Advances in Animal and Veterinary Sciences
Research Article
Pathomorphological and Morphometric Studies of Bovine Liver Infected with Fascioles
Foat Karimov1*, Evgeny Skovorodin1, Valiyn Gimranov1, Aidar Bagautdinov1, Alexey Zhukov2
1Federal State Budgetary Educational Institution of Higher Education Bashkir State Agrarian University, Ufa, 450001, Russian Federation; 2Federal State Budgetary Educational Institution of Higher Education, Orenburg State Agrarian University, Orenburg, 460014, Russian Federation.
Abstract | The paper presents the studies on morphological changes in cattle with a different number of fascioles in liver (up to 70 fascioles and up to 250 specimen). A total of 127 heads of 2-4-year-old cattle from the farms of the Republic of Bashkortostan were examined. The diagnosis of fasciolosis was based on epizootological data, clinical indicators, and detection of fasciola eggs in faeces by successive washes of faeces. After slaughter 3 groups of 5 animals were selected based on pathoanatomic, helminthological diagnosis and fasciola invasion intensity. The first group included parasite-free animals, the second group – cattle with an infestation rate of 70 worms/head, the third group – animals with infestation rate of 250 parasites/head. Histological studies were conducted on liver samples in 10% neutral formalin with embedding them in paraffin blocks. After dewaxing 5-7 micrometres thick sections were stained with hematoxylin and eosin and coloured according to Van Gieson’s method for connective tissue examination. Morphometric indicators were studied with an ocular ruler. The data was processed on the Pentium OS with Microsoft Word and Excel software and the Student’s t-test results validation. The infected bull calves have an enlarged liver size, dense liver texture and tuberous surface, the thickened capsule, thick, sinuous, expanded yellow-white bile ducts cords. Histological and morphometric methods identified structural changes in hepatocytes, hepatic lobules and the microcirculatory system of the liver. Pathological changes in the liver depend significantly on the average number of parasites found on a single infested animal. The work provides a fasciolosis prevention and treatment recommendation, taking into account the manifested pathological liver processes.
Keywords | Fascioles, Bile ducts, Hepatic lobules, Sinusoid capillaries, Liver
Received | July 29, 2020; Accepted | December 03, 2020; Published | January 15, 2021
*Correspondence | Foat Karimov, Department of Morphology, Pathology, Pharmacy and Non-Communicable Diseases, Federal State Budgetary Educational Institution of Higher Education Bashkir State Agrarian University, 50-letia Octyabrya str., 34, Ufa, 450001, Russian Federation; Email: karimov.foat@rambler.ru
Citation | Karimov F, Skovorodin E, Gimranov V, Bagautdinov A, Zhukov A (2021). Pathomorphological and morphometric studies of bovine liver infected with fascioles. Adv. Anim. Vet. Sci. 9(3): 372-378.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.372.378
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Karimov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Helminths are one of the main sources of zoonosis. They require practitioners to have a deep knowledge of the general epizootology of helminthoses as well as new methods and means of combating parasitic worms (Chen et al., 2019).
Many helminthosis, including trematosis, developed parasitic foci with a pronounced territorial affiliation. Large areas of ruminant infestation with helminthosis like fasciolosis worldwide makes it possible to consider these parasitic diseases to be the global environmental and food problems. References prove high incidence of fasciolosis in many European countries, with an infestation rate of more than 50% in some livestock farms (Beesley et al., 2018). The most affected with fasciolosis are Australia (Elliott et al., 2015) Iran (Khademvatan et al., 2019), Turke (Yibar et al., 2015), China (Ai-Ling et al., 2017), Brazil (Molento et al., 2018; Pritsch and Molento, 2018) as well as many countries in Asia and Africa (Belay et al., 2012).
