Research Journal for Veterinary Practitioners
Research Article
Comparative Analysis of Two Serodiagnostic Assays for Detection of Antibodies Against Peste- des- Petits Ruminants (PPR) from Non-Vaccinated Sheep and Goats
Tahira Hanif1*, Jawaria Ali Khan2, Aamer Bin Zahur3, Asma Latif3, Aman Ullah3, Muhammad Avais2, Aftab Ahmad Anjum2, Nabeela Haneef4, Muhammad Abdul Rauf Malik2
1Department of Clinical Medicine and Surgery, University of Veterinary and Animal sciences, Lahore, Pakistan; 2University of Veterinary and Animal Sciences, Lahore, Pakistan; 3Animal Health Laboratories, Animal Sciences Institute, NARC, Islamabad, Pakistan, 4PMAS ARID Agriculture University Rawalpindi, Pakistan.
Abstract | Peste des Petits ruminants (PPR) is an acute, extremely fatal and frequently transmissible viral disease of small ruminants. Passive haemagglutination (PHA) and cELISA were conducted for detection of PPR from sera samples at NARC Islamabad. A total number of sera samples n=597 were collected from non-vaccinated goats and sheep. In this study, the results of passive haemagglutination (PHA) were compared against competitive ELISA, as cELISA was considered as gold standard. So, 354 (59.1%) of sera samples were found positive by cELISA and 331(55.3%) were found positive by PHA. However, both tests declared, 292 (48.8%) of sera samples were positive for PPRV antibodies. Kappa value (k=0.654) indicated perfect strong correlation between PHA and cELISA. It was observed that, the sensitivity and specificity of PHA was 82.4% and 84% respectively. In this study, we found that PHA is an economical and rapid serological assay for detection of PPRV antibodies.
Keywords | PHA, sensitivity, specificity, cELISA, Kappa statistics
Received | October 10, 2019; Accepted | December 23, 2019; Published | December 30, 2019
*Correspondence | Tahira Hanif, Department of Clinical Medicine and Surgery, University of Veterinary and Animal sciences, Lahore, Pakistan; Email: dr.tahirahanif@gmail.com
Citation | Hanif T, Khan JA, Zahur AB, Latif A, Ullah A, Avais M, Anjum AA, Haneef N, Malik MAR (2019). Comparative analysis of two serodiagnostic assays for detection of antibodies against peste- des- petits ruminants (ppr) from non-vaccinated sheep and goats. Res J. Vet. Pract. 7(4): 83-87.
DOI | http://dx.doi.org/10.17582/journal.rjvp2019/7.4.83.87
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Hanif et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peste des petits ruminants (PPR) is an extremely transmissible and economically important disease of small ruminants caused by PPR virus that belongs to genus Morbillivirus and family Paramyxoviridae. Buffalo and cattle are sub clinically infected, a huge number of wild species within the order Artiodactyla also infected by PPR virus (Parida et al., 2015). PPR is endemic in large areas of Asia, the Middle East and Africa, and it is still spreading globally, with emergence particularly reported in Mongolia, China, Georgia and most recently within European union in Bulgaria (Parida et al., 2015; Altan et al., 2019).
Serologically PPRV consists of a single serotype, genetically it is divided into four distinct lineages (I-IV) (Kumar et al., 2014). The main hosts of PPR are goats and sheep, though disease in wild small ruminants and camel has also been investigated (Abubakar et al., 2011; Aguilar et al., 2018; Mahapatra et al., 2015; Khalafalla et al., 2010). PPR virus transmission between animals such as sheep and goats can occur through aerosols, inhalation and direct contact with contaminated water, feed troughs, nasal and ocular discharges and faces. (Zhur et al., 2009).
PPR has been detected in Pakistan since 1991, previously on the basis of clinical signs and symptoms (Athar et al., 1995; Amjad et al., 1996) and later by using the advanced diagnostic methods like polymerase Chain Reaction (PCR) and Enzyme Linked Immuno-Sorbent Assay (ELISA) (Khan et al., 2007; Abubakar et al., 2008; Munir et al., 2012). However definitive diagnosis always relies on laboratory confirmation.
Sheep and goats are mostly reared in remote and rural areas of Pakistan and have a major role to cope up with meat and wool need of the country. Unfortunately, PPR is a fast spreading, lethal disease of mainly sheep and goats leading to the death of millions of domestic small ruminants. If the disease remains incurable it results in greater economic loses of poor farmers. Earlier possible diagnosis of PPR is crucial in implementing control measures.
PPRV and measles virus (MV) are unique among morbilliviruses that contain haemagglutination activity and tissue homogenate from PPR affected animal could agglutinate piglet red blood cells. (Wosu, 1985). So, the aim of this study is serological detection of PPR virus antibodies and to evaluate sensitivity and specificity of passive hemagglutination (PHA) comparative to the OIE recommended competitive enzyme linked immunosorbent assay (cELISA) (Libeau et al., 1995).
