Advances in Animal and Veterinary Sciences
Research Article
Plasma D-dimer Concentration in Horses with Colic
Heba El-Zahar1, Yasmin Bayoumi1, Shimaa Shalaby2, Heidrun Gehlen3, Tarek Shety1*
1Animal Medicine Dept., Faculty of Veterinary Medicine, Zagazig University, Egypt; 2Physiology Dept., Faculty of Veterinary Medicine, Zagazig University, Egypt; 3Equine Clinic, Faculty of Veterinary Medicine, Freie Universität Berlin, Berlin, Germany.
Abstract | The aim of this study was to measure the concentration of the plasma D-dimer in healthy horses and in horses with colic and to compare it before and after therapy. Thirty-two horses were included; 22 horses showed signs of abdominal pain. Horses were grouped according to clinical and laboratory examination results into healthy horses (Group I, n=10); horses with colic associated with enteritis and/or colitis (Group II, n=16) were presented with moderate intermittent abdominal pain with increased peristalsis and increased frequency of defecation; horses with colic associated with impaction of the intestine (Group III, n=6) were presented with severe abdominal pain and decreased fecal output. There was significant increase in the concentration of plasma D-dimer in horses with impaction of the intestine and horses with enteritis and/or colitis compared to clinically healthy horses. Therapeutic trials resulted in improvement of the D-dimer concentration where the concentration of plasma D-dimer was significantly decreased in horses with enteritis and/or colitis and in horses with impaction of the intestine after therapy compared to its concentration before therapy. In conclusion, D-dimer is a sensitive marker for detection of excessive fibrinolysis in horses. Higher concentration of plasma D-dimer is observed in severe cases of colic and might reflect poor prognosis.
Keywords | Horse, Colic, Ultrasonography, D-dimer
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 17, 2017; Accepted | December 11, 2017; Published | December 28, 2017
*Correspondence | Dr Tarek Shety, Animal Medicine Dept., Faculty of Veterinary Medicine, Zagazig University, Egypt; Email: tarekshety@gmail.com
Citation | El-Zahar H, Bayoumi Y, Shalaby S, Gehlen H, Shety T (2018). Plasma d-dimer concentration in horses with colic. Adv. Anim. Vet. Sci. 6(1): 27-32.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.27.32
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 El-Zahar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The equine abdominal pain is one of the most important conditions affecting horses and may lead to death (Salem et al., 2017). The most important process that might lead to death of horses due to colic is the haemostatic dysfunction which resulted in vein thrombosis (Prasse et al., 1993; Collatos et al., 1994). Haemostasis involves fibrin deposition and its removal from circulation, the end products of fibrinolysis is D-dimer which is considered the most sensitive marker of coagulopathies in human medicine especially deep vein thrombosis (Mousa et al., 2017). D-dimer is formed by the breakdown of cross-linked fibrin by plasminogen during finbrinolysis (Stokol, 2003). In equine medicine, it has been used as an important tool for detection of abnormal coagulation process in horses with colic (Welch et al., 1992). Previous studies on equine colic have proven the importance of fibrinolysis markers for evaluating the hypercoagulation state (Sandholm et al. , 1995; Goldman et al., 1995; Armengou et al., 2008; Delgado et al., 2009a and b; Cesarini et al., 2010; Cesarini et al., 2014; Cesarini et al., 2016).
The objective of the present study was to measure the concentration of the plasma D-dimer in healthy horses and in those with colic and to compare its concentration before and after therapy.
Materials and methods
Animals
A total of 32 horses examined from the period between July 2015 and July 2017 were included in the present study. Horses were grouped according to the results of the general clinical examination and the diagnosis. Ten horses were apparently healthy used as control horses (Group I) and 22 horses were presented with signs of colic included horses with enteritis and/or colitis (Group II, n=16), horses with impaction of the intestine (Group III, n=6). Horses with lesions other than gastrointestinal disorders were not included. Colicky horses were presented with signs of uncontrolled abdominal pain, tachycardia and congested mucous membrane. Case history, complete physical examination, laboratory analysis, abdominal ultrasonography, rectal examination and postmortem for horses that died were performed. The owners signed client consent before samples collection and animal handling. The study was approved by the Animal Care Committee at Zagazig University.