Fasciolosis causes significant economic losses to animal breeding due to reduced milk yields and weight gains, liver utilization, lower specific and non-specific resistance in livestock, higher susceptibility to pathogenic agents, and decreased quality of vaccination (Belay et al., 2012; Mehmood et al., 2017; Molento et al., 2018; Yibar et al., 2015). Fasciolosis is not only a veterinary problem, but also a medical one. Some works quote that the number of people infected with fasciolosis is increasing in the world every year (Al-Mahmood and Al-Sabaawy, 2019; Matsuda et al., 2019). Fascioles mainly parasitize the liver.
The liver is one of the most vital organs that provide data on metabolism. This organ receives nutrients from the gastrointestinal tract (Chernitskiy et al., 2019). It is the very place where the most concentrated and active processes of metabolism occur. The liver synthesizes complex organic substances such as proteins, glycogens, and others. Here non-oxidized and toxic compounds are neutralized, and complex compounds are broken down into simpler ones (Said, 2018).
Fascioles at the stage of maritogony, as co-agents of the functioning parasitic system, live in the liver and gall bladder of ruminants and cause severe, often irreversible pathological changes in organs and tissues. They often result in the death of animals at the stage of acute course of the painful process (Ai-Ling et al., 2017; Balqis et al., 2013).
Fasciolosis is characterized by secondary immunodeficiency states, manifested by the suppression of specific and non-specific resistance of the body. The leading role in the helminthoses pathogenesis belongs to secondary factors responsible for pathological processes in the body, including immunopathological, toxic and allergic reactions that lead to disorders of the neurohumoral and enzymatic systems of the body. All this brings in metabolic disorders and, as a result, a decrease in the productivity of animals (Ruiz-Campillo et al., 2017). Modern biological and veterinary science has accumulated extensive experience in conducting preventive and curative measures against fasciolosis. There are numerous publications on this issue. However, there are few deep, subtle studies of the liver, the main target of fascioles (Vorobyev et al., 2019). This paper aimed to study pathomorphological changes in the cattle liver with different infection rates of fasciolosis. The obtained data in the study of the fasciola effect on the structural unit of the liver, hepatic lobules, their hepatocytes, the microcirculatory bed of the liver and the stroma, will make a significant contribution to clarifying the pathogenesis of animal fasciolosis.
MATERIALS AND METHODS
Characteristics of experimental animals
The spread of fasciola infestation was preliminary studied in livestock farms of the Tatyshlinsky district of the Republic of Bashkortostan. The experimental part of the studies, the development of experimental groups of animals, cattle feeding and housing were guided by the methodological provisions and rules for animal breeding. All experiments were conducted in accordance with the legislation (European Convention for the protection of vertebrates; European Convention for the protection of vertebrates used for experimental and other scientific purposes; guidelines for the care and use of laboratory animals and based on the report of the Committee on ethics in the field of animal research (no.9 of 23.02.2015).
Infected animals were identified based on epizootological data, clinical signs and directly by detecting parasite eggs in animal faeces. A total of 127 heads of cattle were examined. Fasciola eggs were detected by successive washes of faeces (Fulleborn’s method). During the experiments, 3-5 grams of faeces were collected in a glass and suspended with up to 50 ml of water. The resulting liquid solution was sieved through a grid into another glass. After 5 minutes the solution was decanted and refilled with water. These sequential washes were repeated 3-4 times. Then the sediment was put portionwise on the slide and analyzed under a microscope. Post-mortem diagnosis was made based on pathoanatomic changes and detected fascioles in the liver. The presence of fascioles was checked by cutting the liver, pressing on its surface. The contents of the bile ducts were scanned for fascioles. The animals were divided into groups after the slaughter from the results of pathoanatomic studies and the infection rate. The cattle were slaughtered within the technological time after 18-24 hours of fasting. According to the results of post-mortem diagnosis, 15 heads of bulls aged from 2 to 4 years were selected. The animals were divided into three groups of five heads each. The first group of non-invasive animals served as control. The second group consisted of animals with a low infestation rate of up to 70 fascioles in the liver; the third group included highly infected cattle with more than 70 parasites in the liver.
