Age and Growth of Triplophysa (Hedinichthys) yarkandensis (Day, 1877) in the Tarim River in Xinjiang, China
Age and Growth of Triplophysa (Hedinichthys) yarkandensis (Day, 1877) in the Tarim River in Xinjiang, China
Xinyue Wang1, Shengao Chen1,2*, Fangze Zi1, Jianmin Ge1, Desheng Chang1, Yong Song1 and Congxin Xie2
1College of Life Sciences and Technology, Tarim University, Tarim Research Center of Rare Fishes, Alar Xinjiang 843300, China
2College of Fisheries, Huazhong Agricultural University, Wuhan Hubei 430070, China
ABSTRACT
Influenced by water pollution and flow modification, Triplophysa (Hedinichthys) yarkandensis (Day, 1877) may become an indigenous endangered fish species in the Tarim River. In order to study its population characteristics and improve the conservation measures, 940 specimens of T. yarkandensis were collected in 2018 and 2020 from the Alar Section of Tarim River. Otoliths were chosen as the main age structure in this study. Results showed that the rings of T. yarkandensis formed once per year from March to May. The ages ranged from 1+ to 10+, with 2.5+ to 6.5+ predominating in the total specimens. Among them, 5+ predominated for males and 4+ to 6+ predominated for females respectively. The standard length (SL) of the specimens ranged from 30.0 to 195.0 mm, and the body weight (BW) ranged from 3.40 to 114.00 g. The predominating SL ranged from 75.0 to 125.0 mm, accounting for 51.81% of the specimens. Length-weight relationship was described as W = 0.0355 × L 2.5916 (R2 = 0.8736, n = 940). Growth was described by the von Bertalanffy equation: Lt = 184.79 (1−e−0.1026 (t +1.0458)), Wt = 68.08 (1−e−0.1026 (t + 1.0458))2.5916 for male and Lt = 236.63 (1−e−0.0900 (t + 0.1831)), Wt = 129.16 (1−e−0.0900 (t + 0.1831))2.5916 for female. Age at inflection point for male and female were 8.24 and 10.39 years, respectively. The goal of this study is to better understand the population’s makeup and migratory patterns in order to exploit and develop germplasm resources of T. yarkandensis. Subsequent efforts should be made to prevent overfishing of young fish by limiting the minimum fishing individuals, and to protect older individuals by banning fishing during the breeding period.
Article Information
Received 31 October 2022
Revised 25 December 2022
Accepted 13 January 2023
Available online 29 March 2023
(early access)
Published 08 June 2024
Authors’ Contribution
XYW drafted the manuscript. FZZ and JMG in the design of the study and performed the statistical analysis. DSC and YS conceived of the study, and participated in its design and coordination. CXX and SAC have given final approval of the version to be published. All authors read and approved the final manuscript.
Key words
Triplophysa yarkandensis, Tarim River, Otolith
DOI: https://dx.doi.org/10.17582/journal.pjz/20221031021052
* Corresponding author: shengao@taru.edu.cn
0030-9923/2024/0004-1771 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Age determination is the basis for studying the biology and population ecology of fish (Yin, 1995). Age can be determined by three methods: Direct observation, statistics and analyses on catch, and structural analyses on calcification (Ye, 2002). Scales, vertebrae, otoliths, opercular, cleithra, actinosts, basioccipital bones, and hyoid bones are usually used for the observation, analysis, and estimation on age (Wu, 1975; Xie et al., 1994). Previous studies have found out that it is not reasonable to choose scales for those old and slow-growing fishes. Their scales are easy to get lost which results in underestimation (Shen et al., 2001). For different fishes, different age structures should be utilized. High-accurate structure should be taken as the principal material and others as the supporting materials. Therefore, it is necessary and crucial to select the best and the most suitable material for age determination (Polat et al., 2001). Otoliths have been proved to be the most commonly accurate age structure for fish so far. Ring marks in otoliths grow steadily no matter how widely the living environment changes (Sponaugle, 2009), because it is closely correlated with the growth of fish (Casselman, 1990; Campana, 2001; Horn, 2002; Chen et al., 2009). Pannella (1971) found the daily increment on otolith, which laid a solid foundation for the studies on the relationship between otoliths and the growth of fish.
