Research Journal for Veterinary Practitioners
Research Article
Seroprevalence of Camel Brucellosis and Molecular Characterization of Brucella melitensis Recovered from Dromedary Camels in Egypt
Hosein Hosein Ibrahim1, Sherin Rouby1*, Ahmed Menshawy1, Nabila Ghazy2
1Department of Veterinary Medicine, College of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Veterinary Serum and Vaccines Research Institute (VSVR), Abbassia, Egypt.
Abstract | The present study was delineated for investigation of the seroprevalence of brucellosis in camels and characterization of the causative Brucellae affecting camels in Egypt on bacteriological and molecular basis. A serological study using 1126 blood samples collected from Dromedary camels was carried out. The modified Rose Bengal Plate Test (mRBPT) and competitive ELISA (cELISA) were used as screening and confirmatory tests, respectively. The overall sero-prevalence of Brucella antibodies was 4.17% and 3.73% as detected by the mRBPT and c-ELISA respectively. Lymph nodes, testicular tissues, udder tissues and milk from seropositive positive camels were used for isolation of Brucella organisms. Brucella melitensis biovar 3 was isolated from milk of two she- camels. The bacteriological findings suggested that camels were infected from cattle or sheep and goats where Brucella melitensis biovar 3 is prevalent among large and small ruminants in Egypt. The mRBPT was suitable for screening camel sera for brucellosis, but the cELISA was more specific. It is likely that the tendency to raise large flocks of sheep along with the camels in Egypt contributed towards Brucella melitensis infection among camels. DNA extracts of two Brucella melitensis positive milk samples were tested using conventional and Bruce ladder multiplex PCR. Three fragments of 587 bp, 1071 bp and 1682 bp sizes were amplified using multiplex Bruce ladder PCR confirming the bacteriological findings.
Keywords | Brucellosis, Camel, ELISA, PCR, RBPT
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | April 10, 2016; Accepted | April 17, 2016; Published | April 20, 2016
*Correspondence | Sherin Rouby, Department of Veterinary Medicine, College of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt; Email: sh_alrouby@yahoo.com
Citation | Hosein HI, Rouby S, Menshawy A, Ghazy N (2016). Seroprevalence of camel brucellosis and molecular characterization of Brucella melitensis recovered from dromedary camels in Egypt. Res. J. Vet. Pract. 4(1): 17-24.
DOI | http://dx.doi.org/10.14737/journal.rjvp/2016/4.1.17.24
ISSN | 2308-2798
Copyright © 2016 Hosein et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The camel (Camelus dromedarius, one-humped camel) plays an important socio-economic role within the pastoral and agricultural system in dry and semi dry zones of Asia and Africa (Gwida et al., 2011).
Brucellosis is considered by the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and Office International des Épizootics (OIE) as one of the most widespread diseases in the world (Schelling et al., 2003).
During the last few years, camel brucellosis has been a subject for many researches in many countries of the world especially those rearing racing camels such as the Arabian Gulf countries as well as other countries where camels constitute an important part of their livestock in many African and Asian countries (Yasmin and Remya, 2011). However, in many countries of the world, there are no sufficient data about the real status of camel brucellosis due to lack of scientific research conducted on such animal species. Globally, this disease is under-reported because of its vague clinical symptoms, difficult laboratory diagnosis and lack of familiarity of the medical professionals (WHO, FAO report, 2006).
Brucellosis of domestic animals including camels causes considerable economic losses due to abortion and infertility (Ocholi et al., 2005). In addition the disease can generally cause significant loss of productivity through late first calving age, long calving interval time, low herd fertility and comparatively low milk production in camels (Gessese et al., 2014). Moreover, brucellosis causes a serious illness in man especially that contact with infected animals and those consume infected animal products and are considered as one of the great public health problem all over the world (Marei et al., 2011; Sayour et al., 2015; Shimol et al., 2012). During investigations conducted by Radwan et al. (1995) it was found that brucellosis was diagnosed in 30% of the camel handlers and milkers and the same Brucella melitensis biovars were cultured from aborted sheep and goats sharing the same premises. Therefore the intense alertness which is directed nowadays toward brucellosis all over the world can be justified by the economic impact of the disease, the ease with which the disease transmits among animal population, the substantial difficulties associated with its control and finally the public health significance of the disease.
