepancies found. This will provide useful insights into any potential allelopathic interactions between Okra seeds and Moringa extract. Studying the allelopathic relationship between Moringa and Okra is crucial because it sheds light on the numerous ways that chemical interactions between distinct plant species can affect one another. The fields of agriculture and ecology both depend on this knowledge. Our capacity to effectively manage and protect biodiversity is improved by our understanding of allelopathy in natural ecosystems. Examining these interactions provides information about sustainable agricultural practices and ecological health in general.
Experimental setup
Fifteen petri platees were collected, each measuring 10cm in diameter, and thoroughly cleaned and dried them before commencement of the experiment. Two tissue layers were then carefully put within each petri plate and ten seeds of okra were put in each petri plate. Each plate was labeled with the appropriate treatment group. Five treatments, each consisting of varying concentrations of Moringa aqueous extract (control, 25%, 50%, 75%, 100%) was applied to their respective petri plate with a 10-milliliter dropper for each distinct concentration.
Following treatments were used in the experiments:
- Treatment 1: 25% Moringa aqueous extract
- Treatment 2: 50% Moringa aqueous extract
- Treatment 3: 75% Moringa aqueous extract
- Treatment 4: 100% Moringa aqueous extract
- Treatment 5: 0% (Control as distilled water)
Data collection procedure
Okra seeds were divided into five groups, each treated with one of the extract concentrations. We thoroughly mixed each extract solution before utilizing it. A control group was treated with distilled water only to compare the effects. Ten sound seeds were taken and placed into sterile 15cm plastic Petri plates with a single layer of tissue paper on the bottom. Three milliliters of the test solution were added to the Petri plates to suitably hydrate them. Along with a control in which the tissue paper was wet with 3ml of distilled water, this was set up in three replicates (i.e., R1, R2, and R3). Over the course of seven days in May 2024, the complete setup was inspected to record a variety of metrics, including the number of seeds that germinated, the length of the roots and shoots, the seedlings, and shoots, among other things, all while maintaining a normal temperature and light level. Radicals showing up was an indication of germination. The percentage of seeds that sprouted is called germination. The shoot length is observed after the predetermined time frame (7 days). The seedling vigor index were calculated from the shoot growth. The length of shoot was measured by a ruler. Detail data for each respective parameters was recorded by the following procedure.
Parameters
The germination indices were studied by using the following equations.
Germination percentage
When a seedling appears in a petri plate, counting begins immediately, and measurements are taken daily thereafter. The number of visible seedlings was tallied. The measurement was carried out using the Smith and Millet (1964) formula until there was no more rise. Seed germination was counted every day for seven days. Seeds germinated when the visible radical length reached two millimeters.
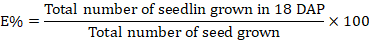
Germination index (GI)
It is the estimate of emergence rate of seedlings and was calculated as described in Association of Official Seed Analysis (Rice, 1960).
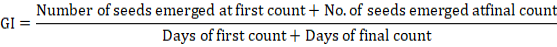
Mean germination rate (MGR)
The following formula from Ranal and Santana (2006) was used to compute the mean germination rate (MGR), which is defined as the reciprocal of the mean germination time.
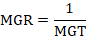
Germination rate index (GRI)
Emergence rate index for each treatment and replication was calculated as emergence index divided by emergence percentage.

Energy of germination
Energy of emergence was computed the method as delineated by Ruan et al. (2002). It is the percentage of emerged seedlings three days after sowing.
Mean germination time (MET)
Mean germination time(MGT) was calculated the following formula of Eills and Roberts (1981).
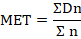
Where, n is the number of seeds, which were germinated correspondent to the day. D observation (not the accumulated number), and D n is number of days counted from the beginning of germination.
Coefficient of velocity of germination (CVG)
Coefficient of velocity of germination (CVG) was calculated the following formula of Scott et al. (1984).
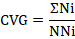
Where, N is the number of seeds germinated on day 1 and T is the number of days from seedling correspondence to N.