Triplophysa, widely spread in inland waters on plateaus in the middle parts of Asia, is indispensable to the fish fauna on Qinghai-Tibet Plateau (He et al., 2008), having special adaptability to life on plateau (He, 1996). Triplophysa (Hedinichthys) yarkandensis (Day 1877) (Cyprinidformes, Cobitidae, Triplophysa), is an endemic fish species in the Tarim River. Locally known as Goutou (Dog-head). Since 1960s, population structure has been disrupted by human activities, biological invasion, water pollution and other factors. This caused the decline in the numbers of endemic fishes (Wang, 1995; Olden et al., 2010; Xiong et al., 2015).
T. yarkandensis may become another indigenous endangered fish species in the Tarim River after Aspiorhynchus laticeps and Schizothorax biddulphi (Zhu, 1989; Chen, 2012). Recently, the research of T. yarkandensis mainly focuses on growth and reproduction, culture and toxicity test, genetic diversity and interspecific differences (Zhao, 1989; Zeng and Tang, 2010). As a plateau fish, the T. yarkandensis has a slower growth rate, smaller size and greater salt tolerance, and has specific economic value and unique ecological significance.
In this study, otoliths were chosen for age determination. Relationship between otoliths and body length/age was described. The von Bertalanffy growth model was fitted and its parameters were compared with results of other studies. The potential for population development and characteristics of the T. yarkandensis population growth in the Alar Section of the Tarim River were identified. It served as a foundation for the correct prediction on the development prospect of the population and the reasonable conservation and effective utilization of the fish resources.
Materials and Methods
Sampling
Periodic sampling was carried out in February, May, August and November from 2018 to 2020, using a variety of gear, including drift gill net (mesh size 2cm), set gill net (mesh size 2cm) and net cage (mesh size 2cm). 940 T. yarkandensis individuals were collected in the Alar Section of the upper of Tarim River (Figs. 1A, B).
All specimens were measured at the site for standard-length (SL, mm), weight (W, g), gonad weight (WG, g), visceral weight (WV, g) and eviscerated weight (WE, g). SL was accurate to 1.0 mm; while W, WG, WV, and WE to 0.01 g. Biological dissection was performed on site. The left lapillus otoliths, and the 6th to 7th vertebrae and opercular were selected as age identification materials. All of them were washed, dried, wrapped in filter paper and put in 2-milliliter plastic centrifuge tubes. The specimens which were taken successfully for all three structures were 891 out of 940.
Preparation and examination of age structures
Otoliths
Lapillus was embedded in nail enamel and mounted on a glass slide with the convex side pointing upwards. After dried for 12 h, lapillus was ground with 1,000 to 2,000 grit wet sandpaper. Frequent microscopic observation and
adjustment were made during the grounding process until the core was reached. Then lapillus was polished. When one side was finished, acetone was applied to dissolve the nail enamel. Lapillus was turned over, mounted, dried, ground and polished in the same way (Xiong et al., 2006; Li et al., 2008; Ma, 2011). When the core plane was available, it was observed and shot under anatomical lens Leica EZ4D and Niko80i at 10×/0.3 magnification. And then the radius of lapillus at 100 um scale were measured using Image-Pro Plus 6.0 (Fig. 1C).
Previous studies have demonstrated that otolith size and weight etc. are highly associated with the growth traits. Only the radius of the sectioned lapillus was measured as the otolith of T. yarkandensis was extremely small.
Vertebrae
As the vertebrae of T. yarkandensis were relatively small, it took only 2 to 5 min to boil them in the boiling water. After the boiling, vertebrae were taken out, dried, and soaked in 1% hydrogen peroxide for 24 to 48 h. Then the 6th and 7th vertebrae were removed and put under anatomical lens Motic SMZ-168 for observation and taking photos. If not clear, dimethyl could be added as transparent reagent. If still not clearly read, the vertebrae could be adjusted for a better observation angle (Fig. 1C).
Opercular
Both sides of opercular were taken and put in hot water to boil for 1 min. They were cleaned for residual tissue, dried, and soaked in 1% hydrogen peroxide for 24 to 48 h. When all the above had been completed, the opercular were examined and photographed using an anatomical lens, the Motic SMZ-168.