Histological and morphometric studies of the liver
Histological and morphometric studies were conducted on liver samples of control and experimental groups of animals. The collected samples were fixed in 10% neutral formalin. Then they were processed with acetone-xylene, embedded in paraffin blocks and cut into 5-7 micrometres thick sections. Sections after dewaxing were stained with hematoxylin and eosin, the connective tissue was coloured by Van Gieson’s method. Then random microphotos of histological preparations were taken with the OLYMPUS XC30 digital camera based on the OLYMPUS SH41 microscope (Japan) with SWH X10 zoom and the UPLanFL X10, X20, X40 lenses (at least 10 fields of view in each histological section).
Comparative assessment of the hepatic lobule size, the hepatocyte, their nuclei; the diameter of the bile ducts and the thickness of the lining epithelium; as well as elements of the microcirculatory bed were studied by histomorphocytometric measurements using an ocular ruler. The graduation scale mark of the ocular ruler was calculated by a stage micrometer. It was used for periodic standardizing of the ocular ruler.
Statistical analysis
The resulting digital data was processed on the Pentium OS using a package of standard Microsoft Word and Excel software. The results of morphometric studies of hepatic lobules, hepatocytes, their nuclei, and blood vessels (Table 1) were validated using the Student’s t-test.
RESULTS
Liver structure of the control animals
There were no signs of pathology in the liver of non-infected animals. The organ is not enlarged; the edges are sharp. On the section, the structure of the organ is uniform, brown in colour, the pattern of the lobular structure is expressed.
Histological liver samples of the control animals, the lobule parenchyma is represented by hepatocytes, arranged in the form of plates. The cytoplasm has a granular structure. Hepatocytes are densely arranged with clear contours and their cytolemma is well defined. Hepatocyte nuclei, as a rule, occupy a central position, they contain numerous well-marked nucleoli. The stroma is moderately prominent and consists of soft fibers of loose connective tissue (Figure 1A).
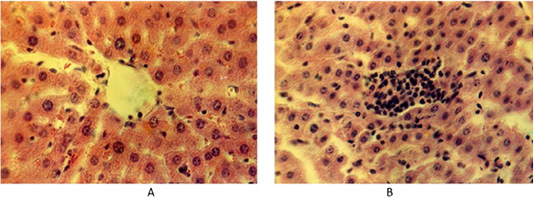
Figure 1: (A) Liver of the control group of animals. Hematoxylin and eosin. Image scale 400:1. (B) Accumulation of lymphoid tissue inside the liver lobules at fasciolosis in the second group of weakly infected animals. Hematoxylin and eosin. Image scale 400:1.
The main morphometric indicators of hepatic lobules, hepatocytes and elements of the microcirculatory bed are presented in Table 1.
The size of hepatocytes in the peripheral areas of the hepatic lobules is small and large in the middle. A high index of the nuclear-cytoplasmic ratio (NCR) was found in peripheral hepatocytes, and a minimum one in central hepatocytes. Sinusoidal capillaries between the lobules are moderately developed with a width of 4.6 µm and a length of 109 µm. There are structural elements of triads in the lobule stroma. The area of the stroma under the triads is within 0.16 µm2. In the bile ducts, the height of the lining epithelium with a border is 3.00±0.025 µm. Cylindrical epithelial cells of the bile ducts are arranged in a single row.
Liver structure of the second group animals (up to 70 fascioles)
In the second group of animals, spontaneously infected with fasciolosis, there were up to 70 parasitic warms in the liver, the liver is slightly enlarged, its color is reddish-brown. The figure of the lobular structure of the liver is shaded. There are whitish cords of various widths and lengths on the visceral surface of the left lobe, the bile ducts are noticeably enlarged with a thickened wall. The liver cut in the parenchyma disclosed many small and medium-sized bile ducts with a reduced lumen and thickened walls from which a greenish liquid with parasites flowed out.
