Activation Solutions Effect on Sperm Motility and Fertilization Rates of Oreochromis niloticus
Research Article
Activation Solutions Effect on Sperm Motility and Fertilization Rates of Oreochromis niloticus
Aziz Ahmed1*, Anila Naz Soomro2, Muhammad Ramzan Ali1, Muhammad Aleem Khan1 and Hasina Basharat1
1Aquaculture and Fisheries Program, National Agriculture Research Centre (NARC), Park Road, Islamabad, Pakistan; 2Department of Freshwater Biology and Fisheries University of Sindh Jamshoro, Sindh, Pakistan.
Abstract | Fish sperm-activating media are chemical solutions having certain properties now thought to be a prime indicator for the peculiarity of spermatozoa. Present research evaluated the effect of two laboratory-prepared media and one commercially available media i.e., ACTi fish while control is freshwater on sperm quality parameters i.e., percent motility, motility interval, sustainability, and fertilization rates in Nile tilapia (Oreochromis niloticus). The experimental design was CRD with four treatments and three replicates. Lab prepared (Activation media A and B), one commercially available (Activation media C) and freshwater served as control. After the pre-developed broods were selected based on maturity approaches and collected sperm samples were diluted in the ratio of 1:30 fold media and divided into different aliquots before applying them to in vitro fertilization. It was clear from the results of the current study that, the commercially available activation media C showed a highest significant effect on percent sperm motility i.e., 89.66%±2.88, motility interval 300sec±2.67%, Viability: 74.48±0.39% and fertilization rates: 90.04±1.70% followed by activation media A. motility: 78.24±1.90, interval: 280sec±1.65 viability: 68.21±0.64 and fertilization rates: 78.01±1.81 while B. motility: 68.62±1.08, interval: 250sec.54±0.75%, viability:58.02±0.59 and fertilization rates 70.60±1.02 respectively. The lowest percentage of motility and fertilization rates were recorded in milt aliquots treated with fresh water i.e., control: 53.10±1.02 and 69.60±1.05. Our results indicated, commercially available activation media improved efficiency of sperm motility and fertilization rates.
Received | January 08, 2024; Accepted | February 13, 2024; Published | February 29, 2024
*Correspondence | Aziz Ahmed, Aquaculture and Fisheries Program, National Agriculture Research Centre (NARC), Park Road, Islamabad, Pakistan; Email: azizglt_73@yahoo.com
Citation | Ahmed, A., A.N. Soomro, M.R. Ali, M.A. Khan and H. Basharat. 2024. Activation solutions effect on sperm motility and fertilization rates of Oreochromis niloticus. Sarhad Journal of Agriculture, 40(1): 246-253.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.1.246.253
Keywords | Sperm motility (SM), Sperm viability (SV), Sperm motility interval (SMI); Fertilization rates, ACTi fish solution, Tilapia fish
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The activation of fish sperm and the length of motility are significantly subjective by numerous aspects including temperature, ocean and freshwater ionic composition, several activating solutions and ovarian fluid (Le et al., 2011; Dzyuba and Sosson, 2014) Manly reproductive performance rely on the quality of gametes both sperm and eggs (Bobe and Labbe, 2010). Control of gametes eminence plays an essential role in the aquaculture industry for the fabrication of categories by previously highly profitable attentiveness and as well as latent candidate species (Cabrita et al., 2014; Samarin et al., 2017). While in vitro loading of fish sperm quality can suffer considerable reductions in motility, viability, plasma membrane integrity, and mitochondrial membrane potential (Contreras et al., 2017; Risopatron et al., 2018). The ionic composition, as well as the ultimate osmolality, are critical factors in the activation of sperm (Alavi et al., 2007). Several studies were carried out to investigate the physiological structures of sperm quality (Boryshpolets et al., 2009). This is the principal feature that contributes to productive fertilization rates and the progress of sustainable offspring (Rurangewa et al., 2004).
Valuation of fish sperm quality criteria such as motility is presently used as a tool for aquaculture exploration and is an old technique (Victor et al., 2019). Generally, all the males cannot contribute equally to the gene pool resulting in sperm variability and low fertility (Bekkevold et al., 2002).