Analysis of the formation cycle of rings
March to May is the peak breeding period of T. yarkandensis. The formation cycle of rings was analyzed through marginal increments (MIR) (Haas and Recksiek, 1995). The equation is as follows:
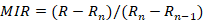
Where R is the radius of the hard structure, Rn is the radius from the core to the last ring on the outside, and Rn–1 is the radius from the core to the penultimate ring on the outside.
Photos used in the above analysis were taken under microscope Niko80i at 10×/0.3 magnification and measured at 100 um scale using Image-Pro Plus 6.0.
Age validation
Age was validated according to the ring number on the hard structure. In this study, the first ring was considered one year of age from where age increases (Massutí et al., 2000). Samples were assessed for age independently by two readers. If the ages assigned by each reader agreed, then the ages were considered valid. Otherwise, the two readers re-examined the structure together and reached an agreement. In case the second results still varied widely, the sample had to be abandoned (Liu et al., 2009).
Relationship between length and weight
In this study, a common equation was applied to analyze the characteristics of growth. Measurements of length and weight between sexes were used for Kolmogorov-Smirnov test and significance analysis (Cazorla and Sidorkewicj, 2009; Ma, 2011). A t-test was used to compare growth parameters b and 3 (Pauly, 1984) to estimate whether T. yarkandensis grow at a constant speed.
The Power-exponential relationship between length and weight was shown in the following equation:
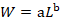
Growth equation
The characteristics of the growth were described by the classic von Bertalanffy growth model:
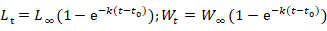
Where Lt is the length at age t of individual fish, Wt is the weight at age t, L∞ is the maximum attainable length, W∞ is the maximum attainable weight, t0 is the hypothetical age at which length and weight were zero, and k is the curvature of the growth curve.
In order to further predict the growth trend, the growth rate and acceleration equations were obtained by first order and second order derivation of the growth equation. The growth trend of fish was fitted by the measured age data or the retrogradation age data. And the growth rate was slower when it approached an asymptotic value (Fei and Zhang, 1990; Yin, 1995; Pauly, 1981).
The growth performance index was calculated using the equation: Ø = lgk+2lgL∞ (Munro and Pauly, 1983).
Data processing
All statistical analyses were performed using SPSS 16.0 and Origin 8.0.
Results
Frequency distributions of length and weight
The frequency distributions of SL were shown in Figure 2A. The length ranged from 30.0 to 195.0 mm and the mean SL was 96.5±17.0 mm. The predominating SL ranged from 75.0 to 125.0 mm, accounting for 51.81% of the whole specimens. There were 485 males whose length ranging from 65.0 to 162.0 mm, and 412 females ranging from 30.0 to 190.0 mm. The length difference between the sexes was not significant (Kolmogorov-Smirnov test, F = 0.972, p > 0.05).
The frequency distributions of W were shown in Figure 2B. The weight ranged from 3.40 to 114.04 g and the mean weight was 14.25±7.29 g. The visceral weight ranged from 0.07 to 12.14 g, and the evisceral weight ranged from 1.06 to 101.59 g with the mean weight of 12.77±8.52 g. The predominating weight ranged from 5.00 to 20.00 g, proportion of those above 30.00 g decreasing as their weight increases. The weight of the indeterminate ranged from 12.57 to 85.48 g. In the whole specimens, the weight of male from 3.40 to 50.99 g and female ranged from 3.59 to 114.04 g. Weight between sexes differ significantly in different ages Kolmogorov-Smirnov test, F = 23.362, p < 0.05).
Frequency distributions of age
The vertebrae and opercular were found to be less useful for determining the age of T. yarkandensis, especially for older fish, and were only utilized as an additional aid for identification in cases of otolith loss. 891 were successfully read for ages in the 940 specimens, and the rest 49 were unsuccessfully read (including 6 females and 43 indeterminate). Age frequency distribution of T. yarkandensis was shown in Figure 2C. The youngest individual in the capture population was 1 year of age, the oldest was 10 years, and the preponderant was 2.5 to 6.5 years. The predominant male was 5 years and female were 4 to 6 years of age.
