The Journal of Advances in Parasitology
Review Article
Overview of Strongyloidiasis: A Neglected Tropical Disease
Muhammed Hossain*, Md. Jamal Uddin Bhuiyan
Department of Parasitology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Sylhet-3100, Bangladesh.
Abstract | Strongyloidiasis infection caused by Strongyloides stercoralis is still an elusive disease in spite of recent advances with experiments on animal models. As it is endemic in developing countries such as Asia, Africa, Southeast Asia, Bangladesh, Central and South America but still poses a threat to the developed world owing to its peculiar trait of autoinfection, hyper-infection syndrome involving pulmonary and gastrointestinal system and disseminated infection with involvement of other organs like liver, brain and lungs. It can occur asymptomatically which confuses the clinicians during diagnosis as it has no gold standard diagnostic techniques although luciferase immune-precipitation system shows increased sensitivity and specificity to detect S. stercoralis specific antibody titter in serum and PCR method to detect DNA in fecal samples. Ivermectin and albendazole are recommended anthelmintic for strongyloides infection. This review will be focused on current published research on improved diagnostic techniques for strongyloidiasis detection and immune mechanism thought to be responsible for hyper-infection along with epidemiology, pathogenesis, clinical manifestations, diagnosis, treatment, prevention and control. S. stercoralis is still a global health concerned topic that is undermined in many countries. Novel diagnostic methods are expected to improve epidemiological studies, treatment, control and prevention of strongyloidiasis. Further studies are needed to open the mechanism of severe clinical manifestations of human strongyloidiasis.
Keywords | Strongyloidiasis, hyper-infection, immune-compromised, neglected and overview
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | March 21, 2016; Accepted | May 27, 2016; Published | June 09, 2016
*Correspondence | Muhammed Hossain, Department of Parasitology, Faculty of Veterinary and Animal Science, Sylhet Agricultural University, Sylhet-3100, Bangladesh; Email: bmhossain34sau@gmail.com
Citation | Hossain M, Bhuiyan MJU (2016). Overview of strongyloidiasis: A neglected tropical disease. J. Adv. Parasitol. 3(3): 93-103.
DOI | http://dx.doi.org/10.14737/journal.jap/2016/3.3.93.103
ISSN | 2311-4096
Copyright © 2016 Hossain and Bhuiyan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The threadworm Strongyloides stercoralis is a common intestinal nematode affects 30-100 million people worldwide (Bethony et al., 2006; Olsen et al., 2009). S. stercoralis is only parasite of soil-transmitted helminths (STH) group which can cause auto infection and thus ultimately lead to high parasite intensity specifically in immune-compromised individuals (Basile et al., 2010; Marcos et al., 2008; Vadlamudi et al., 2006). Strongyloidiasis is endemic in areas where sanitation conditions are poor and where the milieu is warm and humid (Hall et al., 1994) such as Asia, Africa, Southeast Asia, Bangladesh, Central and South America (Adedayo et al., 2002; Ericsson et al., 2001; Segarra-Newnham, 2007). Other species of Strongyloides, S. fuelleborni and S. kellyi can cause human strongyloidiasis but is less common and mainly found in Africa and Papua New Guinea (Siddiqui and Berk, 2001). S. stercoralis is unique in its ability to replicate in the human host permitting ongoing cycles of autoinfection. Strongyloidiasis can consequently persist for decades without further exposure to exogenous infection (Weller and Nutman, 2010).
Molecular and serological studies ensured its continuous existence in Bangladesh (Hossain et al., 2016a, 2016b, 2016c; Khair et al., 2016; Hall et al., 1994; Sultana et al., 2012). Severe complication with clumsy infection of strongyloidiasis may lead to substantial mortality as high as 87% (Ericsson et al., 2001). Chronic S. stercoralis infections can be asymptomatic or cause cutaneous, gastrointestinal or pulmonary symptoms (Siddiqui and Berk, 2001). Most of the infections may remain asymptomatic (Fardet et al., 2007; Foreman et al., 2006; Jr et al., 2010) but diarrhea and abdominal pain are the most common symptoms (Grove, 1995; Lim et al., 2004). The common dermatological aspects of chronic strongyloidiasis are itching and rash (Ly et al., 2003).