Mean daily germination (MDG)
Mean daily germination (MDG) was calculated the following formula of Scott et al. (1984):
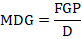
Where, FGP is a final germination percent, D is day of maximum germination (experiment period).
Seed vigor index (SVI)
Seed vigor index is a measure that combines both germination percentage and seedling growth to assess seed quality and vigor. It is often calculated by using the formula:
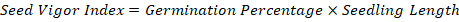
Germination inhibition/ stimulation percentage (GI-S%)
The following equation was used to determine the percent inhibition or stimulation of germination (Soliman et al., 2016).
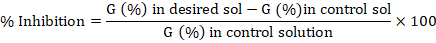
Where the term germination percentage in desired solution refers to the proportion of seeds that germinate successfully after being subjected to a specific treatment or solution under investigation. Furthermore, germination percentage in control refers to the proportion of seeds that successfully sprouted in the absence of any treatment or solution under standard or control conditions.
Shoot length inhibition/ stimulation percentage (SLI-S%)
the following Shoot length stimulation or inhibition of okra was calculated by using the following equation as outlined by Turk et al. (2003).
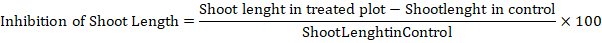
Where the mean length of the shoot measured in the experimental group or plot that underwent a given intervention, treatment, or condition is referred to as the term shoot length in treated plot. Furthermore, term shoot length in control refers to the average length of shoots in the control plot that received either regular treatment or no treatment at all.
Statistical analysis
Data was analyzed by using MS Excel 2010 and Statistix 8.1. To determine the significance of various treatments, all data were statistically evaluated using a one-way analysis of variance (ANOVA) suitable for a completely randomized design (CRD). The Fisher’s protected Least was used to distinguish the means. Turkey Pairwise comparison was employed to ensure a 99% confidence in the mean value separation by applying a significant difference (LSD) test α = 0.01.
Results and Discussion
One-way analysis of variance (ANOVA) indicated F-Fisher values with level of significance showed that various aqueous extract of moringa significantly affected the germination percentage, germination energy, germination rate index, mean germination time, mean germination rate, mean daily germination, seed vigor index, and germination and shoot inhibition or stimulation percentages of okra seeds (Table 1). However, coefficient of velocity of germination shows no significant response to the given treatments. The results of ANOVA show that various concentration of moringa extract considerably affected germination parameters of okra seeds. The effect was highly significant on the germination percentage, germination energy, and other germination indices. This could be possibly due to the bioactive compounds of the moringa extracts, which influence the physiological processes of seeds. In particular, a higher germination rate and vigor index observed at lower concentrations of 25% and 50% suggest that Moringa extracts at low levels increase the metabolic activity in seeds and improve physiological conditions that are favorable for germination. In contrast, higher concentrations (75% and 100%) seem to cause inhibitory effects, most likely resulting from the potentially toxic influence or osmotic stress due to high concentrated extracts which might interfere with the normal development of seed growth.
Table 1: F-Fisher values of different treatments resulting from analysis of variance (ANOVA).
|
Parameters |
F-fisher value/ significance |
|
Degree of freedom (DF) |
4 |
|
Germination % (GP) |
26.5 *** |
|
Germination energy (GE) |
17.2 *** |
|
Germination rate index (GRI) |
39.7 *** |
|
Mean germination time (MGT) |
654 *** |
|
Mean germination rate (MGR) |
703 *** |
|
Mean daily germination (MDG) |
26.5 *** |
|
Coefficient of velocity of germination (CVG) |
3.21 ns |
|
Shoot length (SL) |
9.43 *** |
|
Seed vigor Index (SVI) |
8.89 ** |
|
Germination inhibition or stimulation (GI-S %) |
20.5 *** |
|
Shoot Inhibition or stimulation (SI-S %) |
10.2 ** |
F-Fisher values are referred to Bliss-transformed data; DF: degrees of freedom; ***, ** and * indicate statistical significance at P ≤ 0.001, P≤ 0.01 and P ≤ 0.05, respectively; ns: not significant.