Relationship between length and weight
The power-functional relationship between SL and W of the total 940 specimens were described by the following equations:
Indeterminate population: W = 0.0376 × L2.5484 (R2 =0.8910, n = 43)
Male population: W = 0.0321 × L2.6362 (R2 = 0.8595, n = 485)
Female population: W = 0.0369 × L2.5772 (R2 = 0.8766, n = 412)
Overall population: W = 0.0355 × L 2.5916 (R2 = 0.8736, n = 940)
Through Kolmogorov-Smirnov test, we found that the length-weight relationship between sexes was not significantly different (F = 0.857, p > 0.05). The b value of the overall population of T. yarkandensis was significantly different from 3 (t = 5.699, p < 0.05), which suggesting that T. yarkandensis grew allometricly (Fig. 3A, B).
Relationship between otolith radius and body length/age of fish
The relationship between radius of the major axis of otolith and body length/age was analysed (Fig. 4). The fitting equation of otolith radius (OR) and body length was power function with low correlated: OR = 7.9688L0.1101 (R2 = 0.0033). While the fitting equation of otolith radius and logarithmic age (A) represented linear relationship: OR = 293.56A+9336.6 (R2 = 0.0689). Above means otolith radius increases as body length and age increases.
Growth equation
Length data of individual age classes of both sexes of T. yarkandensis were shown in Table I and Figure 5. Mean length between male and female in the same age class did not differ significantly (independent sample t-test, p > 0.05, except 3+ and 6+). In age class 3+ and 6+, mean length of female differed significantly (p < 0.05). Length differences in the same age class were also significant as T. yarkandensis grow older. They were used to fit growth curves.
The von Bertalanffy growth equations were fitted to measured SLs as following:
Male population: Lt = 184.79 (1−e−0.1026 (t +1.0458));
Female population: Lt = 236.63 (1−e−0.0900 (t + 0.1831)).
The growth equation of weight could be obtained from the above:
Male population: Wt = 68.08 (1−e−0.1026 (t + 1.0458))2.5916;
Female population: Wt = 129.16 (1−e−0.0900 (t +
0.1831))2.5916.
The growth performance index (Ø) of male and female were 3.6457 and 4.7106, respectively.
Table I. Number of specimens and mean ± S.D. and range of standard length at age of T.yarkandensis.
|
Age |
Male (n=485) |
Female (n=412) |
||||
|
n |
Mean±SD (cm) |
Range (cm) |
n |
Mean±SD (cm) |
Range (cm) |
|
|
X |
6 |
10.75±1.43 |
7.9-13.2 |
|||
|
1 |
2 |
7.45±0.05 |
7.4-7.5 |
|||
|
2 |
53 |
9.28±1.17 |
6.5-13.1 |
15 |
8.77±1.93 |
3.0-13.6 |
|
3 |
124 |
8.98±1.02 |
6.5-13.2 |
77 |
10.01±1.68 |
6.8-19.5 |
|
4 |
176 |
9.35±1.22 |
6.7-13.9 |
84 |
8.59±1.05 |
6.7-15.0 |
|
5 |
109 |
9.38±1.41 |
6.8-16.2 |
91 |
9.38±1.44 |
6.4-19.5 |
|
6 |
15 |
8.49±0.74 |
7.6-10.2 |
79 |
10.24±2.30 |
6.7-19.4 |
|
7 |
4 |
8.30±0.70 |
7.2-9.5 |
42 |
11.45±3.13 |
7.2-19.5 |
|
8 |
1 |
7.9 |
10.8-18.2 |
16 |
13.46±1.64 |
7.2-13.2 |
|
9 |
1 |
12.4 |
||||
|
10 |
2 |
13.3±5.50 |
7.8-18.8 |
|||
Growth rate and acceleration
Performing first-order derivative and second-order derivative based on the above length and weight growth equation. The following equations of growth rate and acceleration were obtained (Fig. 6).