MATERIAL AND METHODS
This study was conducted at the Animal Health Research Laboratories at National Agriculture Research Center (NARC) Islamabad, Pakistan during September 2018 to June 2019.
Sample Collection
A total number of sera samples 597 were collected including sheep (n=247) and goats (n=350) from six villages of Mithi. These samples were donated by national veterinary laboratories, National agriculture research center Islamabad. These sera samples were collected randomly from animals showed symptoms, erosion in mouth, nasal and ocular discharges, foul-smelling diarrhea suggestive of PPR disease as well as from apparently healthy animals. Number of samples (597) were selected to validate the results of passive haemagglution (PHA). Samples were collected from non-vaccinated animals of all ages.
Sample Processing
The blood was collected from jugular vein of animals. Then the blood was allowed to clot in cold boxes with ice packets. These samples were centrifuged and transferred into sterilized tubes that were placed on ice packets while transferring to the National Agricultural Research Center (NARC) Islamabad. Then these sera samples were kept at -20ºC in sterilized 2ml cryovials till further analysis.
Competitive ELISA
The competitive ELISA kit was used manufactured by Innovative Diagnostics (ID. vet), CIRAD, France, for the detection of PPRV antibodies This kit was used according to the manufacturer’s instructions, provided with the kit. The ELISA micro -plate was read with ELISA reader at 450nm filter (Libeau et al., 1995).
Passive Haemagglutination (PHA) Test
This test was conducted to determine the antibodies against PPRV from serum samples according to the method designed by (Ishag et al., 2014).
Preparation of Sensitized Chicken Red Blood Cells (RBCs)
Briefly sensitization of chicken RBCs was done as described by (Ishag et al., 2014) with some modification. A mixture was prepared by using an equal volume of 3% formalin solution and 10% RBCs suspension. This prepared mixture was incubated overnight at 37°c after that the RBCs centrifuged for 15 minutes at 100g and washed 3 times with PBS to remove formalin. Then 5% RBCs suspension was prepared in PBS. An equal volume of 5% RBCs suspension was added to freshly prepared tannic acid at a concentration of 1; 20,000 prepared in PBS and this mixture was incubated for half an hour at 37°c. Tanned formalized RBCs were washed two times with PBS and treated directly by adding PPR antigen, incubated at room temperature for one hour and washed twice with PBS and again washed twice with PBS having 1% inactivated horse serum. In PBS 1% coated tanned RBCs were resuspended, stored at 4°c until used.
Procedure
Briefly, two-fold serial dilution of sera samples were made in 30µl PBS with PH 6.9 in U bottomed micro titer plate, then suspension of 30µl of 1% sensitized RBCs were added. The plate was agitated to mix the reactants, incubated at 4°c and readings was taken after 4 hours. The formation of thin uniform layer of erythrocytes on bottom of the wells considered as positive agglutination pattern. However, the formation of tear or round button of erythrocytes considered as negative pattern.
Statistical Analysis
The overall agreement between the results of PHA and cELISA was determined using Kappa statistics (Dohoo, 2009). Receiver operating characteristic (ROC) curve of sensitivity was plotted against 1-specificity and area under curve was calculated by STATA 11.2 software. (Thrusfield, 2005).
RESULTS
Out of the results of 597 tested sera samples, both tests agreed on the status of 497 samples; 292 were positive by both tests and 204 were negative by both tests. However, both tests showed a strong agreement; of 331 samples that
Table 1: Comparative sensitivity and specificity of passive haemagglutination and competitive ELISA
| c-ELISA | Total | ||
| Positive |
Negative |
||
| PHA | 292 | 39 |
331 |
| 62 | 204 |
266 |
|
| 354 | 243 |
597 |
were positive by PHA, while 292 were positive by cELISA. Similarly, PHA declared 266 samples as negative; of these 266, only 204 were negative by cELISA. (Table 1). The overall agreement between c-ELISA and PHA was 83.08% (292+204/597) (Table 2). However, it was observed that the sensitivity and specificity of PHA was 82.4%(292/354) and 84%(204/243) respectively. Kappa value indicated (k=0.654) perfect strong correlation between PHA and cELISA. These findings suggest a strong sensitivity and specificity of PHA for detection of PPR disease as is evident by the area (0.9716) under ROC curve (Figure 1).