Mean data presented in Table 2 showed that the control treatment resulted maximum germination percentage (93.3%), followed by and 80% with 25% concentration, while that of the 50% moringa concentration was 76.7%. For energy germination, the 25% concentration showed the value slightly inferior to 73.3%, while the value for the control and at 50% concentration with 93.3%. The germination rate index (GRI) was highest in the control group at 22.7, whereas it was 18.2 in the 25% concentration and 13.9 in the 50% concentration. Mean germination time (MGT) was shortest in the control at 4.1 days, similar to the 25% concentration at 4.2 days, while in the 75% concentration, maximum MGT was observed at 4.8 days. The highest mean germination rate was found in the controls at 0.245, followed by 0.24 in the 25% concentration and 0.227 at 50% concentration. Finally, MDG reached its highest value in the control (13.3), followed by the 25% and 50% concentration with (11.4 and 11, respectively) which are statistically comparable. The 75% and 100% concentration always involved the lowest values for the different indices, denoting negative effects on germination with increased concentration. The control treatment shows the highest germination. This certainly reveals the most suitable condition for seed germination with no interference from exogenous extracts. The reduction in the germination indices with an increase in the concentration of moringa extract approves with the dose-depended response, wherein low to moderate levels of the given concentration stimulant the biological processes however the high concentration has inhibitory effect. Moreover, maximum mean germination time and a lower germination rate index support the fact that excess of moringa extract concentration could interfere with the seed’s ability to use water efficiently or absorb necessary nutrients. In a comparable investigation Nwangburuka (2012) studied the effect of different concentrations of Moringa leaf extract on okra seed germination and seedling growth. Although the various concentrations failed to increase any of the indices tested in this study, including germination percent, length of seedling, or vigor index, low-concentration extracts of Moringa expressed some promise in reducing fungal populations on seeds, therefore indirectly favoring its germination.
Data presented in Table 3 shows effect of various concentrations of moringa aqueous extract on okra seed germination. The data show that coefficient of
Table 2: Effect of different concentration of moringa aqueous extract on germination %, germination energy, germination rate index, mean germination time, mean germination rate and mean degree germination of okra seed.
|
Treatments |
Germination % (GP) |
Germination energy (GE) |
Germination rate index (GRI) |
Mean germination time (MGT) |
Mean germination rate (MGT) |
Mean degree germination (MDG) |
|
25% |
80 a |
73.3 ab |
18.2 ab |
4.2 b |
0.24 ab |
11.4 a |
|
50% |
76.7 a |
76.7 ab |
13.9 b |
4.4 b |
0.227 b |
11 a |
|
75% |
43.3 b |
40 bc |
5.5 c |
4.8 a |
0.208 c |
6.2 b |
|
100% |
0 c |
0 c |
0 c |
0 c |
0 d |
0 c |
|
Control |
93.3 a |
93.3 a |
22.7 a |
4.1 b |
0.245 a |
13.3 a |
|
LSD (0.01) |
32.732 |
40.088 |
0.3462 |
0.0176 |
4.6762 |
42.594 |
Mean values followed by different letters are statistically significant at 1% level of probability.
Table 3: Effect of different concentration of moringa aqueous extract on coefficient of velocity of germination, shoot length, seed vigor index, germination Inhibition or stimulation, shoot length inhibition or stimulation of okra seed.