Cross transmission can occur between cattle, sheep, goats, camels and other species (Ghanem et al., 2009). Camels are not known to be primary hosts of Brucella organisms, but they are susceptible to both Brucella abortus and Brucella melitensis (Abbas and Agab, 2002; Cooper, 1991; Gwida et al., 2012; Seifert, 1996). In pregnant camels, the bacteria localize in the placenta and are most abundant in abortion material (up to 1013) including the fetal stomach, vaginal discharge and colostrum (Millar and Stack, 2012). Therefore camels may constitute an important source of infection not only to susceptible camels but also to other animal species including cattle, sheep and goats especially when they are reared in contact with each other’s. Consequently, infection rate in camels depends upon the infection rate in primary hosts in contact with them.
Brucella infected animals are usually kept for breeding despite the fact that congenital infection is a major epidemiological mean of spread of the disease and it is well known that as high as 20% of calves born by infected heifers could be found persistently infected with Brucellae (Nielsen and Duncan, 1990).
Camel brucellosis has been reported in Egypt for the first time by Ahmed (1939) and since that time the prevalence of the disease continued to appear with fluctuation which may be attributed to the fact that a large number of camels are continuously introduced to Egypt from many African countries. Camels may be also infected due to rearing of camels in close association with cattle and sheep especially in villages.
Therefore, the present work aimed at determining the prevalence of brucellosis in camels in Egypt and characterization of the causative Brucellae on bacteriological and molecular basis.
Materials and methods
Study Areas
The study was conducted during 2014-2015 in different localities including Upper and Lower Egypt (Figure 1).
Animals
A total of 1126 apparently healthy dromedary camels of 3 to 8 years old were employed in this study. These animals included 956 males and 170 females from different localities. All camels were clinically normal at the time of sampling and according to the owners; none had previously shown clinical signs of brucellosis.
Blood Sera
About 10 ml of blood was drawn from a jugular vein of each apparently healthy animal using a vacutanier tube. Tubes were incubated over night at 4ºC and serum was then separated by centrifugation. The collected sera were labelled and stored at -20ºC until used.
Samples for Brucella Isolation
Lymph nodes, testicular tissues of 128 seropositive male camels and udder tissues and milk of 26 seropositive she-camels were collected from slaughtered camels at abattoirs for isolation of Brucella organisms. Tissues were homogenized with a sterilized pestle and mortar using sterilized sand and phosphate buffered saline (PBS). Milk samples were centrifuged at 3,000 revolutions per minute (rpm) for 5 min to obtain sediment and cream and in order to concentrate the bacteria.
Serological Examination
All sera were screened by Rose Bengal plate test (RBPT) for the presence of Brucella agglutinins. RBPT was used in a modified test (mRBPT) with 25μL antigen (Veterinary Sera and Vaccines Research Institute, Abbasia, Cairo, Egypt) and 75μL serum as described by Blasco et al. (1994). Results were considered positive when there was any degree of visible agglutination. The positive samples with RBPT were further confirmed by the competitive enzyme linked immunosorbent assay (c-ELISA), (CVL, New Haw, Weybridge, surrey KT15 3NB UK) according to the manufactory instruction. The cut-off for determining sero-positivity was calculated as ≥60% of the mean of the Optical Density (OD) of the four conjugates control wells and antibody titers were calculated as binding ratio using the formula defined by the c-ELISA kit:
Banding ratio = Mean of six negative control wells/ Mean of six positive control wells
Brucella Strains
Reference brucella strains employed in this study were supplied by the Central Veterinary Laboratory, Weybridge, Surrey KT15 3NB, UK (Table 1).
Table 1: Lyophilized reference Brucella strains
|
Species |
Biovar |
Strain |
ATCC1 |
NCTC2 |
|
Brucella melitensis |
1 |
16M |
23456 |
10094 |
|
Rev.1 |
||||
|
3 |
Ether |
23458 |
10509 |
|
|
Brucella abortus |
1 |
544 |
23448 |
10093 |
|
S19 |
||||
|
RB51 |
||||
|
Brucella suis |
1 |
1330 |
23444 |
10316 |
ATCC1: American Type Culture Collection, USA; NCTC2: National Collection of Type Cultures, UK
Brucella melitensis biovar 3 field isolate was previously isolated and identified from cattle (collection culture of department of Veterinary Medicine Beni Suef University).