Therefore, the proper assessment of sperm quality parameters including motility and duration as well as viability and concentration is necessary to realize bias concerning discrete male fertility latent. However, there are numerous inventions that can offer a consistent sperm study (Kime et al., 2004), Computer-assisted sperm analysis (CASA) (Gallego et al., 2018).
The Nile Tilapia are gaining popularity in the culture system due to their rapid development, consumer desire for high-quality flesh with few spines, and great export potential. Nile Tilapia, a high-value fish species, has been successfully introduced into Pakistan’s aquaculture system (Rab et al., 2008).
Nile Tilapia (Oreochromis niloticus) breeding for seed stock production using pond culture, pen culture, and aquarium culture methods (NASS). Any artificial media that imparts improved activation of sperm and prevents their exposure to high osmotic conditions increases sperm motility for a longer period of time. (Cosson, 2004).
The assessment and establishment of fish sperm quality characteristics will substantially facilitate the use of various aquaculture reproductive technologies. (Alavi, 2008).
The study compared different parameters i.e., sperm mobility, viability, and concentration on fertilization dimensions in Nile tilapia using two types of laboratories prepared to activation solutions, e.g., potassium chloride (KCl) including sodium chloride (NaCl) and Tris, to control, i.e., distilled water. Sperm total motility and sperm viability are highly correlated; therefore, using sperm total motility as the basis for viability evaluation is a more objective, straightforward, and efficient way to determine the viability of the sperm.
Materials and Methods
Study area
The research was conducted between May to August 2021 at the National Agricultural Research Centre (NARC), Islamabad, which is located in the Potohar neighborhood near Check Shahzad NIH and is run by the Pakistan Agricultural Research Council (PARC), Animal Science Institute (ASI), and Aquaculture and Fisheries Programme (AFP). The Programme has access to three well-equipped research facilities: Fish microbiology, fish limnology, and fish nutrition labs. Concrete raceways, hatching troughs, fiberglass circular tanks, and tube wells are also available, as are channel catfish and tilapia fish seed-producing hatcheries.
Experimental design
The current study aimed to investigate the effects of two distinct sperm motility activation solutions or media, such as A and B, and C with distilled water (control), on sperm mobility or motility and the rate of egg fertilization in Nile Tilapia (Oreochromis niloticus) locally bred. The experiment’s CRD (Complete Randomized Design) consisted of three treatments (A, B, and control) and three replicates.
Protocol
Two laboratory-prepared A and B activating solutions were used as directed by Bastami et al. (2010) and Bastami et al. (2010), with different chemical (Merck) conformations and different potassium k+ ion concentrations, including chemical Tris in 10 milliliters (mL) of distil water, while C was ACTi fish that was readily available commercially and Freshwater served as the control.
35 mM of NaCl, 10 mM of KCl and 30 mM of Tris.
45mM of NaCl, 15 mM of KCl and 30 mM of Tris
ACTi fish (commercially available)
Collection of gametes (sperm and eggs) from mature Nile Tilapia
The brooders, which included 8 males and 8 females with an average weight of 550–650 gm, were chosen based on maturity approaches. All of the fish samples were healthy, practically disease-free, and fully ripened at the time of the experiment, which took place during the spawning season, which runs from May to August. As soon as the fish were captured, they were taken to the lab for dissections and the collection of gametes for assessing sperm motility and egg fertilization.
Dilution of sperm samples
Prepared the diluter solution earlier. The sperm samples were diluted in each activator solution 30 times at 40 oC by taking 1 µL of sperm and 29 µL of activation solution. It is worth noting that the dilution ratio is sperm sample specific based on estimated sperm concentration obtained in the pre-analysis of motility using software (Sperm Class Analyzer®, Morfo Version 1.1; Images, Barcelona, Spain) to calculate the best samples with the correct concentration.
Evaluation of sperm motility parameters
- Set up motility modules of software select the properties and choose the desired parameter before starting an analysis.
- Choose the frame rate and number of pictures per second.
- Negative contrast is required for the correct reconstruction of spermatozoa pathways.