|
Treatments |
Coefficient of velocity of germination (COVG) |
Shoot length (SL) |
Seed vigor index (SVI) |
Germination inhibition or stimulation (GI-S %) |
Shoot length inhibition or stimulation (SLI-S %) |
|
25% |
13.7 ab |
52.8 a |
4323.2 a |
-13.7 ab |
10.9 a |
|
50% |
14.8 ab |
39.5 ab |
2998.6 a |
-17.4 ab |
-17.7 ab |
|
75% |
45.6 a |
10.8 bc |
758.3 ab |
-52.6 b |
-70.5 bc |
|
100% |
0 b |
0 c |
0 b |
-100 c |
-100 c |
|
Control |
11.1 ab |
47.5 a |
4451.5 a |
0 a |
0 a |
|
LSD (0.01) |
NS |
34.115 |
1711.8 |
39.94 |
66.854 |
Mean values followed by different letters are statistically significant at 1% level of probability.
velocity of germination show highest value (45.6) at 75% concentration of moringa extract with lowest value (0) at concentration of 100%. Shoot length is highest at 52.8 cm with 25% concentration and lowest (0 cm) with 100% the concentration. The SVI peaked at 4323.2 at the 25% concentration, with 100% concentration show the lowest value (0). Germination inhibition or stimulation is most profound at -100% with the 100% concentration, indicating the highest degree of inhibition; the 25% concentration showed an inhibition of -13.7%. For SLI-S, the 100% concentration exhibits the greatest inhibition at -100%. At the 75% concentration, it was -70.5%. If non-significant differences are noted, this means that variations between those particular treatments do not produce an effect that is statistically significant.
Coefficient of velocity of germination was highest at a concentration of 75%, which may suggest some kind of initial stimulatory effect on the rate of germination with low values at 100% which indicates severe inhibition. The results on shoot length and SVI further affirm that the low concentration, possibly 25%, has positive effects on seedling growth, while higher concentrations reduce seed growth and vigor, likely due to toxic effects or imbalanced nutrient uptake. High inhibition percentages at higher extract concentrations inhibitory effects which determine it beyond a certain threshold. However, the moringa extracts are more inhibitory than beneficial. Moringa extracts have potential in improving germination and seedling vigor at their lower concentrations, and show poor germination at higher concentrations. In a similar study Ali et al. (2024) reported that Moringa leaf extract significantly improved okra growth. Though the major focus was on growth and not germination, however, the findings suggest that Moringa extract can enhance overall plant health, likely to be positively related to its effect on improving germination rates at 6% aqueous extract, however at high concentration it negatively affect plant growth. This biphasic response shows the requirement for optimal dosing to benefit from the positive effects of moringa extracts without inducing an inhibitory response. This should be taken into consideration when future studies are designed to establish the exact concentration that could achieve the maximum stimulation of germination and seedling health for possible application in agriculture.
Conclusions and Recommendations
In light of the results it is concluded that lower doses of Moringa extract (25% and 50%) may encourage or maintain germination and growth, whereas higher doses (75% and 100%) hinder them. The control group shows perfect development and germination as a point of reference. To increase okra seed germination and growth, the proper concentration of extract must be used. While higher doses (75% and 100%) of Moringa extract impede germination and growth, low to moderate dose (25% and 50%) might promote or maintain them. As a reference, the control group demonstrates ideal development and germination. Selecting the right extract concentration is essential for improving the germination and growth of Okra seeds. The above result indicates that the seed germination of Okra seeds affected by different aqueous concentration of Moringa. As the concentration of Moringa was increased, the seed germination of Okra was decreased. Lowest germination (0%) was recorded, when Moringa was used at 100%. This reveals that there are certain chemicals in the leaves of Moringa that negatively affect the seed germination, energy and growth of okra seed. In conclusion, there are notable allelopathic effects of Moringa aqueous extract on okra seedling development and germination. Despite the fact that Moringa has many applications, care should be taken while growing Okra or other delicate crops in agricultural settings. To isolate and identify the particular allelochemicals involved and investigate their methods of action, more investigation is required. This work contributes to sustainable agricultural practices by offering insightful information on the possible application of Moringa extracts in crop productivity and weed management.
Acknowledgements
The partial financial support of Higher Education Commission, Islamabad through Project No. HEC-NRPU# 16722 entitled “Exploring the potential of wild plants in dry zones of southern Punjab and Khyber Pakhtunkhwa” is greatly acknowledged.