Histological examination of the sick animals’ liver showed that the architectonics of the hepatocyte lobule structure remained unchanged, although the interlobular space expanded. Some hepatocytes have specific changes as dystrophy and necrobiosis in different combinations. There were separate areas of hepatic lobules with a significant destructive process. There are prominent histological changes in blood vessels in the liver. The interlobular and central veins of the liver are infiltrated by white blood cells. There are many lymphoid cells in the area of the liver triad. Lymphoid cells also penetrate along the sinusoid capillaries of the liver lobes. They form small clusters of lymphoid cells inside the liver lobes (Figure 1B).
In this case, the lymphoid cells are located close to each other. At the same time, there are areas with a diffuse location of lymphocytes, macrophages and plasma cells among the liver plates and hepatocytes.
The histological examination showed an enlarged lumen of central veins due to the thickened wall up to 6.6 µm. The lobule structure of the hepatocytes is destroyed and preserved only in certain fields of view. The area of hepatocytes in the central zone of the lobules is noticeably larger than in the control group of animals. The size of the nucleus in liver cells tends to increase. There are numerous hepatocytes without nuclei. The remaining nuclei were located closer to the periphery of the cell. This arrangement of liver cell nuclei is also found in remaining hepatic plates. In such areas, there are hepatocytes with larger nuclei. The peripheral lobules consist of square-oblong hepatocytes. The area of the liver lobules is significantly larger than that of the control group of animals. Around the central vein of the liver lobules, there were assembled round-angular and round-oblong lymphoid cells with a compact nucleus or nucleoli on the area of up to 667 µm2 (Figure 2).
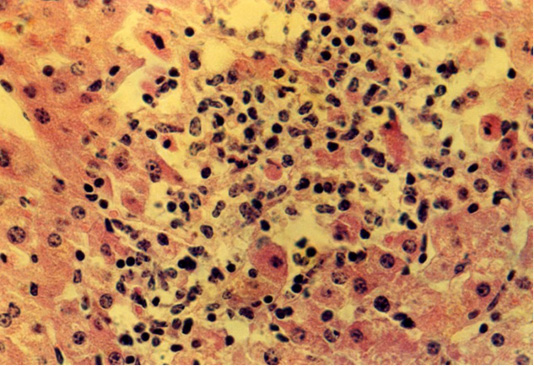
Figure 2: Isolated location of hepatocytes amid lymphocytes and macrophages at fasciolosis of the second group animals. Hematoxylin and eosin. Image scale 400:1.
Liver structure of the second group animals (up to 250 fascioles)
The third group of animals had up to 250 fascioles, their liver was significantly enlarged, the liver borders were blunt, rounded and tuberous. The liver texture was very dense. Its surface is unevenly colored from light to yellowish-brown. There were many thin bluish-gray cords with growing connective tissue on the Glisson’s capsule and under it, making an irregular grid pattern. In some areas, they form foci of continuous thickening of the capsule, in the form of limited white spots. There are thick, sinuous, expanded yellow-white cords of bile ducts with a diameter of up to 2-3 cm at the cut and outside. The liver has a smooth lobular structure, distended bile ducts are filled with mature fascioles in the amount of several tens to 250 specimens with a semi-liquid greenish-brown mass. Bile ducts have a dense texture with a large diameter with the wall thickness reaching up to 5 mm. Around them, there was an expanded connective tissue in the form of grayish cords (Figure 3A).
Histological studies of the liver in highly infected animals have shown that the plate structure of hepatocytes is broken. Due to a decrease in the amount of glycogen and the accumulation of fat inclusions in hepatocytes, there were nodes of necrobiotic hepatocytes among healthy liver tissue in the liver parenchyma. These small foci of parenchymal necrosis with the destructed argyrophilic stroma had focal infiltrates from macrophages, lymphocytes, and neutrophils. There were prominent proliferation and hypertrophy of Kupffer’s cells. Separate foci of the remaining plate structure of hepatocytes had destructed morphological structures. In such areas, hepatocytes are large in size with large nuclei. Individual hepatocytes were almost round, square, and square-oval in shape. The nuclei have a compact chromatin structure. The stroma occupies a large area, being up to 10 times bigger the size of the stroma in the liver of the control and second groups of animals. In the stroma, the interlobular capillaries are expanded, full-blooded, and the blood flow is slowed down (Figure 3B).