Bacteriological Examination
Tissue homogenates and milk cream and sediment mixture were cultured on tryptose agar medium with antibiotics selective antibiotic supplement of Ewalt et al. (1983), (Oxoid, Hampshire, UK) according to Alton et al. (1988). Plates were incubated at 37°C in an atmosphere of 10% CO2 and examined daily for 10 days for growth. Milk samples were also emulsified in PBS and used for inoculation of Guinea pigs (one ml suspension intramuscularly). The Guinea pigs were euthanized after 28 to 35 days and their sera were examined for Brucella antibodies using RBPT. Spleens from serologically positive Guinea pigs were removed, macerated by sterile forceps, cultured on tryptose agar media, and examined in the same way as the primary cultures. Isolates were identified as Brucella according to the method of Alton et al. (1988), Ewalt et al. (2001) and OIE Terrestrial Manual (2012).
PCR Testing
DNA extracts of two Brucella positive milk samples were examined by conventional PCR according to Bricker (2002) using a universal primer (Biosearch Technologies, South McDowell Boulevard, Petaluma, USA) for amplification of target gene (Immunodominant antigen, gene bp26), (Table 2). In addition, INgene Bruce ladder (INgene Bruce ladder V®: Batch No 180515 , Ingenasa, Madrid, Spain) according to Garcia-Yoldi et al. (2006) was used employing five primers pairs (Table 3), designed on the strain-specific genetic differences. It was used in multiplex PCR for molecular typing of different Brucella species on species level. The PCR amplification was carried out using Labnet® Multigen Gradient thermal cycler, Catalog TC9600-G- 230V, Labnet international, Inc.
Table 2: Primer sets for conventional PCR
|
Primer |
Sequence (5'–3') |
Amplicon size (bp) |
|
BMEI0535f BMEI0535r |
GCG-CAT-TCT-TCG-GTT-ATG-AA CGC-AGG-CGA-AAA-CAG-CTA-TAA |
450 |
Table 3: Primer sets for Bruce ladder multiplex PCR
|
Primer |
Sequence (5'–3') |
Amplicon size (bp) |
|
BMEI0998f BMEI0997r |
ATC-CTA-TTG-CCC-CGA-TAA-GG GCT-TCG-CAT-TTT-CAC-TGT-AGC |
1682 |
|
BMEII0843f BMEII0844r |
TTT-ACA-CAG-GCA-ATC-CAG-CA GCG-TCC-AGT-TGT-TGT-TGA-TG |
1071 |
|
BMEII0428f BMEII0428r |
GCC-GCT-ATT-ATG-TGG-ACT-GG AAT-GAC-TTC-ACG-GTC-GTT-CG |
587 |
|
BR0953f BR0953r |
GGA-ACA-CTA-CGC-CAC-CTT-GT GAT-GGA-GCA-AAC-GCT-GAA-G |
272 |
|
BMEI0752f BMEI0752r |
CAG-GCA-AAC-CCT-CAG-AAG-C GAT-GTG-GTA-ACG-CAC-ACC-AA |
218 |
Edison, NJ, USA). The cycling conditions were four minutes at 95°C for initial heating, 35 cycles of 45 Seconds at 94°C, 45 Seconds at 60°C, 60 Sec at 72°C and final extension for seven minutes at 72°C. The PCR amplicons were analysed by running 10 µl of the PCR products in 1% agarose gel stained with ethidium bromide (0.5µg/mL). Thereafter, gels were photographed under UV illumination using gel documentation and analysis system. Brucella species was determined according to molecular size of the amplified products using DNA ladder (100 bp and 1Kb), (Biomatik ® Code No. M7123 and M7508. Biomatik Corporation Ontario, Canada.
Results
During 2014-2015 a total of 1126 apparently healthy dromedary camels (956 males and 170 females) were serologically examined using the modified Rose Bengal Plate Test (mRBPT) and competitive ELISA (cELISA). The results of the current study have indicated an overall Sero-prevalence rate of Brucella antibodies 4.17% and 3.73.0% respectively (Table 4). The prevalence was 3.70% in males and 5.88% in females using the mRBPT, while it was 3.35% in males and 5.88% in females using the cELISA.