Novelty Statement
The present study highlights the allelopathic potentials of Moringa oleifera L. aqueous extract on okra seed germination and seedling growth. Most of the previous works have focused on the nutritional and medicinal values of Moringa, while our study pioneers an investigation on its phytotoxic effects on an important vegetable crop (Okra). The findings reveal that Moringa extract has dual effect which is particularly dose dependent, low to medium concentrations stimulated germination and seedling development, whereas high concentrations were found to show a noticeable inhibiting effect. The present investigation shows the allelopathic nature of Moringa extract which could expose new perspectives for its application in sustainable weed management and crop production strategies.
Author’s Contribution
Mahisba Waris: Performed research work, data collection, data entry and write-up.
Muhammad Azim Khan: Supervision, Funding acquisition, Investigation, proofreading.
Muhammad Fawad: Research conceptualization, methodology and manuscript writing.
Nabeela Jafer: Supervision, manuscript review and editing.
Rashid Hussain: Resources, manuscript review, editing and proofreading.
Haseeb Ahmad: Resources, data validation.
Conflict of interest
The authors have declared no conflict of interest.
References
Adetuyi, F.O., Osagie, A.U. and Adekunle, A.T., 2008. Effect of postharvest storage techniques on the nutritional properties of Benin indigenous okra (Abelmoschus esculentus L.) Moench. Pak. J. Nutr., 7(5): 652-657. https://doi.org/10.3923/pjn.2008.652.657
Ain, Q., Mushtaq, W., Shadab, M. and Siddiqui, M.B., 2023. Allelopathy: An alternative tool for sustainable agriculture. Physiol. Mol. Biol. Plants, 29(4): 495-511. https://doi.org/10.1007/s12298-023-01305-9
Ali, G.H., Taweel, G.E.E. and Ali, M.A., 2004. The cytotoxicity and antimicrobial efficiency of Moringa oleifera L. seeds extracts. Int. J. Environ. Stud., 61(6): 699-708. https://doi.org/10.1080/0020723042000189877
Ali, W., Khan, M.N., Nabi, G., Rahman, S., Sattar, S., Khan, M.F., Rahman, S., Zubair, S., Ain, Q., Sabeeh, M. and Ali, A., 2024. Effect of moringa leaf extract and its solution application forms on growth and yield of Okra. Sarhad J. Agric., 40(2): 407-417. https://doi.org/10.17582/journal.sja/2024/40.2.407.417
Awodele, O., Oreagba, I.A., Odoma, S., da Silva, J.A.T., Osunkalu, V.O., 2012. Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J. Ethnopharmacol., 139(2): 330-336. https://doi.org/10.1016/j.jep.2011.10.008
Balakumbahan, R., Ravindran, C. and Sivakumar, V., 2024. Standardization of propagation techniques in annual moringa (Moringa oleifera Lam.) for enhancing crop uniformity. J. Adv. Biol. Biotechnol., 27(7): 1315-1320. https://doi.org/10.9734/jabb/2024/v27i71093
Baloch, A.F., 1994. Vegetable crops horticulture. pp. 502-503.
Basnet, S., Lamichane, P., Meheta, A. and Rajbanshi, Y., 2023. A review on biochemical, nutritional and medicinal properties of okra. International congresses of Turkish science and technology publishing, pp. 46-53.
Das, S., Nandi, G. and Ghosh, L.K., 2019. Okra and its various applications in drug delivery, food technology, health care and pharmacological aspects. A review. J. Pharma. Sci. Res., 11(6): 2139-2147.
De-Albuquerque, M.B.D., Santos, R.C.D., Lima, L.M., Filho, P.D.A.M., Nogueira, R.J.M.C., Camara, C.A.G.D. and Ramos, A.D.R., 2010. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev., 31: 379-395. https://doi.org/10.1051/agro/2010031
Dixit, S., Tripathi, A. and Kumar, P., 2016. Medicinal properties of Moringa oleifera: A review. Int. J. Educ. Sci. Res. Rev., 3(2): 173-185.