Male: dL/dt = 18.96 e−0.1026 (t + 1.0458);
dL2/dt 2= −1.95 e−0.1026 (t + 1.0458);
dW/dt = 18.10 e−0.1026 (t + 1.0458) (1– e−0.1026 (t + 1.0458)1.5916;
dW2/dt2 = 1.86 e−0.1026 (t + 1.0458) (1– e−0.1026 (t + 1.0458)0.5916
(2.5916×e−0.1026(t + 1.0458) –1);
Female: dL/dt = 21.30 e−0.0900 (t + 0.1831);
dL2/dt2 = −1.92 e−0.0900 (t + 0.1831);
dW/dt = 30.13 e−0.0900 (t + 0.1831) (1– e−0.0900 (t + 0.1831)1.5916;
dW2/dt2 = 2.71 e−0.0900 (t + 0.1831) (1–e−0.0900 (t +
0.1831)0.5916(2.5916×e−0.0900 (t + 0.1831) –1).
Male and female T. yarkandensis had similar variation trends in the growth rate and acceleration of length and weight (Fig. 5-6). Age at inflection point for male (ti) was 8.24, length and weight of which was 113.5 mm and 19.26 g, while for female (ti) was 10.39, length and weight of which was 145.2 mm and 36.47 g.
We found no inflection point on the curves of growth rate and acceleration of length. Growth rate and acceleration were negatively correlated with age increase.
The weight grew sustainably and the trend was flat at the inflection point. While the acceleration of weight increases first and then decreases, representing a slow decline. As for female, arriving at the maximum when the age was two, which was key to the inflection point of weight. As for male, there was no rising trend but a slow drop, leveling off when age was zero.
Discussion
There has always been disputed about the relationship between the size of otolith or scale and the body length of fish (Yin, 1995). By taking measurements on otolith through different axes, we could find that otolith grow all the time but on a non-uniform rate (Fowler, 1990). For old or slow-growing fish, continuous distal deposition of otolith resulted in non-uniform growth of body length (Boehlert, 1985; Reñones et al., 2007; Ma, 2011), which will reduce the accuracy of back-calculation of length (Campana, 1990).
From the growth equation of T. yarkandensis, we found the age at inflection point was relatively old. 10+ were the most numerous in the specimens and around 9+ for male and female. The relationship between coefficient k and asymptotic length and asymptotic weight was positive, suggesting that T. yarkandensis is a fast-growing, long-lived Triplophysa although the growth is slow compared to other fish (Table II). The maximum length and weight of female were bigger than those of male, and the k value of female was smaller than that of male. This was closely related to the big change in the aquatic environment of the Tarim River and the different maturity of individual male and female.
The finding of longevity of T. yarkandensis was similar to that of Zhang et al. (2010). The reasons for different growth parameters obtained by different authors might be the following (Table II): (1) different distributions of length and weight as a consequence of different mesh sizes and number of specimens; (2) different sampling locations, areas and seasons; (3) different methods of fitting growth equations (mainly by back-calculation and field measurement of length). And studies of Cailliet and Goldman (2004) demonstrated that if there are a lack of sufficient number of samples of younger fish or older fish to apply the established model, t0 will be instability. This can lead to inaccurate inferred growth models.
Table II. Growth parameters of Triplophysa.
