The Journal of Advances in Parasitology
Research Article
Epidemiological Study of Sheep Anaplasmosis (Anaplasma ovis Infection) in Kairouan, Central Tunisia
Mohamed Gharbi1*, Houda Omri1, Mohamed Jedidi1, Samir Zorii2, Mohamed Aziz Darghouth1
1Laboratoire de Parasitologie, École Nationale de Médecine Vétérinaire, Université de Manouba, 2020 Sidi Thabet, Tunisia; 2Office des Terres Domaniales, Agro-Combinat El Alem, Tunisia.
Abstract | Specific diagnosis of infection with Anaplasma ovis is difficult, if based only on clinical signs. A cross-sectional survey was carried out in 26 flocks totalling 8,049 ear tagged Barbarin sheep. All the animals were clinically examined and Giemsa stained blood smears were performed. A total number of 282 Anaplasma ovis infected sheep were examined for clinical symptoms. The cumulative incidence of sheep anaplasmosis was 11.6%, rickettsiaemia was significantly higher in symptomatic (median=16/50 field microscope) compared to asymptomatic animals (median=6/50 field microscope) (p<0.001). Symptoms of anaplasmosis were pale mucosa (84.4%), anorexia (25%), prostration (51.6%), nasal discharge (18.3%), respiratory distress (22.6%) and fever (100%). Clinical cases with no mortality, and positive blood smears, only occurred in lambs, and adults were apparently immune. The situation may therefore be considered as enzootic stable. We recommend for field veterinarians to suspect erythrocytic anaplasmosis in regions where Rhipicephalus turanicus is present in lambs with prostration, pale mucosa, and fever.
Keywords | Anaplasma ovis, Sheep, Erythrocytic anaplasmosis, Clinical symptoms, Tunisia
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | May 31, 2015; Revised | June 11, 2015; Accepted | June 12, 2015; Published | June 16, 2015
*Correspondence | Mohamed Gharbi, Laboratoire de Parasitologie, École Nationale de Médecine Vétérinaire, Univ. Manouba, 2020 Sidi Thabet, Tunisia; Email: gharbim2000@yahoo.fr
Citation | Gharbi M, Omri H, Jedidi M, Zorii S, Darghouth MA (2015). Epidemiological study of sheep anaplasmosis (Anaplasma ovis infection) in Kairouan, Central Tunisia. J. Adv. Parasitol. 2(2): 30-33.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.2.30.34
ISSN | 2311-4096
Copyright © 2015 Gharbi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sheep production in Tunisia (North Africa) is an important sector, involving 4,044,000 females; the density of sheep in Tunisia is the highest in Africa (40 sheep/km²) (Ministère de l’Agriculture et des Ressources Hydrauliques, 2006). This species contributes to 50.3% of national red meat production (Ministère de l’Agriculture et des Ressources Hydrauliques, 2006). Sheep are facing several health problems with high prevalence such as cystic echinococcosis, brucellosis, toxoplasmosis, gastrointestinal and respiratory nematodes, fasciolosis, etc. (Akkari et al., 2011; Akkari et al., 2012; Darghouth and Gharbi, 2011; Lahmar et al., 2012). The sheep population is mainly owned by small farmers with poor financial resources, since farmers having a land surface comprised between 0 and 10 ha own 70% of the sheep (Ministère de l’Agriculture et des Ressources Hydrauliques, 2006).
According to Friedhoff (1997) sheep anaplasmosis due to Anaplasma ovis infection is a subclinical infection with little economic importance; Camus and Uilenberg (2010) also consider it to be usually subclinical or moderately virulent. It is frequently misdiagnosed due to the absence of specific symptoms and consequently its confusion with other diseases. According to Klabi (2011) this long-lasting infection may nevertheless cause an important decrease of weight gain, estimated in carrier lambs as over 14%. Ovine erythrocytic anaplasmosis seems to be an emergent disease in some Tunisian regions (North and Centre). Recently, Chochlakis et al. (2010) reported on what may be a human case of A. ovis infection.
We report herein a cross-sectional epidemioclinical survey carried out in farms where clinical cases of sheep anaplasmosis were reported for the first time in 2003.
Material and methods
This cross-sectional survey was carried out during the summer season (from June to late August) concerned 26 flocks totalling 8,049 ear tagged Barbarin sheep (kept within a distance of about 3 km) in the Governorate of Kairouan (Central Tunisia, 35-40N; 010-06E). This locality is characterized by a semi-arid climate with a mean annual rainfall of 290 mm and mean monthly minimum and maximum temperatures of 13 and 36°C during December and July respectively.
The animals pasture from late March till early September in stubbles of wheat, maize, and barley. The rest of the year, they graze in natural poor pastures. During the lambing period (from September to November), ewes receive complementation with concentrate and oaten hay. Acaricide treatments (diazinon) are implemented on animals once a year in early summer. The sheep are treated against gastro-intestinal and respiratory nematodes with a subcutaneous injection of ivermectin at the conventional dose of 0.2 mg/kg.