Mobility duration
Sperm mobility time is measured as the length of time it takes for all spermatozoa cells to go from fully mobilized to entirely dead. A 30 µL sample of diluted sperm (01 µL milt + 29 µL activating solution = 30 µL) was reserved from a chosen aliquot on a glass slide, and using a cover slip, the motility time was monitored under a 100 X magnification. In laboratory settings, the parameters of motility and its duration were both monitored at regular intervals of one second. Samples were tested every one to two seconds to gauge the percentage of mobilized spermatozoa until every last one was discovered to be dead.
Sperm viability
A 0.4% solution of trypan blue was prepared by dissolving four milligrams (mg) of trypan in one milliliter (mL) of distilled water. This solution was used to assess the viability of sperm. The stain was continuously filtered before use. On a glass slide, a smear was formed by combining 1µL of sperm and 1µL of stain. The mixture was then allowed to air dry for 2–5 minutes before being examined under a microscope (Optika, Italy) at a magnification of 40. Live spermatozoa with intact membranes remained uncolored when milt was added to the stain, whereas dead sperm with damaged membranes stained blue. Sperm viability was further calculated by using the following formula (Vasan, 2011).
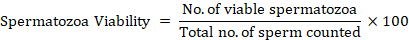
Concentration/micro liter
As per the recommended approach, the concentration of spermatozoa was determined using a Neuberger hemocytometer (Improved Neuberger, Germany) with the use of a micropipette, The hemocytometer was filled with a total of 30 µL of diluted solution (01 µL of milt + 29 µL of medium). During loading, the pipette’s tip was positioned on the hemocytometer’s V-shaped groove. To stop the spermatozoa from moving, the sample was put in a lab setting. The spermatozoa were counted to determine the concentration under 40 X magnification in the four corner squares and central Square. The following formula was used to calculate the concentration of sperm per microliter (Hala et al., 2009; Fatihah et al 2014a, b).
Viable sperm cells per square x dilution factor x 104
Eggs collection and fertilization rates
Following the collection of sperm from male tilapia and division into three aliquots according to the determined sperm concentration/microliter, eggs from chosen females were divided into three of one gram each, totaling nearly 220 eggs. Then a full 31 µL of ready solution was added. In practically all the treatments, various amounts of milt were utilized to maintain the spermatozoa concentration per microliter.
Visual evaluation of fertilization rates was performed in the current study by comparing the proportion of eyed eggs to the total number of eggs after fertilization. Eggs were combined with this diluted milk. For optimum fertilization, new water was added after 5 minutes of mixing, and it was left to stand for 10-15 minutes. Dead or unfertilized eggs appear impermeable, whereas water-hardened and fertilized eggs were brilliant and translucent in colour, measuring three to five millimeters in diameter. Fertilization rates were determined by following this formula (Khara, et al., 2014).
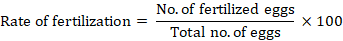
Statistical analysis
The data was presented as means and standard deviations (S.D). Effects of media were assessed using a one-way ANOVA (variances of analysis) and Duncan’s Multiple Range Test (DMRT) with the Statistix 8.1 for statistically significant p < 0.05 comparisons of average means.
Results and Discussion
Effectiveness of two lab-prepared sperm mobility activation media on the viability of sperm and the rate of egg fertilization in commercially available ACTi fish was examined in comparison to distilled water, the control, in Nile tilapia (Oreochromis niloticus).
Sperm mobility
The current study’s findings demonstrated that inside the experimental activation media within three treatments and when compared to the control i.e., freshwater the highest values found significant p<0.05 effects with in ACTi fish C i.e., 89.72%±3.08 followed by A i.e., 78.24±1.90 while B. 68.62±1.08 and control i.e., 53.10±1.02. Data is also shown in (Figure 1) with means ± Standard Deviation (S.D).
Sperm mobility interval
Calculated in seconds, duration recorded with four treatments and high values also found in ACTI fish i.e., 300sec±2.67% following by A. 280sec±1.65, B. 250sec. 154±0.75% significantly p < 0.05 dissimilar from each other. Presented in (Figure 2) with means ± Standard Deviation (S.D).