|
Species |
Location |
L∞ |
W∞ |
t0 |
ti |
Source |
|
T. yarkandensis |
Tarim River |
184.8♂ 236.6♀ |
68.08♂ 129.16♀ |
-1.0458♂ -0.1831♀ |
3.4645♂ 4.7106♀ |
this study |
|
T. yarkandensis |
Yarkand River |
166.5742 |
56.6625 |
-1.319 |
3.9548 |
Wang, 2022 |
|
T. yarkandensis |
Hotan River |
302.772 |
310.845 |
-0.4608 |
9.0710 |
Wang, 2022 |
|
Triplophysa bombifrons |
Tarim River |
633.1 |
585.64 |
-1.4371 |
13.71 |
Yao et al., 2018 |
|
Triplophysa orientalis |
Yarlung Tsangpo River |
125.19♂ 151.659♀ |
18.787♂ 31.496♀ |
-0.0695♂ -0.01786♀ |
5.83♂ 8.19♀ |
Li et al., 2016 |
|
Triplophysa tenuis |
Kaidu |
237.5 |
97.42 |
-0.7 |
9.14 |
Jin et al., 2020 |
|
Triplophysa stenura |
Nujiang River |
246.943 |
132.03 |
0.1689 |
18.45 |
Deng et al., 2010 |
|
Triplophysa siluroides |
Beichuanhe Bssin |
103.2 |
9.24 |
-0.8345 |
2.14 |
Yao et al., 2019 |
|
Triplophysa stewarti |
Lake Chugutso |
138.91 |
28.179 |
-2.895 |
3.65 |
Tian et al., 2022 |
|
Triplophysa markehenensis |
The Dadu River |
173.1241 |
60.7531 |
-0.5328 |
6.25 |
Zhang et al., 2010 |
The growth coefficient k was useful during the assessment of the potential sensitivity of fish resources (Musick, 1999; Li et al., 2009). This study showed that the k value of male was higher than that of female. Those slow-growing, long-lived, late-maturing fish, such as T. yarkandensis, were very sensitive to the environment. High mortality and fast consumption of resources make the recruitment rate slower than expected (Musick, 1999). These characteristics were probably due to the adaptation to extreme environments of low water temperature and lack of food.
Conclusion
In this study, T. yarkandensis of Tarim River was slow-growing, long lived and the individual was relatively small-sized. The fullness was asynchronous and uneven and the population structure was not stable. They cause the fish resource was limited. For those fish, it is very difficult to recover when the stock is depleted. In conclusion, as an endemic fish, T. yarkandensis deserves our attention and proper conservation. It was quite necessary to set up a set of scientific fisheries management measures for the conservation of T. yarkandensis.
Acknowledgments
We would like to thank our colleagues of the Fishery Department. Thanks to LetPub for providing the language touch-ups for this article.
Funding
This study was funded by the Natural Science Foundation of China (no. 31360635), the Key Lab Program of Animal Science and Technology Corps of Tarim (no. HNLH202006), the special Agriculture and Rural Finance Project (Investigation on Fishery Resources and Environment in Key Waters of Northwest China).
Ethical statement
This research was conducted in accordance with ethics committee procedures of animal experiments.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Boehlert, G.W., 1985. Using objective criteria and multiple regression models for age determination in fishes. Fish. Bull., 83: 103-117.
Cailliet, G.M., and Goldman, K.J., 2004. Age determination and validation in Chondrichthyan fishes. Biol. Sharks Relatives, 10: 399-447. https://doi.org/10.1201/9780203491317.pt3
Campana, S.E., 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol., 59: 197-242. https://doi.org/10.1111/j.1095-8649.2001.tb00127.x
Campana, S.E., 1990. How reliable are growth back-calculations based on otoliths? Can. J. Fish. aquat. Sci.., 47: 2219-2227. https://doi.org/10.1139/f90-246
Casselman, J.M., 1990. Growth and relative size of calcified structures of fish. Trans. Am. Fish. Soc., 119: 673-688. https://doi.org/10.1577/1548-8659(1990)119<0673:GARSOC>2.3.CO;2
Cazorla, A.L., and Sidorkewicj, N., 2009. Age and growth of the largemouth perch Percichthys colhuapiensis in the Negro River, Argentine Patagonia. Fish. Res.., 92: 169-179. https://doi.org/10.1016/j.fishres.2008.01.016
Chen, Z.M., Li, W.X., and Yang, J.X., 2009. A new miniature species of the genus Triplophysa (Balitoridae:Nemacheilinae) from Yunnan, China. Zool. Anz., 248: 85–91. https://doi.org/10.1016/j.jcz.2009.02.001
Chen, S.A., 2012. Study on population ecology of Triplophysa yarkandensis (Day) in Tarim River. Master’s Thesis, Huazhong Agricultural University, Wuhan, China.
Deng, H.T., Yue, X.J., Chen, D.Q., Tian, H.W., and Liu, S.P., 2010. Growth characteristics and feed habit of Triplophysa stenura in Nujiang River. Freshw.Fish., 40: 26-33.
Fei, H.N., and Zhang, S.Q., 1990. Aquatic resource science. China Science and Technology Press, Beijing.