Table 4: Seroprevalence of camel Brucellosis using modified Rose Bengal Plate test and competitive enzyme linked immunosorbent assay in Egypt
|
Number of blood serum samples |
Positive for mRBPT a |
Positive for cELISA b |
|
956 (males) 170 (females) 1126 (Total) |
37(3.70%) 10(5.88%) 47 (4.17%) |
32(3.35%) 10(5.88%) 42 (3.73%) |
amodified Rose Bengal Plate test; bcompetitive enzyme linked immunosorbent assay
Brucella organisms could be detected only indirectly from Guinea pigs inoculated by she-camel’s milk samples. Two (7.69%) she-camel’s milk samples out of 26 seropositive she-camels revealed positive cultures from the spleens of inoculated Guinea pigs. On the other hand, all tissue specimens and milk samples revealed negative results by direct isolation. The two Brucella cultures showed typical characteristics for the genus Brucella. Colonies were smooth elevated, transparent, and convex, with intact borders, brilliant surface and have a honey colour under transmitted light. Typing of the two Brucella isolates recovered in this study revealed Brucella melitensis biovar 3 (Table 5).
Conventional PCR in this study confirmed the presence of genetic material of genus Brucella in the two she-camel’s milk and Brucella melitensis culture DNA extracts through amplification of target gene (Immunodominant antigen, gene bp26) with amplification of the fragment of 450 bp, (Figure 2).
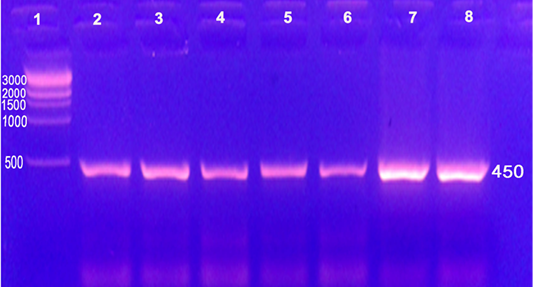
Figure 2: Conventional PCR used for detection of Brucella DNA from camels on genus level with amplification of the fragment of 450 bp
Lane 1: 1 kb DNA ladder (500, 1000, 1500, 2000, 3000bp); Lane 2: Strain 19 vaccine; Lane 3: RB51 vaccine; Lane 4: Rev1 vaccine; Lane 5, 6: she-camel’s milk (Brucella melitensis); Lane 7: Brucella melitensis culture isolated from she-camel’s milk; Lane 8: Brucella melitensis culture field isolate
Bruce ladder multiplex PCR confirmed the presence of Brucella melitensis in the two she-camel’s milk and Brucella melitensis culture DNA extracts as the test has amplified three fragments of 587 bp, 1071 bp and 1682 bp sizes (Figure 3).
Lane 1: 1 kb DNA ladder (500, 1000, 1500, 2000, 3000bp); Lane 2: Rev1 vaccine (218 bp, 587 bp, 1071 bp and 1682 bp); Lane 3: Brucella suis (272 bp and 587 bp); Lane 4: Brucella melitensis culture isolated from she-camel’s milk (587 bp, 1071 bp and 1682 bp); Lane 5: she-camel’s milk (Brucella melitensis) (587 bp, 1071 bp and 1682 bp); Lane 6: Brucella melitensis culture field isolate (587 bp, 1071 bp and 1682 bp)
Discussion
The overall sero-prevalence of Brucella antibodies in camels using the modified Rose Bengal Plate Test (mRBPT) and competitive ELISA (cELISA) was 4.17% and 3.73.0%, respectively (Table 4). The prevalence was 3.70% in males and 5.88% in females using the mRBPT, while it was
Table 5: Phenotypic characteristics of two Brucella isolates (Brucella melitensis biovar 3) recovered from milk of she-camels
|
Brucella isolates |
CO2 |
H2S |
Urease |
Growth on dyes |
Lysis by Tb phage |
Monospecific sera |
Conclusion |
|||||||
|
Thionin |
Fuchsin |
RTD |
RTD 104 |
A |
M |
R |
||||||||
|
a |
b |
a |
b |
|||||||||||
|
Two Brucella isolates recovered from milk |
- |
- |
+ in 20 hrs |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
- |
B. melitensis 3 |
|
|
Reference strains |
||||||||||||||
|
B. melitensis Ether |
- |
- |
+ in 18-24 hr. |
+ |
+ |
+ |
+ |
- |
- |
+ |
+ |
- |
B. melitensis 3 |
|
|
B. abortus544 |
- |
+ |
+ in 2 hrs |
- |
- |
+ |
+ |
+ |
+ |
+ |
- |
- |
B. abortus 1 |
|
|
B. Suis1330 |
- |
+++ |
++ in < 15min. |
+ |
+ |
- |
- |
- |
+ |
+ |
- |
- |
B. suis 1 |
|
RTD: routine test dilution; Tp: Tbilisi (Tb); a: 1:50000; b: 1:100000; A: anti Brucella abortus; M: anti Brucella melitensis; R: rough brucella antiserum