Sperm sustainability
Sustainability or viability of sperm cells also recorded highest in ACTi fish C i.e., 74.48±0.41% followed by A. 68.21±0.64 and B. 58.02±0.59 with average means ± Standard Deviation (S.D) in (Figure 3). Significantly p < 0.05 indicates that within the media A, B and C viability differs significantly.
Sperm concentration/microliter
In order to calculate the fertilization rate, the almost equal concentration of sperm in each aliquot of dilution medium was adjusted using sperm concentration. For medium A, B, and C, the concentration values were 2.50 x 104±0.76, 2.50 x 104±0.14, and 1.93 x 104±0.45.
Fertilization rates
The ACTi fish C had the highest fertilization rates under the nearly equalized concentration of spermatozoa, measuring 91.21±2.81%. Media A and B came in second and third, respectively, with 79.23±2.63, 71.58±2.14, and freshwater (control) measuring 69.11±2.09. Based on statistical analysis, it was noted that there was a substantial variation of p < 0.05 in the fertilization rates across all treatments. Data also presented with means ± Standard Deviations (S.D) in (Figure 4).
According to the findings of the current study, the kind of activating solutions (AS) utilized has a substantial impact on the mobility of milt and the rate of fertilization in Nile Tilapia. The success of fertilization is strongly correlated with the quality of gametes (Bobe and Labbe, 2010), the activating solution employed (Zarski et al., 2012), the sperm to egg ratio (Linhart et al., 2014), and the period active sperm and active eggs are in contact (Bozynski and Liley, 2003). Numerous research projects examining the effects of various activation media on spermatozoa motility activation, (Billard 1995; Ohta et al., 1997). However, little has been discovered about how different activating solutions alter fertilization efficacy (Linhart et al., 2014; Zarski et al., 2012).
In the presence of semen plasma or isotonic solutions, the spermatozoa of most freshwater fish species are immobile, and they must be diluted with a suitable medium to produce good motility. Applying several solutions to the in vitro fertilization of crucian carp (Zarski et al., 2012), Carassius (L). After dilution (0 h), sperm motility was high (range 82.4-94.5%) in all ASP variants and even 97.8% in the control group with fresh sperm while with the passage of time motility significantly decreased. Cejko et al. (2018) investigated the effect of sodium and potassium, along with an appropriate PH, on the motility activation of common carp L. sperm during short-term storage in artificial seminal plasma. In the current investigation, fresh sperm motility was observed to be 81.66% utilizing activation media that contained 10 milli liters (ml) of pure water, 50 milli molars (mM) of NaCl, 30 milli molars (mM) of KCl, and 30 milli molars (mM) of Tris.
The longest period of sperm motility, 570 seconds, was attained in the current investigation. Duration was reached in Cyprinus carpio and Danio rerio up to between 10 and 15 minutes using the same sort of activation material (Jing et al., 2009; Bastami et al., 2010). Other findings, such as 540s using sodium citrate, were also obtained (Cosson et al., 2008). In addition to sperm viability testing, the percentage of spermatozoa that were alive and dead was determined using a trypan blue stain method (0.5 percent) milt trial, and the error was discovered using a 40-X powerful microscope. Similar investigations were still ongoing by (Rurangewa et al., 2004), who calculated the number of full (viable and damaged red-stained) spermatozoa under a microscope and recommended the stain of sperm. According to (Morisawa et al., 1983), k-ions increased sperm viability and sperm ambition quickly at an absorption beneath in the seminal plasma, where Na ions and the none- electrolyte were minus operational (Sule and Adikwu, 2004) suggested that larger eggs take a developed sperm viability in comparison to smaller eggs since they are species-specific and larger.
In the current study, Nile tilapia fertilization rates were found to be around 84.98 % with mean standard deviations of 3.11% in media (B). Hossain et al. (2012) described the linked effects of significantly high fertilization rates or 85.9%. With therapies, it can be higher than usual (Strasser et al., 2010). The object may be identified by the significant changes in hormone dosages, as well as by the size of the brooder and seasonal differences (Nwokoye et al., 2007). The proportion of eggs to sperm and the superiority of the gonads are directly related to fertility rates (Bobe and Labbe, 2010). Linhart (2006), the length of time that active sperm interact with active eggs (Lily et al., 2003), and the activating agent used (Zarski, 2012a). Rurangwa et al., (2004) the optimum ratio of spermatozoa: Eggs (1500:1) for artificial insemination of Nile Tilapia, gave fertilization and hatching rates of 81 and 69%, respectively.