Fowler, A., 1990. Validation of annual growth increments in the otoliths of a small, tropical coral reef fish. Mar. Ecol. Prog. Ser., 64: 25-38. https://doi.org/10.3354/meps064025
Haas, R.E., and Recksiek, C.W., 1995. Age verification of winter flounder in Narragansett Bay. Trans. Am. Fish. Soc., 124: 103-111. https://doi.org/10.1577/1548-8659(1995)124<0103:AVOWFI>2.3.CO;2
He, C.C., 1996. Two species of fish with the highest altitude and their distribution. Sichuan J. Zool., 15: 116-117.
He, C.L., Zhang, X.Y., Hou, F.X., Zhang, X.F., and Song, Z.B., 2008. Threatened fishes of the world: Triplophysa siluroides (Herzenstein 1888) (Balitoridae). Environ. Biol. Fishes., 83: 305-305. https://doi.org/10.1007/s10641-008-9339-5
Horn, P., 2002. Age and growth of Patagonian toothfish (Dissostichuseleginoides) and Antarctic toothfish (D. mawsoni) in waters from the New Zealand subantarctic to the Ross Sea, Antarctica. Fish. Res., 56: 275-228. https://doi.org/10.1016/S0165-7836(01)00325-3
Jin, S.S., Wang, X.Y., Lin, X., Chen, S.A., Liu, M.C., and Xie, C.X., 2020. Age and growth of Triplophysa tenuis in Kaidu River, Xinjiang. Xinjiang Agric. Sci., 57: 181-189.
Li, H., Shen, J.Z., Liu, Q.G., Liu, Y., Zhao, Y.J., Ma, X.F., Wang, Y.B., Liu, J., and Zhu, X.Q., 2009. Comparative studies on four calcified structures for age determination of roach Rutilus rutilus in Ulungur Lake, Xinjiang Uigur Autonomous Region, China. J. Shanghai Ocean Univ., 18: 295-301.
Li, L.T., Yang, X.F., Yang, R.B., Fan, Q.X., Wei, K.J., and Jiang, H., 2016. Age structure and growth characteristics of Triplophysa orientalis in the middle of the Yarlung Tsangpo River, Tibet. J. Huazhong Agric. Univ., 35: 117-123.
Liu, K.M., Lee, M.L., Joung, S.J., and Chang, Y.C., 2009. Age and growth estimates of the sharptail mola, Masturus lanceolatus, in waters of eastern Taiwan. Fish. Res., 95: 154-160. https://doi.org/10.1016/j.fishres.2008.08.013
Ma, B.S., 2011. Study on the biology and population dynamics of Schizothorax o’connori. Doctoral Dissertation of Huazhong Agricultural University.
Massutí, E., Morales-Nin, B., and Moranta J., 2000. Age and growth of blue-mouth, Helicolenus dactylopterus (Osteichthyes: Scorpaenidae), in the western mediterranean. Fish. Res., 46:165-176. https://doi.org/10.1016/S0165-7836(00)00143-0
Munro, J.D., and Pauly, D., 1983. A simple method for comparing the growth of fishes and invertebrates. Fishbyte, 1: 5-6
Musick, J.A., 1999. Ecology and conservation of long-lived marine animals. Life in the slow lane: Ecology and conservation of long-lived marine animals University of California. Am. Fish. Soc., 23: 1-10. https://doi.org/10.47886/9781888569155.ch1
Olden, J.D., Kennard, M.J., Leprieur, F., Tedesco, P.A., Winemiller, K.O., and García-Berthou, E., 2010. Conservation biogeography of freshwater fishes: Recent progress and future challenges. Divers Distrib., 16: 496–513. https://doi.org/10.1111/j.1472-4642.2010.00655.x
Pannella, G., 1971. Fish otoliths: Daily growth layers and periodical patterns. Science, 173: 1124-1127. https://doi.org/10.1126/science.173.4002.1124
Pauly, D., 1981. The relationship between gill surface area and growth performance in fish: A generalization of von Bertalanffy’s theory of growth. Meeresforsch, 28: 251-282.
Pauly, D.P., 1984. Fish population dynamics in tropical waters: A manual for use with programmable calculators. World Fish, UK.