Sample collection
Blood samples were collected from the jugular vein through jugular venipuncture into 3.2% coagulation sodium citrate tubes (Vacuette®, Greiner Bio-One Middle East, Egypt). Whole blood samples were used for complete blood count (CBC) including hemoglobin concentration (Hgb), erythrocyte count (RBCs), erythrocyte indices including mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) with an automatic analyzer (HA-Vet Clindiag, Clindiag Systems B.V.B.A, Belgium). Leucocytic count and differential count was performed by staining blood smears with Giemsa stain. Plasma was collected by centrifugation of samples at 3000 rpm/10 min and used for D-dimer assay. Blood samples were collected from each horse in two instance; one during the first clinical examination and the second samples after 10 days during follow-up. Samples were stored under -20oC until analyzed.
Measurement of Plasma D-Dimer Concentration
D-dimer was measured in plasma samples using chemiluminescent microparticle immunoassay (Abbott, Architect i2000, Abbott Diagnostics, Illinois, USA) according to manufacturer’s instruction. The results of D-dimer are expressed as ng/mL.
Ultrasonographic Examination
Ultrasound examination of the abdomen was performed for all horses included in the present study. Each animal was prepared for ultrasound examination. Ultrasound coupling gel was used to facilitate ultrasound beam penetration. Ultrasound scanner (WED, WELLD®, Shenzhen Well D Medical Electronics Co., Ltd., China) equipped with trans-abdominal scanning head (3.5 MHz) was used in the present study. All abdominal organs including stomach, small intestine, large intestine and peritoneal cavity were examined according to methods reported by Shety (2007).
Statistical analysis
Data were collected and analyzed using SPSS software (SPSS for windows, Version 17.0). The data are expressed as mean ± standard error of mean (SEM). The laboratory data in healthy horses was compared with those with enteritis and/or colitis and horses with impaction of the intestine using one way ANOVA followed by Duncan Post Hoc Multiple Comparison. In addition, the D-dimer concentration before and after therapy were compared using Wilcoxon rank test for paired measures. The quantitative measures including age, weight and height were presented in the results as mean ± SEM for descriptive purposes. While, qualitative data like gender and diagnosis, descriptive statistics was based on frequencies and percentages. Significance was set at P<0.05.
Results
During the study period, 32 horses met the inclusion criteria were included in the present study. The age of the whole population was ranged from 3-11 years (mean age of 7.5±1.3 years). The whole population was belonged to draft breeds. The gender distribution was 19 mare (59.5%), 10 stallion (31%) and 3 geldings (9.5%). The diagnosis was based on the results of clinical examination, laboratory analysis, rectal examination, ultrasonographic examinations and in two cases was supported by postmortem findings. Ten horses (31.25%) were control horses (Group I); diseased horses included 16 horses (50%) with severe colic due to enteritis and/or colitis (Group II) and 6 horses (18.75%) with colic due to impaction of the intestine (Group III) (Table 1).
The horses in the control group (Group I) were apparently healthy horses with no evidence of gastrointestinal disorders, normal clinical examination results and normal laboratory results. Horses with signs of intermittent moderate abdominal pain, loss of appetite, soft feces, straining during defecation, increased peristaltic activity, increased frequency of defecation and in some case enterogastric reflux were assigned to enteritis and/or colitis group (Group II). Horses with signs of reduced fecal output, abdominal distension, stretching, rolling was compatible with impaction of the intestine (Group III) and on rectal examination an impacted intestinal segment was palpated.
The clinical examination was performed for all horses. The results of clinical examination revealed no significant changes in the mean values of temperature (p=0.976) and capillary refill time (p=0.988), and significant changes in heart rate (p=0.036) and respiration rate (p=0.014). Temperature (oC) was 37.6 ± 0.4, 37.9 ± 0.9 and 38.1 ± 1.1 in control horses, horses with enteritis and/or colitis and horses with impaction of the intestine, respectively. Heart rate (beats per min) was 34 ± 5.2, 48 ± 10.2 and 61 ± 7.4 in control horses, horses with enteritis and/or colitis and ho-
Table 1: Selected clinical parameters observed during clinical examination of healthy horses (Group I), horses with colic associated with enteritis and/or colitis (Group II) and horses with colic associated with impaction of the intestine (Group III). Values are expressed as mean ± SEM.