Dubey, D.K., Dora, J., Kumar, A., Gulsan, R.K., 2013. A multipurpose tree-Moringa oleifera. Int. J. Pharma. Chem. Sci., 2(1): 415-423.
Einhellig, F.A., 2018. Allelopathy a natural protection, allelochemicals. In: Handbook of natural pesticides: Methods, CRC Press. pp. 161-200.
Ellis, R.H. and Roberts, E.H., 1980. Improved equations for the prediction of seed longevity. Annals Bot., 45: 13-30. https://doi.org/10.1093/oxfordjournals.aob.a085797
Farooq, M., Wahid, A., Basra, S.M.A. and Din, I.U., 2009a. Improving water relations and gas exchange with brassinosteroids in rice under drought stress. J. Agron. Crop Sci., 195: 262–269. https://doi.org/10.1111/j.1439-037X.2009.00368.x
Farooq, M., Jabran, K., Cheema, Z.A., Wahid, A. and Siddique, K.H.M., 2011b. The role of allelopathy in agricultural pest management. Pest Manage. Sci., 67: 493–506. https://doi.org/10.1002/ps.2091
Foidl, N., Makkar, H.P.S. and Becker, K., 2001. The potential of Moringa oleifera for agricultural and industrial uses. In: Miracle tree: The multiple attributes of moringa, pp. 45-76.
Fouad, E.A., Elnaga, A.S.A., and Kandil, M.M. 2019. Antibacterial efficacy of Moringa oleifera leaf extract against pyogenic bacteria isolated from a dromedary camel (Camelus dromedarius) abscess. Vet. World, 12(6): 802.
Fuglie, L.J., 2000. New uses of moringa studied in Nicaragua: ECHO’s technical network site-networking global hunger solutions. ECHO, Nicaragua.
Gadzirayi, C.T., Mudyiwa, S.M., Mupangwa, J.F., and Gotosa, J. 2013. Cultivation practices and utilisation of Moringa oleifera provenances by smallholder farmers: Case of Zimbabwe. Sarhad J. Agric.
Koul, B. and Chase, N., 2015. Moringa oleifera Lam.: Panacea to several maladies. J. Chem. Pharma. Res., 7(6): 687-707.
Kumar, S., Dagnoko, S., Haougui, A., Ratnadass, A., Pasternak, N. and Kouame, C., 2010. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res., 5: 35900-3598.
Mashamaite, C.V., Ngcobo, B.L., Manyevere, A., Bertling, I. and Fawole, O.A., 2022. Assessing the usefulness of Moringa oleifera leaf extract as a biostimulant to supplement synthetic fertilizers: A review. Plants, 11: 2214. https://doi.org/10.3390/plants11172214
Mehboob, W., Rehman, H., Basra, S.M.A. and Afzal, I., 2011. Role of seed priming in improving performance of spring maize. Available at: http://cropmanagementseminar.edu.pk (accessed 2 June 2011).
Narwal, S.S., 1994. Allelopathy in crop production. Scientific Publishers, Jodhpur. pp. 288.
Nwangburuka, C.C., 2012. Effect of Moringa oleifera leaf extract and sodium hypochlorite on seedling growth rate and fungal abundance in two accessions of Abelmoschus esculentus (L) Moench. Arch. Appl. Sci. Res., 4: 875-881.
Oyelade, O.J., Ade-Omowaye, B.I.O. and Adeomi, V.F., 2003. Influence of variety on protein, fat contents and some physical characteristics of okra seeds. J. Food Eng., 57(2): 111-114. https://doi.org/10.1016/S0260-8774(02)00279-0
Ranal, M.A. and Santana, D.G., 2006. How and why to measure the germination process? Rev. Brasil. Bot., 29(1): 1-11. https://doi.org/10.1590/S0100-84042006000100002
Rice, W.N. 1960. Development of the cold test for seed evaluation. Proc. Assoc. Off. Seed Anal., 50: 118-123.