3.35% in males and 5.88% in females using the cELISA. The higher prevalence observed in females than in males in this study is consistent with other reports (Blasco et al., 1994; Musa 1995; Omer et al., 2010). The overall prevalence was reduced from 4.17% to 3.73% when c-ELISA was used as a confirmatory test for positive mRBPT samples. This variation could be attributed to false positives obtained by the former test which has been described as highly sensitive but not specific test while the latter is both sensitive and specific test and can eliminate cross reactions due to heterogeneous bacteria and minimize false positive results (Emmerzaal et al., 2002).
Reviewing the literature concerned with the efficacy of the diagnostic procedures commonly used in diagnosis of camel brucellosis, it is obvious that there are many variations in sensitivity and specificity of these procedures in different researches. Also there is a marked difference in sensitivity and reliability of such diagnostic methods in camels and those when applied in other animal species such as cattle and sheep. Such variations and differences may be attributed to probable difference in the pathogenesis and course of the brucellosis in camels. In addition, it may be the result of susceptibility of camels to other Gram-negative organisms which are antigentically related to Brucella organisms such as Yersinia enterocolitica and Pasteurella multocida. Some proteins of Brucella are responsible for serological cross-reactions between Brucella spp. and other bacterial species (Emmerzaal et al., 2002). Cross-reactivity exists to Yersinia enterocolitica O:9, Escherichia hermannii, E. coli O:157, Francisella tularensis, Stenotrophomonas maltophilia, Vibrio cholera O:1, Salmonella serotypes group N (Kagunya and Waiyaki, 1978; Sunaga et al., 1983; Barsoum et al., 1995; Emmerzaal et al., 2002).
The obtained results indicate the high prevalence of Brucella infection among camels in Egypt. The high prevalence of brucellosis may be attributed to the continuous importation of camels from enzootic African countries. In addition, such finding refers to the importance of testing the imported camels in quarantines for brucellosis. Camels may be also infected due to rearing of camels in close association with cattle and sheep especially in villages where the prevalence of brucellosis in these animals is high. Brucellosis has been reported in camels in Egypt by several authors, Ahmed (1939) 1% and 7% in males and females respectively, Hamada et al. (1963) 10.29%; Nada (1984) 21.28%; Hegazy et al. (1998) 9%; El-Seedy et al. (2000) 3.9% by BAPA and 4.9% by RBPT. Variation in the prevalence of the disease obtained by different authors and those reported in this study may be attributed to difference in the rate of exposure, breeding of camels in contact with other infected animal species and difference in the tests employed in each study.
Trials for Brucella isolation from tissue specimens and milk samples of slaughtered camels revealed detection of Brucella organisms from two (7.69%) she-camel’s milk samples out of 26 seropositive she-camels by Guinea pigs inoculation. On the other hand, all tissue specimens and milk samples revealed negative results by direct isolation. Colonies were smooth, elevated, transparent, and convex, with intact borders, brilliant surface and have a honey colour under transmitted light. Failure of isolation of Brucella organisms from tissue specimens and milk samples by direct isolation may be attributed to milk samples contamination which is considered as complicating factor for Brucella isolation due to the fastidious nature of Brucella organisms (Alton et al., 1988). Furthermore, Brucella isolation requires a large number of viable bacteria in clinical samples, proper storage and quick delivery to the diagnostic laboratory (Seleem et al., 2010). Difficulty of isolation of Brucella organisms from camel’s clinical specimens may be also attributed to unknown factors which need further studies especially on the pathogenesis of camel brucellosis. In addition, excretion of the pathogen through milk is intermittent (Wernery et al., 2007).