Conclusions and Recommendations
Sperm total motility and sperm viability are highly correlated; therefore, using sperm total motility as the basis for viability evaluation is a more objective, straightforward, and efficient way to determine the viability of the sperm. It was proved in the study that commercially available ACTi fish media can control sperm motility enhance sperm movement duration and viability and improve fertilization rate in Tilapia fish. Sperm preparation after freezing improves motile sperm count, motility, and viability in frozen-thawed sperm.
Acknowledgments
The authors are grateful to Aquaculture and Fisheries Program for their valuable contribution.
Novelty Statement
Commercially available activation media improved efficiency of sperm motility and fertilization rates.
Author’s Contribution
Aziz Ahmed: Conceptualized and designed the study, performed the experiments. interpreted the results, and wrote the manuscript.
Anila Naz Soomro: Conceptualized and designed the study, wrote the manuscript.
Muhammad Aleem Khan: Assisted in conducting the experiments and analyzed the data.
Muhammad Ramzan Ali: Helped in conducting the experiments and analyzed the data.
Hasina Basharat: Helped in data collection.
Conflict of interest
The authors have declared no conflict of interest.
References
Alavi, S.M.H., M. Rodina, T. Policar, P. Kozak, M. Psenicka and O. Linhart. 2007. Semen of Perca fluviatilis L.: Sperm volume and density, seminal plasma indices and effects of dilution ratio, ions and osmolality on sperm motility. Theriogenology, 68: 276–283. https://doi.org/10.1016/j.theriogenology.2007.05.045
Alavi, S.M.H., 2008. Fish spermatology. Oxford, UK: Alpha Science International. pp. 397-460. ISBN 978-1-84265-369-2
Bastami, K.D., M.R. Imanpour and S.H. Hoseinifar. 2010. Sperm of feral carp Cyprinus carpio: Optimization of activation solution. Aquacult. Int., 18: 771-776. https://doi.org/10.1007/s10499-009-9299-6
Bekkevold, D., M.M. Hansen and V. Loeschcke. 2002. Male reproductive competition in spawning aggregations of cod (Gadus morhua L.). Mol. Ecol., 11(1): 91-102. https://doi.org/10.1046/j.0962-1083.2001.01424.x
Billard, R., J. Cosson, G. Perchec and O. Linhart. 1995. Biology of sperm and artificial reproduction in carp. Aquaculture, 129(1-4): 95-112. https://doi.org/10.1016/0044-8486(94)00231-C
Bobe, J. and C. Labbe. 2010. Egg and sperm quality in fish. Gen. Comp. Endocrinol., 165: 535–548. https://doi.org/10.1016/j.ygcen.2009.02.011
Boryshpolets, S., B. Dzyuba, V. Stejskal and O. Linhart. 2009. Dynamics of ATP and movement in Eurasian perch (Perca fluviatilis L.) sperm in conditions of decreasing osmolality. Theriogenology, 72: 851–859. https://doi.org/10.1016/j.theriogenology.2009.06.005
Bozynski, C. C. and N. R Liley.. 2003. The effect of female presence on spermiation, and of male sexual activity on ready sperm in the male guppy. Anim. Behav., 65(1): 53-58. https://doi.org/10.1006/anbe.2002.2024
Cabrita, E., S. Martínez-Páramo, P.J. Gavaia, M.F. Riesco, D.G. Valcarce, C. Sarasquete, M.P. Herráez and V. Robles. 2014. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture, 432: 389–401. https://doi.org/10.1016/j.aquaculture.2014.04.034
Cejko, B.I., A. Horváth, T. Kollár, E. Kása, J. Lujić, Z. Marinović and R.K. Kowalski. 2018. Optimisation of sodium and potassium concentrations and pH in the artificial seminal plasma of common carp Cyprinus carpio L. Fish Physiol. Biochem., 44: 1435-1442. https://doi.org/10.1007/s10695-018-0491-3
Contreras, P., P. Ulloa, O. Merino, I. Valdebenito, E. Figueroa, J. Farías and J. Risopatrón. 2017. Effect of short-term storage on sperm function in Patagonian blenny (Eleginops maclovinus) sperm. Aquaculture, 481: 58–63. https://doi.org/10.1016/j.aquaculture.2017.08.022
Cosson, J. 2004. The ionic and osmotic factors controlling motility of fish spermatozoa. Aquacult. Int., 12: 69–85.