| Variable | Group I | Group II | Group III | ANOVA Significance | |
| n= | 10 (31.25%) | 16 (50%) | 6 (18.75%) | - | |
| Age (years) | 7.3 ± 0.45 | 4.5 ± 1.5 | 9 ± 0.61 | - | |
| Sex | Mare | 6 | 9 | 4 | - |
| Stallion | 3 | 5 | 2 | - | |
| Gelding | 1 | 2 | - | - | |
|
Temperature (oC) |
37.6 ± 0.4 | 37.9 ± 0.9 | 38.1 ± 1.1 |
P = 0.976 |
|
| Heart rate (beats/min) | 34 ± 5.2 | 48 ± 10.2 | 61 ± 7.4 |
P = 0.036 |
|
| Respiration rate (breaths/min) | 12 ± 3.6 | 28 ± 4.5 | 34 ± 4 |
P = 0.014 |
|
| CRT (sec) | < 2 sec | < 2 sec | < 2 sec |
P = 0.988 |
|
Table 2: Selected hematological parameters measured in clinically healthy horses (Group I), horses with colic associated with enteritis and/or colitis (Group II) and horses with colic associated with impaction of the intestine (Group III). Values are expressed as mean ± SEM.Means having the same superscript are not significantly different from each other.
| Parameters | Group I | Group II | Group III | ANOVA Significance |
|
Erythrocytes (x106/uL) |
7.43 ± 0.3a |
7.52 ± 0.1a |
7.4 ± 0.1a |
0.953 |
| Hemoglobin (g/dL) |
10.7 ± 0.2a |
10.6 ± 0.4a |
11.4 ± 0.4a |
0.972 |
| Hematocrit (%) |
32.6 ± 0.65a |
35.4 ± 0.2b |
35.59 ± 0.8b |
0.003 |
| MCV (fL) |
46.5 ± 1.1a |
45.4 ± 0.4a |
45.92 ± 1.2a |
0.723 |
| MCH (pg) |
14.98 ± 0.3a |
15.12 ± 0.2a |
15.2 ± 0.1a |
0.722 |
| MCHC (%) |
32.45 ± 0.9a |
33.1 ± 0.6a |
33.9 ± 0.7a |
0.945 |
|
Leucocytes (x103/uL) |
6.4 ± 0.25a |
8.42 ± 0.6b |
9.75 ± 0.8c |
0.001 |
| Neutrophils (%) |
55.6 ± 0.2a |
61.8 ± 0.7b |
64.8 ± 0.4c |
0.041 |
| Lymphocytes (%) |
25.6 ± 0.6a |
28.4 ± 0.2b |
38.42 ± 0.6c |
0.038 |
| Monocytes (%) |
2.4 ± 0.1a |
2.5 ± 0.1a |
2.73 ± 0.2a |
0.864 |
| Eosinophils (%) |
1.9 ± 0.4a |
1.6 ± 0.1a |
0.9 ± 0.2a |
0.923 |
| Basophils (%) |
0.3 ± 0.01a |
0.3 ± 0.2a |
0.5 ± 0.02a |
0.862 |
rses with impaction of the intestine, respectively. Respiration rate (breaths per min) was 12 ± 3.6, 28 ± 4.5 and 34 ± 4 in control horses, horses with enteritis and/or colitis and horses with impaction of the intestine, respectively (Table 1).
The results of CBC showed significant changes in hematocrit %, leucocytic count, neutrophils % and lymphocyte % in horses with colic (group II and III) compared to clinically healthy horses. Meanwhile, other parameters including erythrocyte count, hemoglobin, MCV, MCH, MCHC, monocyte %, eosinophils % and basophils % were not significantly changed in all groups (Table 2).
Table 3: Plasma D-dimer concentration in healthy horses (Group I), horses with colic associated with enteritis and/or colitis (Group II) and horses with colic associated with impaction of the intestine (Group III) before and after therapy, values are expressed as mean ± SEM (Min. – Max.). Means having the same superscript are not significantly different from each other.
| D-dimer (ng/mL) | Group I | Group II | Group III | ANOVA Sig. |
| Before therapy |
278.02 ± 29.29a (40 - 825) |
516.89 ± 46.83b (130 - 859) |
1481.60 ± 114.01c (735 - 1639) |
0.001 |
| After therapy | - |
312.26 ± 15.32 (124 - 658) |
468.92 ± 92.15 (368 - 622) |
- |
| 0.040 | 0.000 | Wilcoxon rank test Sig. |
There was significant increase in plasma D-dimer concentration in horses with impaction of the intestine (1481.60 ± 114.01 ng/mL) and in horses with enteritis and/or colitis (516.89 ± 46.83 ng/mL) compared to clinically healthy horses (278.02 ± 29.29 ng/mL). In addition, there was significant decrease in plasma D-dimer concentration in horses with enteritis and/or colitis and in horses with impaction of the intestine after therapy compared to before therapy (Table 3).