In patients with concurrent Human T-cell-lymphocytic virus 1 (HTLV-1) infection or those on corticosteroid therapy, autoinfection can go unchecked and large numbers of invasive Strongyloides larvae may disseminate widely and cause hyper-infection which can be fatal (Weller and Nutman, 2010; Miller et al., 2008; Mirdha, 2009). Other recognized predisposing conditions or risk factors for infection include living in an endemic region, chronic malnutrition, malignancies, organ transplantation, diabetes mellitus, chronic obstructive pulmonary disease (COPD), alcoholism, chronic renal failure and breast milk from an infected mother (Mirdha, 2009; Iriemenam et al., 2010). Therefore the objectives of this study is to provide overall current knowledge on strongyloidiasis.
Distribution
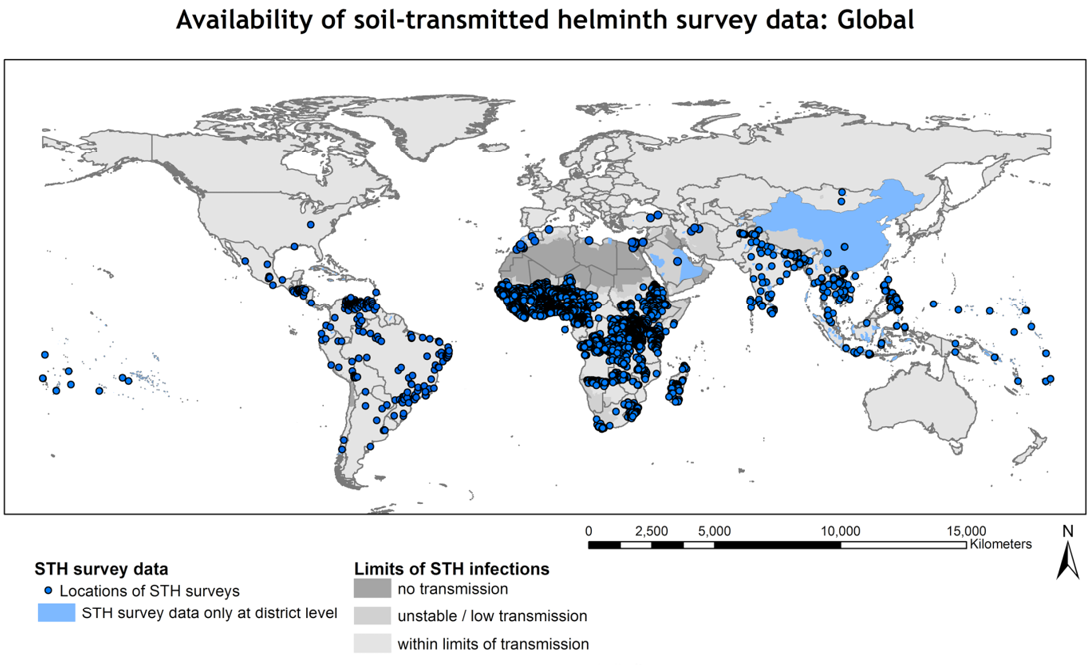
The magnitude of presence of soil-transmitted helminths worldwide has made it surpass the all-time most prevalent parasitic disease, malaria (Fao, 2014).
S. stercoralis has a worldwide distribution and is found in many tropical and subtropical countries including those of Africa, Asia and South America (Cheesbrough, 1982). S. stercoralis is a nematode endemic in humid, tropical regions (Abrescia et al., 2009) including Africa, Southeast Asia, and Latin America (Agarwal et al., 2009) (Figure 1). Strongyloidiasis can consequently persist for decades without further exposure to exogenous infection (Ericsson et al., 2001).