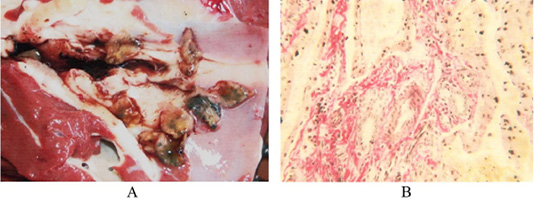
Figure 3: (A) Liver of the third cattle group. Bile ducts with thickened walls and live fascioles, Original. (B) Liver of the third cattle group. An enlarged stroma with dilated capillaries filled with blood. Van Gieson. Image scale 400:1.
Full-blooded sinusoid capillaries expand the space between the liver plates. The arteries’ wall is thickened due to the distended connective tissue. There are clusters of cellular elements with dense nuclei in the hepatic lobules along the central veins, the sinusoids of capillaries and in the triad zone. They had the form of strands 68 x 480 µm or nodules 16,8x34,6 - 178.2 x 190.3 µm (Figure 4).
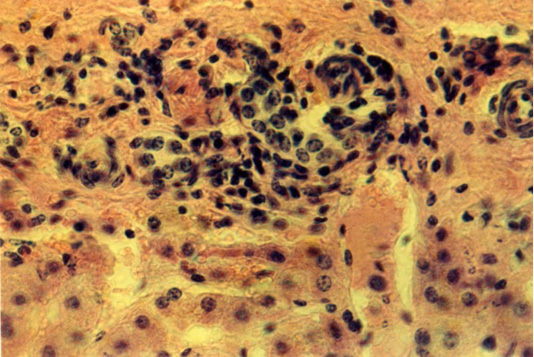
Figure 4: Liver of the third animals group. The lymphoid cells around the triads of the liver. Hematoxylin and eosin. Image scale 400:1.
As a result of hyperplasia of the glandular epithelium, the mucous membrane of the bile ducts is thickened. The bile ducts were star-shaped with folds of epithelium extending into the lumen of the ducts. The results of morphometric research (Table 1) indicate that in animals of the third group, larger size of liver lobules are caused by an increased volume of hepatocyte cytoplasm due to fat infiltration and decomposition of intracellular structures. At the same time, the nuclear volume does not enlarge, and the nuclear-cytoplasmic ratio decreases sharply. This is a sign of a significant decrease in the synthetic activity of hepatocytes. The cause of dystrophic changes in hepatocytes are circulatory disorders and congestion in the bile ducts. This is accompanied by pronounced changes in the liver structure, which are summarized in Table 2.
DISCUSSION
A special feature of our work was a detailed morphological study of the bovine liver, taking into account the intensity of fascioles invasion. The conducted studies involved not only macroscopic and histological examination of the liver in sick animals but also the morphometry of its structural elements. This made it possible to clarify the pathogenesis of this widespread disease.
Helminths, infesting the bovine body, have a harmful effect as toxic substances released in the course of their life (Postevoy et al., 2015) and mechanical influence, injuring the organs of animals. They cause deep path anatomic changes mainly in the liver. During the migration of fasciola larvae from the intestine to the liver, they cause primary changes in the small intestine and, especially, in the duodenum. This translates into desquamation of epithelial cells, proliferation, and catarrhal inflammation of the intestine (Molento et al., 2018).
The conducted studies have shown that severe path morphological changes in the liver are directly related to the intensity of parasite infestation.
The liver of animals with a low infestation rate has rounded edges, which indicates the organ distension, a dense texture, a reddish-brown color. There are prominent small and medium-sized bile ducts with thickened walls and reduced lumen at the liver cut. When pressed, there was a greenish liquid with fascioles 15-25 mm long and up to 8 mm wide.