On ultrasonography, there was significant increase in the intestinal wall thickness in case of enteritis and/or colitis horses with presence of anechoic intestinal contents consistent with excess fluids (Figure 1). In horses with impaction of the intestine there were significant marked increase in the diameter of the intestine, ranged from 4-8.2 cm with decreased motility except in 2 horses that were died, complete atony of intestine were observed in both cases (Figure 2 and 3). This was confirmed in 2 cases that was died after the first clinical examination, postmortem examination was performed, food mass was found engorged in the pelvic flexure of the colon with presence of congested mucosa.
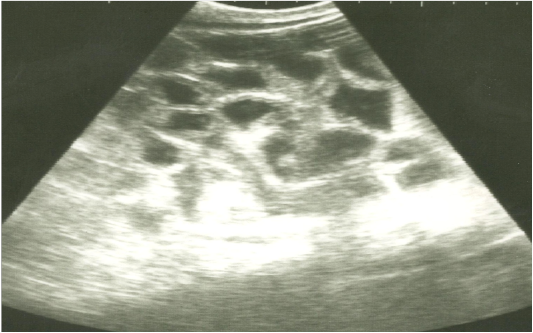
Figure 1: Ultrasonographic image of the left abdomen of horses with colic associated with enteritis and/or colitisusing 3.5 MHz transducer. The intestinal loops appear distended with fluid contents; the intestinal wall is slightly thickenedand corrugated. Increased intestinal motility of the intestinal loops was detected on the screen.
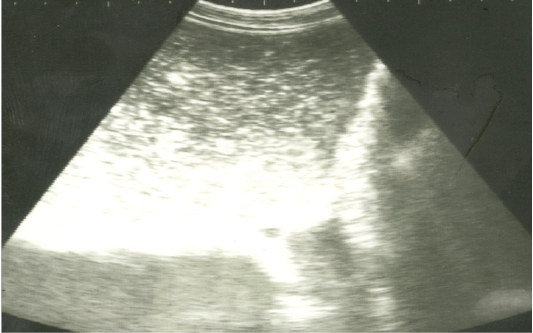
Figure 2: Ultrasonographic image of the left abdomen of horses with colic associated with impaction of the intestineusing 3.5 MHz transducer. There was marked distension of the descending colon with ingested materials that appeared hypo to hyperechoic. The intestinal loop diameter exceeds 8 cm.
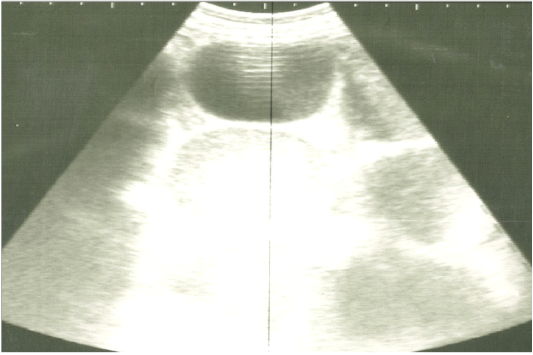
Figure 3: Ultrasonographic image of the left abdomen of horses with colic associated with impaction of the intestine using 3.5 MHz transducer. The intestinal loops were distended with fluid and food particlesand prominent signs of ileus.
Discussion
Equine colic is a challenging for practitioners and for owners, predicting the prognosis of horses with colic reflects the probability of survival for equine colic patients (Furr et al., 1995). There are numerous variables that reflect the health status of a horse with colic including cardiovascular status (heart rate, mucous membrane) and different blood parameters. One of the most important determinants for prognosticating an equine colic patient is the haemostatic profile (Johnstone and Crane, 1986). Haemostatic abnormalities are common in horses with gastrointestinal disease and colic and the severity of abnormalities depends on the cause and the progress of illness (Johnstone and Crane, 1986). A hypercoagulation state is usually developed during the progress of colic in horses and it refers to disseminating intravascular coagulation (DIC) (Cesarini et al., 2010). The hypercoagulation and increased fibrinolysis can be assessed by measuring the concentration of plasma D-dimer, which is very useful in acute gastrointestinal disorders, laminitis and in septic foals (Stokol et al., 2005).