Risk factors
Host Factors
Strongyloidiasis infects wide range of host (cattle, goat, sheep, pig and small mammals like mice) (Haque et al., 2011; Hossain et al., 2015; Dawkins and Grove, 1981) but S. stercoralis, S. fuelleborni and S. kellyi only causes infection in human (Rose, 2008). Female showed higher prevalence of Strongyloides infection than male (Mekonnen et al., 2014; Vonghachack et al., 2015). The elderly persons had higher prevalence than the young participants in Cambodia (Khieu et al., 2013). Strongyloides larvae may disseminate widely and cause hyper-infection in patients which can be fatal (Weller and Nutman, 2010; Miller et al., 2008; Mirdha, 2009). Males, people of white race, residents of chronic care institutions (Proctor et al., 1987) and people working with soil (such as person work in coal mines and farms) are at the greatest risk of acquiring this disease (Hossain et al., 2016; Keiser and Nutman, 2004).
Table 1: Principle features of STHs and S. stercoralis
| Feature | Major soil transmitted helminths | ||||
| A. lumbricoides | T. trichiura | A. duodenale | N. americanus | S. stercoralis | |
| Multiplication within the host/auto infection | - | - | - | - | ++ |
|
Morbidity: acute/chronic |
+/+++ | +/+++ | +/+++ | +/+++ | +/++++ |
| Potential fatality | + | - | ++ | + | ++++ |
| Main diagnostic stage | Egg | Egg | Egg | Egg | Larvae |
| Therapeutic goal | Cure/decrease load | Cure/decrease load | Cure/decrease load | Cure/decrease load | Cure |
| Outcome measurement | Cure rate and egg reduction rate | Cure rate and egg reduction rate | Cure rate and egg reduction rate | Cure rate and egg reduction rate | Cure rate |
Season and Environmental Factors
The seasonal distribution of Strongyloides infection was observed to be highest during rainy season (Hossain et al., 2016a; Hossain, 2015; Cook et al., 2009; Khanum et al., 2014) than summer and winter. During winter the environmental temperatures going low which arrest the development of rhabditiform larvae and continuation of free life cycle (Fauci, 2008). The parasite is mostly confined to the tropics and subtropics (Siddiqui and Berk, 2001). The infection is more prevalent in the Appalachian region mainly rural Tennessee (Concha et al., 2005). S. stercoralis is a soil dwelling nematode and may take one of the two cycles depending on the prevalent conditions and turns parasitic in adverse conditions (Keiser and Nutman, 2004). The environmental temperature and moisture favors the parasitic cycle and transmission to adaptable host (Weller and Nutman, 2010).
Morphology
The identification of S stercoralis mostly focuses on the active feeding stage rhabditiform due to the nature of the parasite’s life cycle (Rose, 2008; Fardet et al., 2006). The rhabditiform larvae are long and slender and can grow up to 630 nm in length and 16 nm in width (Berk et al., 1987). They have a short buccal cavity with a long slender esophagus and have prominent genital primordial. The filariform larvae are the non-feeding stage, they have a longer esophagus and more of a notched tail than the rhabditiform larvae stage (Bianchi et al., 2006). Usually, only the females are found in the intestinal track because of the asexual reproduction therefore, the morphology of male larvae is not significant in a clinical setting (Table 1).