Highly infested animals with 250 fascioles in the liver had deeper changes in the organ. This is due to toxins released by fascioles, which lead to inflammatory processes complicated by fibrous changes in the stroma and hepatic vessels (Table 2).
Table 1: Morphometric indicators of the main vascular and parenchymal structural components of the liver parenchyma in experimental animals.
| No. | Indicators | Group 1 | Group 2 | Group 3 |
| 1 |
The area of hepatic lobules, mm2 |
2.29± 0.07 | 2.46± 0.02* | 2.91± 0.04** |
| 2 |
The area of the hepatocyte, µm2 |
409.45 ±9.81 | 421.98±29.5 | 837.76±32.3** |
| 3 |
The area of the hepatocyte nucleus, µm2 |
62.29 ±2.05 | 67.52 ±7.8 | 74.70 ±3.1* |
| 4 |
The area of the hepatocyte cytoplasm, µm2 |
347.16 ±7.87 | 354.46 ±21.7 | 763.06 ±54.7** |
| 5 | Nuclear-plasma ratio | 0.179± 0.07 | 0.190± 0.09 | 0.097± 0.08** |
| 6 | Diameter of the interlobular vein cross-section, µm | 107.16 ±5.62 | 121.05 ±5.03* | 140.34 ±7.51* |
| 7 | The diameter of the central vein cross-section, µm | 92.0 ±5.05 | 99.79 ±6.05 | 128.43 ±3.16** |
| 8 | The diameter of the interlobular bile ducts, µm | 24.18 ±2.98 | 32.29 ±2.86* | 43.53 ±4.25** |
P < 0.05; *Reliable for the first group; ** Reliable for the first and second groups.
Table 2: Pathomorphological changes in hepatocytes, stroma, blood vessels and bile ducts of the liver.
| No. | Indicators | Group 1 | Group 2 | Group 3 |
| 1 | Granular hepatocyte dystrophy | + | + | + |
| 2 | Hepatocytes fatty dystrophy | - | - | + |
| 3 | Acute venous hyperemia of the liver | - | + | + |
| 4 | Chronic venous hyperemia, liver induration | - | - | ++ |
| 5 | Hyperplasia of the bile duct epithelium | - | + | ++ |
| 6 | Sclerosis of blood vessel walls | - | + | ++ |
| 7 | Fibrinoid swelling of the liver stroma | - | + | ++ |
| 8 | Biliary cirrhosis | - | - | ++ |
-: no changes; +: changes are weakly expressed; ++: changes are expressed.
The liver is greatly enlarged; the texture is very dense. The surface is tuberous, unevenly colored from light to yellowish-brown, there are thick, sinuous cords of bile ducts. The liver capsule is noticeably thickened, there are foci of continuous thickening of the capsule in the form of limited white spots. At the cut, the bile ducts have a dense textuure, some of them are calcified with wall thickness up to 5 mm. The bile ducts are filled with live mature fascioles with a greenish-brown mass.
Histological examination of the liver in weakly infected animals revealed a broken plate structure of the lobules. In some areas of the hepatic lobules there are destructed hepatocytes and necrobiosis in the form of small foci. In these areas, hepatocytes located closer to the centre of the hepatic lobules are completely destroyed, and hepatocytes with destructive signs are located at the periphery. There is also an accumulation of lymphoid cells. Morphometric indicators of the hepatocyte size do not differ much from healthy animals with a slight distension of the lobule areas. There is an expansion of the interstitial sinusoid capillaries and the lumen of the central veins. Expanded sinusoid lumen contains nodules of lymphoid-histiocytic cell type.
A microscopic view at a high infestation rate is characterized by the fact that the destroyed foci of the plate structure of hepatocytes prevail over common ones. Hepatocytes lose their usual morphological structure. They are larger with large nuclei compared to the first and second groups of animals. The overgrown stroma occupies an area several times larger than the size of the liver stroma of the first group of animals. The artery wall is thick due to the growth of connective tissue. Bile ducts with fascioles are large in diameter, the duct walls are dense and reach up to 2-5 mm. The mucous membrane of the bile ducts is thickened as a result of hyperplasia of the glandular epithelium and stroma cells. There are large foci of biliary parasitic cirrhosis with cellular elements in the liver parenchyma.