In the present study, horses were categorized according to results of clinical examination, laboratory analyses and ultrasonographic findings. Horses in group I was clinically healthy and showed normal clinical examination and normal laboratory results. The horses with signs of enteritis and/or colitis (group II) were presented with signs of intermittent moderate abdominal pain, loss of appetite, soft feces, straining during defecation (Makinen et al., 2008). Reduced fecal output, abdominal distension, stretching, rolling and on rectal examination an impacted intestinal segment were palpated these signs were compatible with impaction of the intestine (Group III) (Hallowell, 2008).
The gender variability in the study population included 59.5% mares, which indicate that mares are more liable to colic than stallions and geldings, although, Abutarbush et al. (2005) stated that geldings are more predisposed to colic than mares and stallions which was not attributed to known reasons. On the other hand, Tinker et al. (1997) stated that there were no association between gender and incidence of colic.
The heart rate and the respiration rate were significantly increased in horses with colic compared to healthy horses; this was attributed to cardiovascular involvement and endotoxaemia developed during disease progress beside the effort during colic attacks (Hesselkilde et al., 2014).
The hematocrit values were significantly increased in horses with enteritis and/ colitis and in horses with impaction of the intestine compared to clinically healthy horses, this increase in hematocrit value results in increased whole blood viscosity which may indicate severely compromised regional tissue perfusion with subsequent tissue damage (Andrews et al., 1990). In addition, the increase in hematocrit values might result from remarkable dehydration that occurs during the progress of colic (Parry, 1987).
The abdominal ultrasonography was an important method for providing an insight into the colic patients due to enteritis and/or colitis and those with impaction of the intestine. In horses with enteritis and/or colitis, the small intestine were significantly dilated and imaged ultrasonographically with thickened walls and the contents were fluidier. This was in agreement to those results obtained by Cavalleri et al. (2013). The accumulation of dehydrated ingesta in small diameter portions of the intestine can be palpated during rectal examination of a horse with impaction of the intestine (Plummer, 2009; Monreal et al., 2010). The ultrasonographic image of the intestine in horses with impaction of the intestine revealed marked increase in intestinal diameter with increased motility; this was in agreement with results obtained by Sheats et al. (2010).
The D-dimer was considered a useful prognostic marker for horses with colic and it might detect the severity of colic. The plasma D-dimer concentration in the present study was significantly increased in horses with colic associated with enteritis and/or colitis and those with impaction of the intestine which indicates hypercoagulation state and increased fibrinolysis activity as a consequence of increased fibrin formation, this was in agreement with previous studies by Cesarini et al. (2010); Cesarini et al. (2014). The hypercoagulation state might develop to DIC, thromboembolism and ischemia and this worsens the prognosis (Cotovio et al., 2007). In previous studies D-dimer was measured in peritoneal fluid samples of horses with colic, the results showed increased D-dimer concentration in peritoneal fluid in horses with colic (Delgado et al., 2009a). The increase in D-dimer concentration in peritoneal fluid of horses with colic was recorded in a previous studies by Delgado et al. (2009a and b), which is an indicator of an active fibrinolysis where D-dimer as a fibrin degradation products produced by lysis of cross-linked fibrin by the action of plasmin.
Conclusion
It was concluded that plasma D-dimer concentration was higher in horses with colic compared to clinically healthy horses. Higher concentrations of D-dimer are consistent with poor prognosis. In addition, the ultrasonography of the abdomen provides an accurate insight to diagnose horses with colic. Different inflammation markers are necessary to provide a full insight into the enteritis and colitis patients.
Acknowledgements
The authors would like to thank the Faculty of Veterinary Medicine, Zagazig University for providing the facility to carry out the work.
Conflict of interests
We declare there are no conflicts of interest.
Authors Contribution
All authors are equally contributed to develop the manuscript. H. El-Zahar: designing the methodology, standardizing the protocol and drafting the final version of the article. Y. Bayoumi: discussed the results and revised the manuscript. S. Shalaby: discussed the results and revised the manuscript. H. Gehlen: designing the methodology, discussed the results and revised the manuscript. T. Shety: Administration of the whole research work, Collection of the samples, and drafting the article.
References