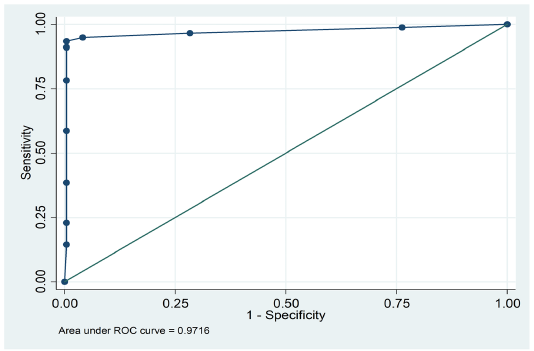
Figure 1: ROC curve displaying sensitivity and specificity of passive haemagglutination (PHA) and competitive ELISA
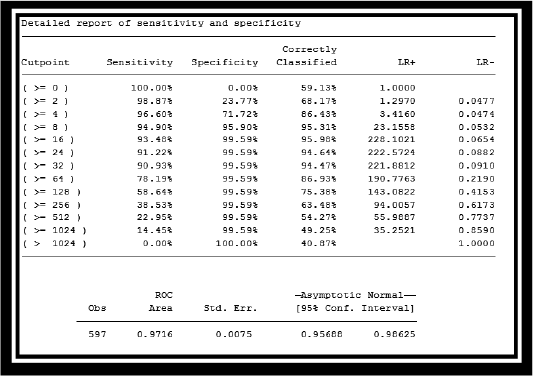
Table 2: Report of sensitivity and specificity of Passive haemagglutination (PHA) and cELISA DISCUSSION
PPR is the major disease of small ruminants that has great economic impact on poor farmers and their livelihoods (Zahur et al., 2011). Numerous molecular and serological laboratory tests are in use for the diagnosis of PPRV, as immunocapture enzyme-linked immunosorbent assay (Ic-ELISA), competitive enzyme-linked immunosorbent assay(cELISA), agar gel immunodiffusion (AGID), isolation on cell culture, polymerase chain reaction (PCR) and haemagglutination inhibition (Khan et al., 2007). Some of these tests are expensive and they cannot perform in less equipped laboratories of rural areas. Haemagglutination (HA) is an inexpensive and simple test and has been used for the detection of PPR (Ezeibe et al., 2004; Wosu, 1985).
PPR diagnosis can be made in live animals using haemagglutination (HA) assay. (Wosu et al., 1991). PPR virus can cause agglutination with red blood cells(RBCs) of some animal species (Ramachandram et al.,1993). In present study, simple haemagglutination (HA) test was modified to passive haemagglutination. (PHA) test for the diagnosis of PPRV from sera samples of goats and sheep.
In this study, erythrocytes were modified by mild treatment with tannic acid and that were used to adsorb soluble antigen on their surface, and then RBCs agglutinate in the presence of antiserum specific for the adsorbed antigen. Collected Sera samples of those sheep and goats were tested that had no history of vaccination prior to sampling or at the time of sampling. These sera samples were inactivated at 56°c for half an hour before PPR detection using PHA. In PHA test, different conditions including PH of the buffer, sensitivity of RBCs of (chicken, sheep and goat), tannic acid concentrations, incubation time and incubation temperature of PHA plates, affecting the sensitivity of the test were monitored.
However, erythrocytes of different animal’s species were used to standardizes the PHA test. PPR virus antigen was coupled to chicken, sheep and goat RBCs in this test. Chicken and sheep RBCs gave the best and comparable results. Chicken RBCs are economical for use in PHA. After collection of blood in Alsever,s solution both fresh and stored erythrocytes were used but the erythrocytes that were stored for at least 3 days at the refrigerator temperature gave excellent results. PH of phosphate buffer saline (PBS) was maintained between 6.8-7.0 and excellent results were found at PH 6.9. In this technique adsorption of antigen to erythrocytes by covalent coupling compound such as tannic acid. But, these compounds often disrupt the cells before antigens can be attached. Therefore, stabilizing reagent formaldehyde was used before sensitization with a coupling compound. Tannic acid was prepared with concentration of 1:10,000,1: 20,000,1: 50,000) to treat erythrocytes as a coupling agent but the best results were found at 1: 20,000, this result was in agreement with the results of (Ishag et al., 2014). It was investigated that many factors that play a role in the coupling reaction with tannic acid, the most significant are tannic acid concentration, antigen concentration, PH level. The reaction temperature and time have little effect on the activity.
The results of passive haemagglutination (PHA) were compared with competitive ELISA, as cELISA was considered as gold standard in this study. Thus, it was found that the sensitivity and specificity of PHA is 82.4% and 84% respectively, these results were found comparable with previous results of (Ishag et al., 2014).
So, it was concluded that passive haemagglutination (PHA) and competitive ELISA are able to detect PPRV antibodies from sera samples. competitive ELISA is a rapid, sensitive and specific assay to detect PPRV antibodies in sera samples, but it is very expensive and not available at small laboratories of Pakistan. PHA is an economical and easy to perform and provide results within a few hours that does not need expensive kits like cELISA. The result of this study suggests that passive hemagglutination can be used as a suitable diagnostic test for detection of PPR disease.
ACKNOWLEDGEMENTS
This work was supported by a project entitled “Development of Indigenous Diagnostic Assay for the Detection of PPR” funded by “Research for Agricultural Development Project” (RADP).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Authors contribution
All the authors contribute equally.
REFERENCES