Cosson, J., A.L. Groison, M. Suquet, C. Fauvel, C. Dreanno and R. Billard. 2008. Studying sperm motility in marine fish: an overview on the state of the art. J. Appl. Ichthyol., 24: 460-486
Dzyuba, V., and J. Cosson. 2014. Motility of fish spermatozoa from external signaling to flagella response. Reprod. Biol., 14(3): 165-175. https://doi.org/10.1016/j.repbio.2013.12.005
Fatihah, S.N., Safiah, J., Abol-Munafi, A.B. and Ikhwanuddin, M., 2014a. Effect of testosterone undecanoate hormone on sperm and its level in the hemolymph of male mud spiny lobster, Panulirus polyphagus. Pak. J. Biol. Sci., 17(7): 937-941. https://doi.org/10.3923/pjbs.2014.937.941
Fatihah, S.N., J. Safiah, A.B. Abol-Munafi and M. Ikhwanuddin. 2014b. Effect of 17 α hydroxyprogesterone and 17 α-hydroxypregnenolone on sperm quality and sperm quantity in male mud spiny lobster (Panulirus polyphagus). Pak. J. Biol. Sci., 17(10): 1124-1129. https://doi.org/10.3923/pjbs.2014.1124.1129
Galego, A. and N.J.F. Astuarino. 2018. Fish sperm motility assessment as a tool for aquaculture research: A historical approach. https://doi.org/10.1111/raq.12253
Hala, D.N., K.V. Look, W.V. Holt and S. Jobling. 2009. Validation of a method for measuring sperm quality and quantity in reproductive toxicity tests with pair-breeding male fat head minnows (Pimephales Promelas). ILAR J., 50: e1-e10. https://doi.org/10.1093/ilar.50.4.E1
Hossain, B., M. Rahman, G. Sarwer, Y. Ali, F. Ahamed, S. Rahman and Y. Hossain. 2012. Comparative study of carp pituitary gland (PG) extract and synthetic hormone ovaprim used in the induced breeding of stinging catfish, Heteropneustes fossilis (Siluriformes: Heteropneustidae). Our Nat., 10(1). https://doi.org/10.3126/on.v10i1.7755
Jiang, M., 2005. Production comparison of channel catfish Ictalurus punctatus, blue catfish I. furcatus, and their hybrids in earthen ponds (Doctoral dissertation). http://hdl.handle.net/10415/502
Jing, R., C. Huang, C. Bai, R. Tanguay and Q. Dong. 2009. Optimization of activation, collection, dilution, and storage methods for zebrafish sperm. Aquaculture, 290(1-2): 165-171. https://doi.org/10.1016/j.aquaculture.2009.02.027
Kime, D.E., F. Olivier and J.P. Nash. 2004. The measurement of sperm and factors affecting sperm quality in cultured fish. Aquaculture, 234(1-4): 1-28. https://doi.org/10.1016/j.aquaculture.2003.12.006
, , , , , and . 2011. Effects of varying dilutions, pH, temperature and cations on sperm motility in fish Larimichthys polyactis. J. Environ. Biol., 32: 271–276.
Linhart, O., M. Rodina and J. Cosson. 2014. 2011, Cryopreservation of sperm in common carp Cyprinus carpio: Sperm motility and hatching success of embryos. Cryobiology, 41: 241–250. https://doi.org/10.1006/cryo.2000.2284
Linhart, O., S.D. Mims, B. Gomelsky, L.I. Cvetkova, J. Cosson, M. Rodina and B. Urbanyi. 2006. Effect of cryoprotectants and male on motility parameters and fertilization rate in paddlefish (Polyodon spathula) frozen-thawed spermatozoa. J. Appl. Ichthyol., 22. ISSN 0175-8659. https://doi.org/10.1111/j.1439-0426.2007.00992.x
Morisawa, M., K. Suzuki and S. Morisawa. 1983. Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. J. Exp. Biol., 107(1): 105-113. https://doi.org/10.1242/jeb.107.1.105
NASS Home Page address: http://www.usda.gov/nass/.