Thus, the mechanical action of parasites, Fasciola hepatica toxins, as well as decay products of the stagnant bile causes significant pathomorphological changes in the wall of the bile passages and the liver tissue. In the initial stage of infection, young migrating fascioles injure the liver tissue and blood capillaries, resulting in inflammation of the liver. In the future, as intoxication increases, morphological changes in the liver are characterized by a broken plate structure of the liver, intralobular alterative manifestations, with the necrosis of individual hepatocytes. In necrotic areas, there is a small accumulation of macrophages, lymphocytes, neutrophils, hypertrophied Kupffer’s cells. There are oedema and expansion of portal tracts infiltrated with their lymphohistiocytic elements and neutrophils. The interlobular and central veins of the liver are infiltrated by white blood cells. Lymphoid cells also penetrate along the sinusoid capillaries of the liver lobes. They form small clusters of lymphoid cells inside the liver lobules (Figure 1B). Matsuda et al. (2019) believe that the formation of lymphoid tissue is the result of permanent immune responses against dead fascioles. At this stage, the degree of microscopic lesions is simple or minor. According to Al-Mahmood and Al-Sabaawy (2019) with timely treatment, the prognosis is favorable with a good reversible process.
At high infestation rates, when a large number of fascioles are parasitized in the liver, pathomorphological changes are aggravated. Enlarged fascioles move from the liver tissue to the bile ducts and clog them. Skovorodin et al. (2019) claim that the body’s intoxication is enhanced by the decomposition of inspissated bile. At this stage of the disease, quite large foci of parenchymal necrosis appear in the hepatic lobules, mainly in the periportal and central zones with the destruction of the argyrophil stroma and focal infiltrates from macrophages, lymphocytes, and neutrophils. There is a proliferation of Kupffer’s cells. The central veins and adjacent sinusoid capillaries were enlarged, filled with red blood cells, and there were frequent foci of diapedesis hemorrhages. Portal tracts are enlarged, edematous, and moderately infiltrated by lymphohistiocytic elements. A histological examination found extended and bile-overfilled bile capillaries and ducts. The thickened wall of the bile ducts occurs is due to the growth of fibrous connective tissue. The mucosa is characterized by cellular infiltration mainly from histiocytes and lymphoid cells. There is an overgrowth of interlobular connective tissue, as well as around the bile ducts. In this stage of liver damage, fibrotic lesions were more intense than inflammatory ones and the prognosis was unfavourable, which is consistent with the literature data (Al-Mahmood and Al-Sabaawy, 2019; Trivilin et al., 2014; Safonov, 2020).
CONCLUSION
Analysis of macroscopic, histological and cytometric liver parameters makes it possible to claim that at fasciolosis there is chronic catarrh of the bile ducts, interstitial hepatitis and, finally, biliary cirrhosis of the liver, resulting from the simultaneous action of pathogenic factors.
The conducted research provides additional information to the main problems in the study of fasciolosis (the need for early diagnosis and treatment of this disease) and clarifies the pathogenesis of this ruminant disease.
The obtained data make it possible to recommend to livestock breeders to carry out preventive and treatment measures against fasciolosis earlier before irreversible pathological changes in the liver. It requires systemic testing of animals for fasciole eggs and deworming with effective and low-toxic drugs. After deworming, it is necessary to conduct pathogenetic therapy aimed at detecting the existing liver pathology with the degree of animals’ invasion taken into account. In the system of diagnostic measures, it is necessary to provide for selective slaughter of diseased animals.
Acknowledgements
Not applicable.
Authors Contribution
F.K. proposed the idea, planned the research, conducted a coprological study and wrote the final version of the article, E. S. researched the liver of animals after slaughter, V. G. conducted a clinical study of animals, A. B. and A. Z. performed histological, morpho-metric and statistical analysis.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