The criteria used for biovar delineation were requirement for additional atmospheric 10% CO2, production of hydrogen sulphide gas, production of urease, growth on media containing the inhibitory dyes thionin and fuchsin, phage typing and agglutination with polyclonal monospecific antisera A and M. Typing of the two Brucella isolates recovered in this study revealed Brucella melitensis biovar 3 (Table 5). Brucella melitensis biovar 3 was previously identified and considered as the prevalent biovar in Egypt as recorded by Salem and Hosein, (1990), Menshawy et al. (2014) and Affi et al. (2015).
Originally Brucella melitensis affects mainly sheep and goats. Such inter-species transmission situation may be the outcome of close contact between (sheep goats and cattle) and camels in this country where Brucella melitensis biovar 3 is the prevalent type in both large and small ruminants. Isolation of Brucella melitensis from camels in this study may be attributed to breeding of in contact with infected cattle, sheep and goats
In spite of the difficulty associated with the isolation of Brucella organisms from camels, the organisms could be isolated from camels in different countries. Brucella meltensis could be isolated from camels in Iran (Zowghi and Ebadi, 1988), Saudia Arabia (Radwan et al., 1992; Radwan et al., 1995), Libya (Gameel et al., 1993) and Egypt (Hegazy et al., 1998).
It is important to refer to the significance of finding Brucella melitensis in the milk of she- camels as brucellosis is easily transmitted to humans by consumption of unpasteurized Brucella infected milk and dairy products (Kiel and Khan 1987; Radwan et al., 1992; Radwan et al., 1995; Sayour et al., 2015). Shimol et al. (2012) described a brucellosis outbreak that affected 15 people who consumed unpasteurized camel milk. Affected people suffered mainly from arthralgia and fever. Consumption of unpasteurized or heat untreated milk especially of camels is still a habit in individuals who rearing camels in many Egyptian villages.
Conventional PCR in this study confirmed the presence of genetic material of genus Brucella in the two she-camel’s milk and Brucella melitensis culture DNA extracts through amplification of target gene (Immunodominant antigen, gene bp26) with amplification of the fragment of 450 bp, (Figure 2).
Bruce ladder multiplex PCR confirmed the presence of Brucella melitensis in the two she-camel’s milk and Brucella melitensis culture DNA extracts as the test has amplified three fragments of 587 bp, 1071 bp and 1682 bp sizes (Figure 3). These findings agreed with those obtained by Waleed et al. (2013) using the same primers. PCR results supported the results of bacteriological examinations and clarified the power of PCR testing for detection of Brucella from clinical samples that could be used effectively in routine diagnosis of brucellosis. The obtained results indicate that molecular detection of Brucella species can be done directly on clinical samples without previous isolation of the organism. In addition, these techniques can be used to complement results obtained from phenotypic tests as reported by Bricker (2002).
Conclusions
The results of this study revealed that camel brucellosis is prevalent in the studied areas in Egypt. Cattle and sheep that reared in close contact with camels in Egypt are incriminated as the source of infection to camels based on isolation of Brucella melitensis biovar 3 which is prevalent in both large and small ruminants. Bruce-ladder PCR assay is recommended for its high discriminatory power of identification of Brucella isolates and can be used without previous isolation of the organism or to complement the results obtained from phenotypic tests. Creation of awareness to camel holders about the economic and zoonotic impact of Brucella infection and its associated risk factors is crucial. In addition there is a need for implementing effective control measures and preventive methods of brucellosis.
Acknowledgements
This study was supported by a grant from the project fund (Development of a DNA based new system for biotyping of Brucella strains) offered by the unit of support and funding of researches and projects, Beni Suef University.
Conflict of interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper. All authors read and approved the final manuscript.
Authors’ Contribution
All authors have made equal substantial contributions to conception, design, collection of samples, execution of serological, bacteriological and PCR assays, interpretation of data and drafting the manuscript. All authors read and approved the final manuscript.
References