Transmission
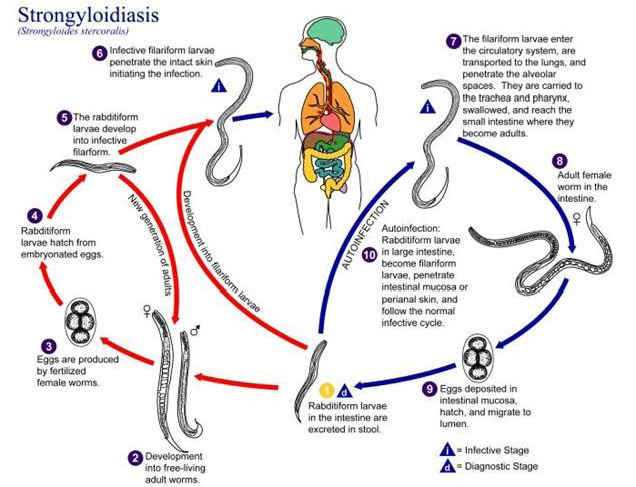
Human acquire the infection through direct skin contact with infective third stage filariform larvae (Montes et al., 2010). Larvae in the soil infect the human host by penetration of intact skin to begin the parasitic cycle (Bianchi et al., 2006). Autoinfection is one of the important characteristic features of the life cycle of S. stercoralis. The various life cycle changes in the case of autoinfection is the rhabditiform larvae instead of being shed in the stool molt twice in the body of the host to become filariform larvae that then penetrate the intestinal wall or perianal skin and reach different organs of the body leading to hyper infection syndrome (Vadlamudi et al., 2006).
Biology of Strngyloidiasis
Strongyloidiasis transmitted through unprotected contact with soil and is endemic in tropical and temperate regions (Schar et al., 2013). Strongyloides has a complex biology with two separate life cycles, the free living cycle and the parasitic cycle (Montes et al., 2010; Iriemenam et al., 2010). Human acquire the infection through direct skin contact with infective third stage larvae (L3) (Figure 2). Filariform larvae in the soil infect the human host by penetration of intact skin to begin the parasitic cycle. The larvae enter circulation are transported to the lungs, penetrate alveolar spaces, ascend the bronchial tree, are swallowed and reach the small bowel (Ericsson et al., 2001; Fauci, 2008). There the parthenogenetic females (capable of reproducing without male) embed in the duodenal mucosa and lay embryonated eggs that hatch in situ and initiate releasing of the rhabditiform larvae in the intestinal wall (Siddiqui and Berk, 2001). The larvae migrate into the lumen and are either passed into feces or mature into filariform larvae, which can infect the intestinal mucosa or skin of the perianal region to restart the parasitic cycle (Fauci, 2008). Rhabditiform larvae passed into feces can become infectious filariform larvae directly or go through a free-living cycle of development in the soil. This adaptability allows for the parasite’s survival in the absence of mammalian hosts (Weller and Nutman, 2010; Iriemenam et al., 2010).
Clinical Features
Strongyloidiasis causes a wide spectrum of clinical features ranging from asymptomatic disease, disease with mild initial symptoms, disease with chronic symptoms and acute exacerbation with hyperinfection or dissemination of larvae involving respiratory and gastrointestinal systems or multiple organ systems respectively. Though fatal hyperinfection
Table 2: Clinical manifestation of Strongyloidiasis in organ system
| Organ System | Symptoms | Signs | Investigation | References |
| Skin | Pruritis & eruption |
Urticaria, angioedema & eruption |
CBS with different count |
Concha et al., 2005 |
| GI |
Abdominal pain, diarrohea, nausea and vomiting |
weight loss, malabsorption & epigastric tenderness |
Stools for parasite, strongyloid antibody titer |
Concha et al., 2005 |
| Pulmonary |
Wheezing, cough, hemoptysis & shortness of breath |
Wheezing and rales |
Chest X-ray, sputum culture for parasites |
Gill et al., 2004 |
| CNS |
Headache, altered mental state, focal seizures & coma |
Meningeal signs & disorientation |
Lumbar puncture and cultures |
Concha et al., 2005 |
| Immune or allergic |
Urticaria or anaphylaxis |
Urticarial rash, larval cutaneous rash |
CBC with differential counts | Concha et al., 2005 |
| Hematological |
Fever, Chills & rigors |
Tachycardia, bacteremia, Septicemia & eosinophilia |
Blood cultures, CBC with differential counts |
Reechaipichitkul & Chuesakoolvanich, 2003 |
| Other (rare) |
Peritonitis, endocarditis, eosinophilic pleural effusion & eosinophilic granulomatous entero-colitis |
- | - | Gutierrez et al., 1996 |
GI: Gastrointestinal; CNS: Central Nervous System; CBC: Complete Blood Count
or dissemination can occur, asymptomatic strongyloidiasis is the most common form of the disease (Concha et al., 2005; Keiser et al., 2005) and often fatal consequences if not treated adequately (Ericsson et al., 2001). The various clinical manifestations are shown in Table 2.