Weekly, the sheep were examined to evaluate their general state, presence of pale mucosa (classified as present or absent), rectal temperature, respiratory and cardiac frequencies and any other symptoms. A clinical case of anaplasmosis was defined as an animal presenting positive blood smears and at least anorexia with prostration. Animals with positive blood smears but no symptoms were classified as carriers.
From each animal, 5 ml of EDTA blood were collected in order to make a Giemsa-stained blood smear. The slides were examined for Anaplasma ovis and the presence of any other haemopathogens (Gharbi and Uilenberg, 2004). The infection intensity was estimated by calculating the number of Anaplasma in 50 microscope fields (each containing approximately 300 erythrocytes).
Weekly prevalence was calculated as follows:
Weekly prevalence (%) = 100 x number of new cases per week/number of examined animals
Comparison of percentages was made by using chi square test, rickettsiaemia was compared with student t test with a significance threshold of 0.05 (Schwartz, 1993).
Results
The only haemopathogen found in blood smears was A. ovis with a peak incidence during late June-early July, a second peak was observed during mid-July. No clinical cases were observed after late August (Figure 1). Clinical cases were observed exclusively in lambs of both sexes with a cumulative incidence of 11.6%, no clinical cases were detected in the other age categories. The disease was more frequent in females than in males (p<0.001) (Table 1); diseased animals presented various symptoms (Table 2). No mortality was observed or reported.
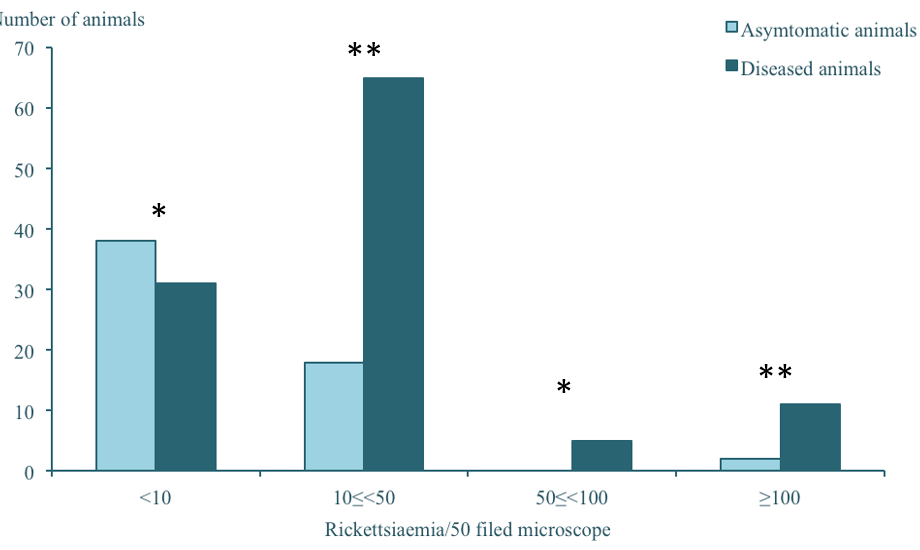
All clinically ill animals had positive Giemsa-stained smears except six, rickettsiaemia varied between 2 rickettsia to 267 per 50 microscope fields. The median rickettsiaemia was estimated to be 16 rickettsia/50 microscope fields (Table 3). They were all lambs; none of the older animals had positive blood smears. Animals without symptoms but with positive blood-smears were considered as healthy carriers of the infection. The rickettsiaemia in these animals was significantly lower than clinically ill animals (p<0.001), it varied between 2 and 300 rickettsia/50 field microscope in one animal (Figure 2). These animals were not in incubation, since these farms were visited periodically (data not shown). No statistically significant relation was observed between rickettsiaemia and the other symptoms.
Table 1: Prevalence of clinical cases in different sheep categories with erythrocytic anaplasmosis
|
Age category |
No. of sheep examined |
No. of positive blood smears |
No. of diseased animals |
Infection prevalence (S.E.) |
Carrier state prevalence (S.E.) |
Disease prevalence (S.E.) |
|
Female lamb |
1,756 |
280 |
224 |
19.14 (0.002) |
3.19 (0.004) |
12.76 (0.008) |
|
Male lamb |
675 |
65 |
58 |
10.65 (0.004) |
1.04 (0.0039) |
8.59 (0.010) |
|
Yearling female lamb |
1,133 |
0 |
0 |
0 |
0 |
0 |
|
Ewe |
4,169 |
0 |
0 |
0 |
0 |
0 |
|
Yearling male lamb |
59 |
0 |
0 |
0 |
0 |
0 |
|
Ram |
257 |
0 |
0 |
0 |
0 |
0 |
S.E.: Standard error
Table 2: Symptoms in sheep with erythrocytic anaplasmosis
|
Range |
|
|
Rectal temperature (°C) |
38.9 -42.5 |
|
Respiratory frequency (cycles/ min.) |
14 - 42 |
|
Cardiac frequency (pulsations/min.) |
96 - 138 |
|
Number of sheep (percentage) |
|
|
Pale mucosa |
291 (84.3) |
|
Prostration |
178 (51.6) |
|
Anorexia |
86 (25) |
|
Respiratory discordance |
78 (22.61) |
|
Nasal discharge |
63 (18.3) |
|
Diarrhea |
1 (0.29) |
Table 3: Comparative indicators of rickettsiaemia in the two sheep groups
|
Rickettsiaemia /50 microscope fields |
||
|
Indicator |
Asymptomatic animals |
Diseased animals |
|
Minimum |
2 |
0 |
|
1st quartile |
6 |
8 |
|
Median |
8 |
16 |
|
3 rd quartile |
12 |
26 |
|
Maximum |
300 |
267 |
*: not statistically significant ; ** : statistically significant
Discussion and conclusions
Ovine anaplasmosis due to A. ovis infection is an under-diagnosed disease; it seems to be an emergent disease in some regions of Tunisia (mainly the North and Centre of Tunisia) (Klabi, 2011).