Polat, N., Bostanci, D., and Yilmaz, S., 2001. Comparable age determination in different bony structures of Pleuronectes flesus luscus Pallas, 1811 inhabiting the Black Sea. Turk. J. Zool., 25: 441-446.
Reñones, O., Piñeiro, C., Mas, X., and Goñi, R., 2007. Age and growth of the dusky grouper Epinephelus marginatus (Lowe 1834) in an exploited population of the western Mediterranean Sea. J. Fish Biol., 71: 346-362. https://doi.org/10.1111/j.1095-8649.2007.01482.x
Shen, J.Z., Cao, W.X., and Cui, Y.B., 2001. Comparison of scale and otolith for estimating age of Carassius auratusl. Acta Hydrobiol. Sin., 25: 462-466.
Sponaugle, S., 2009. Daily otolith increments in the early stages of tropical fish. In: Tropical fish otoliths: Information for assessment, management and ecology. Springer, Dordrecht. pp. 93-132. https://doi.org/10.1007/978-1-4020-5775-5_4
Tian, N.N., Yang, R.B., Tan, B.Z., Zeng, X.L., He, L.Q., Xu, Z.L., Zhu, Z., Liu, H.P., and Yang, X.F., 2022. Age, growth, and reproductive characteristics of Triplophysa stewarti in Lake Chugutso, Tibet. J. Fish. Sci. China, 29: 1013-1021.
Wang, D.Z., 1995. The changes of fishes fauna and protections of aboriginal fishes in the Tarim river. Arid Zone Res., 12: 54-59.
Wang, X.Y., 2022. Age, growth, reproduction and population discrimination of Triplophysa yarkandensis. Master´s thesis of Tarim University.
Wu, Q.J., 1975. Population ecology and maximum sustainable catch of Leiocassis longirostris. J. aquat. Biol., 5: 387-409.
Xie, X.J., Long, T.C., and Cao, Z.D., 1994. Study on the composition and growth in the reproductive population of Silurus meridionalis. J. Southw. China Nor. Univ. (Natl. Sci. Ed.)., 1: 71-78.
Xiong, F., Chen, D.Q., Liu, S.P., Duan, X.B., and Shi, J.Q., 2006. Annuli characteristics of the different ageing meterials of Gymnocypris przewalskii przewalskii (Kessler). Acta Hydrobiol. Sin., 30: 185-191.
Xiong, W., Tao, J., Zhang, D.C., Liu, C.L., He, D.K., and Chen, Y.F., 2015. Length weight relationships for four small fish species caught in wetlands of central Yangtze River, China. J. appl. Ichthyol., 31: 219-220. https://doi.org/10.1111/jai.12484
Yao, N., Ma, L., Jin, S.S., and Chen, S.A., 2019. Growth characteristics of Triplophysa siluroides (HERZENSTEN) in beichuanhe basin of Qinghai. J. Inner Mongol. Agric. Univ. (Natl. Sci. Ed.)., 40: 5-10.
Yao, N., Wang, M., Gong, Y.R., Ju, M.H., Wang, S., Chen, S.A., and Song, Y., 2018. Biological characteristics of the Triplophysa bombifrons in the Tarim River Basin. Jiangsu Agric. Sci., 46: 146-149.
Ye, F.L., 2002. Fish ecology. Guangdong Higher Education Publishing Co., Guangdong.
Yin, M.C., 1995. Fish ecology. China Agriculture Press, China.
Zeng, L., and Tang, W.Q., 2010. Age, body growth and reproductive characteristics of Triplophysa yarkandensis. Chin. J. Zool., 45: 29-38.
Zhang, X.F., He, C.L., and Song, Z.B., 2010. Age and growth of Triplophysa markehenensis from the Markehe River in upper reaches of the Dadu River. Chin. J. Zool., 45: 11-20.
Zhao, K.T., 1989. Ecological survey of Triplophysa yarkandensis in Sogonor fishery. Chin. J. Zool., 24: 41-43.
Zhu, S.Q., 1989. The Nemacheilinae (Cyprinifomes cobitidae) of China. Jiangsu Science and Technology Press, China.
To share on other social networks, click on any share button. What are these?