Acute Infestation
The initial symptoms happen soon after the entry of the infective filariform larvae into the human body from its extra-intestinal migration in the host. Though the acute initial manifestations are not well described (Ly et al., 2003) the following symptoms are noted in some human infections like urticarial rash at the site of entry of the filariform larvae mostly in the legs (Concha et al., 2005; Keiser et al., 2005; Ly et al., 2003) cough and tracheal irritation incurring bronchitis from migration of the larvae through the lungs (Keiser et al., 2005) abdominal cramping with bloating, watery diarrhea and sometimes constipation due to lodging of the larvae and maturation into adult females in the small intestine of the host (Keiser et al., 2005; Muiz, 2003; Sudarshi et al., 2003; Berk et al., 1987) . In fact, the most common complaint noted was abdominal bloating (Sudarshi et al., 2003; Berk et al., 1987). As these initial manifestations are opaque and initiate multiple other diseases, they are often misdiagnosed and treated symptomatically with the host still harboring the parasite leading to a chronic state of the disease.
Chronic Infestation
Even though the chronic form of strongyloidiasis is asymptomatic in most cases, mild symptoms involving pulmonary and gastrointestinal systems can happen (Keiser and Nutman, 2004; Scowden et al., 1978). The various chronic manifestations include nausea, vomiting, epigastric pain with tenderness, intermittent vomiting, diarrhea, constipation, weight loss, asthma-like symptoms, urticaria (Mirdha, 2009) and distinctive larval rash from the subcutaneous migration of larvae (Keiser and Nutman, 2004; Concha et al., 2005; Arthur and Shelley, 1958; Singh, 2002; Smith et al., 1976; Amer et al., 1984; Von and Genta, 1988; Bockers and Bork, 1988). During the asymptomatic stage the only clinical finding is eosinophilia (Keiser and Nutman, 2004; Concha et al., 2005). Unless physicians have high index of assumption based on various factors like residence in endemic areas there is a high likelihood of misdiagnosis.
Hyper Infection
Immunosuppression caused by illness such as HTLV-1 and HIV infection, organ transplantation, and other infectious diseases like kala-azar (Nandy et al., 1995) can increase the risk of hyper-infection syndrome in patients with strongyloidiasis (Concha et al., 2005; Siddiqui and Berk, 2001; Singh, 2002). Hyper-infection syndrome is estimated to happen in 1.5 to 2.5% of the patients with strongyloidiasis (Milder et al., 1981). Several case reports of hyper-infection syndrome have been published in kidney (Mokaddas et al., 2009), heart, lung, intestine, pancreas (Roxby et al., 2009), liver (Vilela et al., 2009; Lichtenberger et al., 2009) and peripheral blood hematopoietic stem cell (Wirk and Wingard, 2009) transplant recipients.
Hyper-infection syndrome is not exactly defined but the assumption is an increase in the number of larvae in the stool and/or sputum along with manifestations confined to respiratory and gastrointestinal systems along with peritoneum (Keiser and Nutman, 2004). The hyper-infection syndrome happens from the enormous multiplication and migration of infective larvae especially in an immunosuppressed state. The intestinal manifestations include severe cramping abdominal pain, watery diarrhea, weight loss, nausea, vomiting and occasionally gastrointestinal bleeding (Keiser and Nutman, 2004). Subacute intestinal obstruction can also be caused by strongyloidiasis (Al-Baharani et al., 1995). The extra-intestinal manifestations include mainly asthma like symptoms such as cough, wheezing and others like pneumonia and pulmonary hemorrhage with diffuse bilateral infiltrates on the chest x ray (Keiser and Nutman, 2004; Concha et al., 2005). Rare conditions like eosinophilic pleural effusions (Roxby et al., 2009; Reechaipichitkul and Chuesakoolvanich, 2003) and eosinophilic granulomatous entero-colitis (Gutierrez et al., 1996) have also been reported in strongyloidiasis. During hyper-infection, the invading filariform larvae transport luminal bacteria to the blood stream and central nervous system leading to bacteremia and meningitis (Agarwal et al., 2009; Mirdha, 2009).