To our knowledge, there is no gold standard for ovine erythrocytic blood smears examination associated with clinical signs should represent the best way to establish a diagnosis of sheep erythrocytic anaplasmosis. The overall prevalence of clinical cases was estimated as 3.5%, affecting exclusively lambs in their first tick season (less than 10 months of age) with a global incidence of 11.6% in the lambs. These prevalences are by far lower than those estimated by PCR in Silica (south-eastern Slovakia) which reached 70.2% (Derdáková et al., 2011); this is logical as prevalence in our study was based solely on Giemsa blood smear examination and clinical observation.
Since the farmers implement oestrus synchronization, lambing occurs exclusively during early autumn. As for several other haemopathogens, the infection intensity depends on the equilibrium between the immune system of the host and the pathogen leading to a continuous fluctuation of infection prevalence and intensity, and they were certainly underestimated in the present survey. The establishment of protective immunity can be explained by the infection of animals during the first months of the life, and consecutive infections boost their immunity. These data can explain the state of enzootic stability since neither positive blood smears nor clinical cases were observed in adult animals despite the fact that both of them were borne on the farms and exposed to the same risk factors (Table 1). The lambs become immunized; the negative blood smears in adults should be explained by the very low rickettsiaemia. Infection was more frequent in female lambs (12.8%) than in male lambs (8.6%), which can be explained by the fact that only female lambs graze, and males are kept in the barns for fattening.
Maximum incidence was observed during late June-early July with a second smaller peak in mid-July and no clinical cases during late August. This period is characterized by high temperatures (peaks of 42°C), favorable for tick activity, a weakness of the immune system of lambs and colostral antibodies dramatically decreasing after the third month. Moreover, during this period, several veterinary actions were performed (vaccinations and injections of ivermectin) which are important risk factors since the veterinarian uses the same syringe for many of the animals.
The symptoms were prostration, anorexia, fever, pale mucosa and less frequently cases of respiratory distress and nasal discharge. The anemia can be due to several other causes such as haemonchosis, fasciolosis or eperythrozoonosis (Mycoplasma ovis infection). All the animals receive periodically ivermectin, the region is arid with no foci of fasciolosis and all the blood samples were negative for Mycoplasma ovis. Hemoglobinuria was not observed in any of the clinical cases, an unusual symptom reported in Hungary (Hornok et al., 2007). No relation was observed between rickettsiaemia and the other symptoms. This is an important finding leading to conclude that there is no threshold of rickettsiaemia to distinguish between carrier and diseased sheep. Hence, we recommend to the field veterinarian to usually associate any of the symptoms cited above with the presence of any level of rickettsiaemia.
Anaplasma ovis has been reported to be transmitted by soft ticks (Ornithodoros lahorensis), hard ticks (Rhipicephalus bursa, R. turanicus and Dermacentor andersoni) and biting Diptera (which act as mechanical vectors), and transmission by sheep keds has been suspected (Friedhoff, 1997; Camus and Uilenberg, 2010; Hornok et al., 2011).
The present study is one of the scarce studies about clinical symptoms of ovine anaplasmosis. Positive blood smears concerned exclusively lambs that become protected thereafter. It would appear from our study that practitioners should take the possibility of erythrocytic anaplasmosis as the cause of disease symptoms in lambs into account.
Acknowledgements
This study was supported by Laboratoire d’épidémiologie des infections enzootiques des herbivores en Tunisie (Ministry of Higher Education, Scientific Research and Information, Technology and Communication, Tunisia) and the Deutsche Forschungsgemeinschaft project ‘‘Molecular epidemiology network for promotion and support of delivery of live vaccines against Theileria parva and Theileria annulata infection in Eastern and Northern Africa’’ (AH 41/7-1). The authors are grateful to Dr Kamel Haouala (Office des Terres Domaniales, Tunisia) for supporting this work.
Conflict of Interest
We declare that we have no conflict of interest.
Author’s Contribution
Mohamed Gharbi performed the analyses and wrote the paper and Houda Omri collected the samples, examined the animals, performed the analyses. Mohamed Jedidi performed the analyses; Samir Zorii collected the samples and Mohamed Aziz Darghouth conceived the study.
References