Ruan, S., Xue, Q. and Tylkowska, K., 2002. Effects of seed priming on germination and health of rice (Oryza sativa L.) seeds. Seed Sci. Technol., 30: 451-458.
Saa, R.W., Fombang, E.N., Ndjantou, E.B. and Njintang, N.Y., 2019. Treatments and uses of Moringa oleifera seeds in human nutrition: A review. Food Sci. Nutr., 7(6): 1911-1919. https://doi.org/10.1002/fsn3.1057
Sayyad, I.M., Ganjiwale, R.O., Gandhare, B.R., and Kediya, A.S. 2024. A review on bioactive components, validated pharmacological application and technological applications of Abelmoschus esculentus Linn. Sarhad J. Agric.
Santos, A.F., Argolo, A.C., Paiva, P.M. and Coelho, L.C., 2012. Antioxidant activity of Moringa oleifera tissue extracts. Phytother. Res., 26(9): 1366-1370. https://doi.org/10.1002/ptr.4591
Scott, S.J., Jones, R.A. and Williams, W.A., 1984. Review of data analysis methods for seed germination. Crop Sci., 24(6): 1192-1199. https://doi.org/10.2135/cropsci1984.0011183X002400060043x
Shahzad, U., Khan, M.A., Jaskani, M.J., Khan, I.A. and Korban, S.S., 2013. Genetic diversity and population structure of Moringa oleifera. Conserv. Genet., 14(6): 1161-1172. https://doi.org/10.1007/s10592-013-0503-x
Singh, P., Chauhan, V., Tiwari, B.K., Chauhan, S.S., Simon, S., Bilal, S. and Abidi, A.B., 2014. An overview on okra (Abelmoschus esculentus) and its importance as a nutritive vegetable in the world. Int. J. Pharma. Biol. Sci., 4(2): 227-233.
Smith, C.W. and Varvil, J.J., 1984. Standard and cool germination tests compared with field emergence in upland cotton 1. Agron. J., 76(4): 587-589. https://doi.org/10.2134/agronj1984.00021962007600040019x
Soliman, M.H., Al-Juhani, R.S., Hashash, M.A., and Al-Juhani, F.M. 2016. Effect of seed priming with salicylic acid on seed germination and seedling growth of broad bean (Vicia faba L.). Int. J. Agric. Technol., 12(6): 1125-1138.
Turk, M.A., Shatnawi, M.K., and Tawaha, A.M. 2003. Inhibitory effects of aqueous extracts of black mustard on germination and growth of alfalfa. Weed Biol. Manag., 3(1): 37-40.
Wei, M.M., Zhao, S.J., Dong, X.M., Wang, Y.J., Fang, C., Wu, P. and Zhou, J.L., 2021. A combination index and glycoproteomics-based approach revealed synergistic anticancer effects of curcuminoids of turmeric against prostate cancer PC3 cells. J. Ethnopharmacol., 267: 113467. https://doi.org/10.1016/j.jep.2020.113467
Yasmeen, A., 2011. Exploring the potential of moringa (Moringa oleifera) leaf extract as a natural plant growth enhancer. 2011. PhD thesis, University of Agriculture, Faisalabad, Pakistan.
Yasmeen, A., Basra, S.M.A., Ahmad, R. and Wahid, A., 2012. Performance of late sown wheat in response to foliar application of Moringa oleifera Lam. leaf extract. Chilean J. Agric. Res., 72(1): 92. https://doi.org/10.4067/S0718-58392012000100015
Yasmeen, A., Basra, S.M.A., Farooq, M., Rehman, H.U. and Hussain, N., 2013. Exogenous application of Moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regul., 69(3): 225-233. https://doi.org/10.1007/s10725-012-9764-5
To share on other social networks, click on any share button. What are these?