Other Manifestations
As most cases of strongyloidiasis are asymptomatic but fatal disseminated infection with involvement of multiple organ systems other than the respiratory and gastrointestinal systems as in hyper-infection syndrome could also occur especially in patients with immunosuppression from systemic steroids (Concha et al., 2005; Siddiqui and Berk, 2001). Chronic infection and malnutrition also predispose to systemic strongyloidiasis (Scowden et al., 1978). The mortality from disseminated infection could be up to 87% (Concha et al., 2005; Reechaipichitkul and Chuesakoolvanich, 2003). The high mortality rate associated with hyper-infection syndrome and disseminated disease is frequently due to secondary bacterial infections (Table 1) (Link and Orenstein, 1999; Siddiqui and Berk, 2001). The disseminated infection occurs when the larval load increases and leading to involvement of multiple organs thereby leading to various manifestations along with severe respiratory and gastrointestinal features as mentioned above (Scowden et al., 1978). The cutaneous manifestations that could occur from dissemination include widespread petechial and puerperal (Von and Genta, 1988). Occasionally this may also present as a pruritic, erythematous and morbiliform eruption (Ly et al., 2003). Involvement of the central nervous system may lead to headache, altered mental state, seizures and rarely coma (Concha et al., 2005).
Diagnosis
There is no gold standard for diagnosing Strongyloidiasis and diagnosis is often delayed or overlooked due to patients presenting with non-specific gastrointestinal complaints (Agrawal et al., 2009). Patients with chronic Strongyloidiasis usually have a low parasite load and irregular larval output making it exceedingly difficult to diagnose (Siddiqui and Berk, 2001). Nowadays widely taken sample for Strongyloidiasis diagnosis are blood and fresh stool. Several diagnostic methods have been compared to detect the presence of Strongyloidiasis including stool examination by Harada Mori filter paper culture, Modified Baermann’s technique, blood agar plate stool culture and Serological test like Enzyme Linked Immuno-Sorbent Assay (ELISA), Indirect Fluorescent Antibody Test (IFAT), Polymerase Chain Reaction (PCR) and gastrointestinal aspirate or biopsy (Greiner et al., 2008).
Direct Smear
This method involves the identification of Strongyloides egg or larvae under microscope from stool samples by normal saline, Eosin or Lugol’s Iodine as emulsifying agents (Cheesbrough, 1982) and formalin ethyl acetate concentration techniques (Siddiqui and Berk, 2001; Kirwan et al., 2009). Stool examination has poor sensitivity with a single sample being positive in only 30 to 50% of cases (Mirdha, 2009) and multiple repeated stool study needed to improve sensitivity (Biggs et al., 2009). A modified formalin-ethyl acetate concentration method resulted in higher recovery rates of S. stercoralis larvae and presumably an improved diagnostic efficiency (Anamnart et al., 2010).