Nwokoye, C.O., L.A. Nwuba and J.E. Eyo. 2007. Induced propagation of African clariid catfish, Heterobranchus bidorsalis (Geoffrey Saint Hillarie, 1809) using synthetic and homoplastic hormones. Afr. J. Biotechnol., 6(23). http://www.academicjournals.org/AJB, https://doi.org/10.5897/AJB2007.000-2430
Ohta, H., H. Kagawa, H. Tanaka, K. Okuzawa, N. Iinuma and K. Hirose. 1997. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol. Biochem., 17: 163-169. https://doi.org/10.1023/A:1007720600588
Rab, A., S.U. Khan, M. Afzal, M.R. Ali and M. Qayyum. 2008. Replacement of fishmeal with soybean meal in diets for channel catfish, Ictalurus punctatus fry introduced in Pakistan. Pak. J. Zool., 40(5): 0030-9923/2008/0005-0341
Risopatrón, J., O. Merino, C. Cheuquemán, E. Figueroa, R. Sánchez, J.G. Farías and I. Valdebenito. 2018. Effect of the age of broodstock males on sperm function during cold storage in the trout (Oncorhynchus mykiss). Andrologia, 50(2): e12857. https://doi.org/10.1111/and.12857
Rurangwa, E., D.E. Kime, F. Ollevier and J.P. Nash. 2004. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture, 234(1-4): 1-28. https://doi.org/10.1016/j.aquaculture.2003.12.006
Samarin, A.M., D. Żarski, K. Palińska-Żarska, S. Krejszeff, M. Blecha, D. Kucharczyk and T. Policar. 2017. In vitro storage of unfertilized eggs of the Eurasian perch and its effect on egg viability rates and the occurrence of larval malformations. Animal, 11(1): 78-83. https://doi.org/10.1017/S1751731116001361
Strasser, P., S. Koh, T. Anniyev, J. Greeley, K. More, C. Yu and A. Nilsson. 2010. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem., 2(6): 454-460. https://doi.org/10.1038/nchem.623
Sule, O.D. and I.A. Adikwu. 2004. Effect of brood stock size on egg and larval size and survival of larvae of the African catfish, Clarias gariepinus under laboratory conditions. J. Aquat. Sci., 19(1): 1-4. https://doi.org/10.4314/jas.v19i1.19957
Vasan, S.S., 2011. Semen analysis and sperm function tests: How much to test? Indian J. Urol. J. Urol. Soc. India, 27(1): 41. https://doi.org/10.4103/0970-1591.78424
Victor, H., B. Zhao, Y. Mu, X. Dai, Z. Wen, Y. Gao and Z. Chu. 2019. Effects of Se-chitosan on the growth performance and intestinal health of the loach Paramisgurnus dabryanus (Sauvage). Aquaculture, 498: 263-270. https://doi.org/10.1016/j.aquaculture.2018.08.067
Żarski, D., A. Horvath, L. Kotrik, K. Targońska, K. Palińska, S. Krejszeff and D. Kucharczyk. 2012. Effect of different activating solutions on the fertilization ability of Eurasian perch, Perca fluviatilis L., eggs. J. Appl. Ichthyol., 28: 967-972. https://doi.org/10.1111/jai.12098
Zarski, D., Á. Horváth, L. Kotrik, K. Targońska, K. Palińska, S. Krejszeff, Z. Bokor, B. Urbányi and D. Kucharczyk., 2014. Application of different activating solutions to in vitro fertilization of crucian carps, carassiu Carassius (L). Aquacult. Int., 22: 173-184. https://doi.org/10.1007/s10499-013-9692-z
To share on other social networks, click on any share button. What are these?