Cultural Techniques
Harada Mori culture techniques provide the morphological identification of Strongyloides larvae which first introduced by (Harada and Mori, 1955) then it is widely used (Vonghachack et al., 2015; Banu et al., 2013; Steinmann et al., 2007). This culture method is more effecitve than direct saline smear and formal ether concentration techniques (Koga et al., 1990; Marchi and Cantos, 2003). Harada Mori techniques require ten days cultivation for getting fully formed larvae. The Baermann method also uses stool to detect parasite larvae. It requires multiple samples to achieve adequate sensitivity (Abrecia et al., 2009; Siddiqui and Berk, 2001). In the agar culture method stool is placed on a nutrient agar plate, incubated for at least 2 days and evaluated for visible tracks created as larvae carry bacteria over the agar (Sultana et al., 2012; Mehraj et al., 2008). Although the agar culture method has a higher sensitivity (96%) than direct fecal smears or the Baermann method and even it is more laborious, time consuming and expensive (Siddiqui and Berk, 2001; Koga et al., 1990; Marchi and Cantos, 2003).
Serology
Diagnosis of strongyloidiasis could be done by serological methods especially in asymptomatic patients with eosinophilia or mildly symptomatic patients. These serological tests are also shown to be useful in the diagnosis of strongyloidiasis even in immune-compromised individuals (de Paul et al., 2000). The serological methods determine the presence of strongyloid antibody in the serum of the human hosts. The antibody could be determined by Enzyme Linked Immuno Sorbent Assay (ELISA) (Ei-Badry, 2009; Huaman et al., 2003; Bailey, 1989), Gelatin Particle Indirect Agglutination (GPIA) (Huaman et al., 2003), Indirect Fluorescent Antibody Test (IFAT) (Machado et al., 2001) and Western Blot Analysis (WBA) (Uparanukraw et al., 1999). Both ELISA and GPIA are useful in the diagnosis of strongyloidiasis with sensitivities of 74.1% and 98.2% respectively and specificity of 100% for both studies. As low titers of strongyloid specific antibodies are noted in hyper-infection along with a low or normal eosinophil count, GPIA is more sensitive than ELISA in detecting the specific immunoglobulin in cases of chronic infection and hyper-infection (Huaman et al., 2003).
PCR
DNA Extraction: Bead beating technique a conventional method for DNA extraction (Salonen et al., 2010) from raw stool samples but now sophisticated methods like QIAGEN DNeasy Blood & Tissue Kit are developed and used widely (Qiagen Inc., Valencia, CA).
Conventional and Real Time PCR: The conventional PCR method established for detection of Strongyloides with two primer sets designed to amplify partial ribosomal DNA of S. stercoralis genome for single and nested PCR. Single PCR method yielded higher efficacy in detecting positive samples. Single PCR method amplifying a short (100bp) target represented more efficacies for detection of S. stercoralis in faecal examination compared to agar plate culture and nested PCR (Hossain et al., 2016b; Moghaddassani et al., 2011). A real-time PCR method developed by (Verweij et al., 2009) to detect S. stercoralis DNA in fecal samples utilizing a primer and probe set from the 18s rRNA gene sequence. The assay was with a high sensitivity and 100% specificity for Strongyloides detection (Taniuchi et al., 2011).
Treatment
The recommended treatment for Strongyloidiasis is either ivermectin (200 mg/kg body weight in a single dose) or albendazole (400 mg daily for 3 days) (Marti et al., 1996; WHO, 2002). In a study in Zanzibar; the effect of the two regimens was compared. A cure rate of 82.9% was achieved for ivermectin while three doses of albendazole cured 45.0% of the infected individuals (Marti et al., 1996). Most helminths control program employs mass drug administration as their principal tool; hence they offer treatment without prior diagnosis.
In areas targeted for lymphatic filariasis treatment, albendazole (400 mg single dose) and ivermectin (150 mg single dose) or diethylcarbamazine are given to the entire population above 4 years old. Albendazole at a dose of 10 mg/kg body weight can be used as an alternative if nothing else is available as it has a lower efficacy (38-45%) (Suputtamongkol et al., 2008; Mirdha, 2009). To minimize costs collaboration between the programs is encouraged and is currently implemented in many countries (Olsen, 2007). Among all these regimens, only the distribution of ivermectin in the frame of lymphatic filariasis control can be expected to result in a meaningful reduction of the prevalence of S. stercoralis. In areas with Strongyloidiasis targeted chemotherapy of high risk groups should be considered. As a minimum all the patients scheduled to receive corticosteroids should be given preventive anthelminthic treatment if they live in or have travelled in potential endemic areas (Fardet et al., 2007).
The efficacy of the treatment depends on many factors like immunodeficiency, co-infection with HTLV-1, use of corticosteroids and presence of bowel ileus that can decrease the efficacy of the drugs used in the treatment of strongyloidiasis (Scowden et al., 1978; Carvalho and Fonseca, 2004). Monitoring the response to treatment could be very difficult with detection of strongyloid larvae in the stool specimen because of the inconsistent shedding of the larvae (Dreyer et al., 1996).
Strategies for Control and Prevention
Personal prevention of Strongyloides infection is aided by the wearing of footwear. This prevents the entry of larvae into the foot. Provision and use of adequate latrines and avoiding the use of untreated human faeces as fertilizer prevents soil from becoming polluted the larvae (Conway et al., 1995). Community level hygienic measures like proper disposal of human excreta, community education about protective and hygienic measures and prompt attempt for treatment of diagnosed cases would help in the prevention of strongyloidiasis (Siddiqui and Berk, 2001). Treatment based control of Strongyloidiasis like Thiabendazole (Bezjak, 1968), Mebendazole (Wolfe and Wershing, 1974) and Ivermectin (Naquira et al., 1989) has over 80% cure rates against adults and larvae of Strongyloides in uncomplicated cases. For control of soil-transmitted helminths the target population is usually school children but regular (e.g., annual) mass treatment of entire communities using one of the four common anthelminthic compounds included in the WHO model list of essential drugs is recommended wherever the prevalence is >50% (WHO, 2006).
Animal studies have suggested a role for innate and adaptive immune mechanisms for controlling Strongyloidiasis. The innate response requires eosinophil to kill Strongyloides larvae, which in turn need cytokine interleukin-5 (IL-5) for their development and activation. Eosinophils serve as antigen presenting cells and are required for an optimal antibody response. The adaptive response involves specific antibody production (IgG and IgE) and granulocytes, which are also needed to kill the larvae (Uparanukraw et al., 1999; Keiser and Nutman, 2004). Helminth infections induce T helper2 (Th2) responses and may also stimulate regulatory T cells (Treg). Th2 cells secrete interleukin-4 (IL-4), IL-5 and other cytokines that promote antibody production by B cells and produce a high level of tissue eosinophilia, mucosal mastocytosis and IgE production and control excessive inflammatory reactions such as that caused by unrestricted T helper1 (Th1) cell-mediated inflammation (Nucci et al., 1995).
Conclusion
Strongyloidiasis infection is still a topic of great concern because of its fatal consequences as it shows hyper infection syndrome and disseminated infection along with a host of other potential complications like gram negative bacteremia and meningitis. As strongyloidiasis mostly chronic and asymptomatic and there is no specific ideal test to diagnose the infection, so this disease still remain elusive in this present era. This disease’s endemic curve is not going down because of its migration from endemic area to non-endemic areas, autoinfection etc. Interim it is warranted to investigate the risk factors involved in Strongyloides infection and screen patients from endemic areas prior to receiving corticosteroid therapy. It is also essential to highlight that prevention effort in endemic countries such as health education campaigns on the disease, proper sanitation through appropriate disposal of fecal material, regular deworming and the use of protective footwear are achievable goals to reducing the occurrence of strongyloidiasis.
Acknowledgments
The authors thankful to Maisha Khair for her cooperation in collection of valuable manuscript needed for giving this article a good shape. Finally would like to thanks to my institutional colleagues for their positive criticism in whole process of writing this manuscript.
Conflict of Interest
The authors declare no conflict of interests.
Authors’ Contribution
Muhammed Hossain has written the manuscript and did all the revision and correction of the manuscript. Md. Jamal Uddin Bhuiyan supervised the manuscript and gave worthy instruction while writing this articles.
References