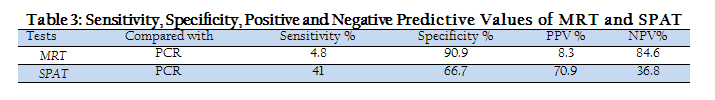Research Journal for Veterinary Practitioners
Research Article
Research Journal for Veterinary Practitioners 2 (1): 5 – 8Comparison of Milk Ring Test; Serum Plate Agglutination Test and Polymerase Chain Reaction for the Detection of Bovine Brucellosis
Shamim Saleha1*, Abdul Basit1, Kashif Rahim1, Muhammad Shahid2, Mirza Ali Khan2
- Department of Microbiology, Kohat University of Science and Technology (KUST), Khyber Pakhtunkhwa, 26000, Pakistan
- Microbiology and Biotechnology Center, Veterinary Research Institute (VRI), Khyber PakhtunKhwa, Peshawar, 25000, Pakistan
*Corresponding author: shamimsaleha@yahoo.com
ARTICLE CITATION:
Saleha S, Basit A, Rahim K, Shahid M and Khan MA (2014). Comparison of milk ring test; serum plate agglutination test and polymerase chain reaction for the detection of bovine brucellosis. Res. j. vet. pract. 2(1): 5 – 8.
Received: 2013–11–10, Revised: 2013–12–01, Accepted: 2013–12–02
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.rjvp/2014/2.1.5.8)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
In the following study, comparative efficacy of Milk Ring Test (MRT), Serum Plate Agglutination Test (SPAT) was determined by calculating the sensitivities and specificities for detection of Brucellosis in bovines from district Peshawar of Khyber PakhtunKhwa, Pakistan. Considering Polymerase Chain Reaction (PCR) as a gold standard diagnostic test, MRT showed very low sensitivity (4.8%), while its specificity was 90.9 %, likewise, SPAT also showed low sensitivity (41 %), while its specificity was 66.7% in bovines. Statistically, high specificities and low sensitivities of MRT and SPAT in bovines recommended the poor efficacy of these tests, when used individually in contrast to PCR. As PCR is more reliable test, while considering its sensitivity for antigen detection as compare to antibody detection, therefore, PCR may be used as a routine screening test in clinical practices in farms animals for detection of Brucellosis.
INTRODUCTION
Brucellosis is considered the most contagious and zoonotic bacterial infection of livestock worldwide (Munir et al., 2010). Brucella abortus and Brucella melitensis are the principal cause of brucellosis in bovines (Radostits et al., 2000; Karaca et al., 2007). These brucella species also cause brucellosis in humans (Gul et al., 2007). In animals, brucellosis frequently results in abortion, birth of weak calves, death of young stock, infertility in males and reduced milk yield in females (Maadi et al., 2011; Abubakar et al., 2011).
There is neither a single diagnostic test available by which a bacterium can be identified as Brucella nor any signs and symptoms of brucellosis are specific, therefore, a combination of cultural, serological and molecular methods is necessary for accurate diagnosis. However, all these methods have serious limitations (Poester et al., 2010; Abubakar et al., 2011). Accurate diagnosis of brucellosis requires Brucella isolation and detection in the laboratory, which is impractical for regular screening of large populations and could not be used as criteria for control and eradication of disease. Serological tests are relatively easy to perform and provide a practical advantage in detecting the prevalence of Brucella infection (Abubakar et al., 2011). The use of the Polymerase Chain Reaction (PCR) to identify Brucella DNA at genus, species and even biovar levels has becoming extended to improve diagnostic tests (Poester et al., 2010; Chimana et al., 2010).
In diagnostic laboratories, sera usually are screened with any simple serological test of high sensitivity. For milk samples, Brucella milk ring test is used for screening and monitoring the dairy herds at regular intervals (Chimana et al., 2010). Rarely, the clinical samples after initial screening by conventional methods are subjected to PCR for confirmation of disease (Abubakar et al., 2011).
In the agriculture based economy of Pakistan livestock accounts for 37.5 percent of agricultural value–addition and about 9.4% of its GDP. Brucellosis infection has a considerable impact on human and animal health as well as on socioeconomic factors where rural income relies mainly on land cultivation and domestic animals farming and people usually live in very close proximity with their livestock (Maadi et al., 2011; Shafee et al., 2011). People practice mixed crop–livestock farms where usually breed 90% buffaloes and 10% cattle, for dairy products and milk production (Afzal and Naqvi, 2004).
In Pakistan, the incidence of brucellosis is increasing day by day, particularly in large dairy herds. Several studies have reported the high incidence rates of brucellosis in livestock farms in government and private sector in different districts and provinces of Pakistan (Abubakar et al., 2010; Hamidullah et al., 2009; Iftikhar et al., 2008; Mukhtar and Kokab, 2008; Omer et al., 2010; Rabab et al., 2000; Shafee et al., 2011). Brucellosis can be controlled in Pakistan by routine screening of domestic livestock and animal vaccination programs (Abubakar et al., 2011). In Pakistan, veterinarians mostly rely on the conventional screening tests due to the lack of more specific diagnostic facilities and financial limitations. Rose Bengal Precipitation Test (RBPT), Serum Plate Agglutination Test (SPAT) and Milk Ring Test (MRT) are the most commonly performed tests at both government and private livestock laboratories in Pakistan (Asif et al., 2009; Gul et al., 2007).
This study was carried to evaluate Milk Ring Test, Serum Plate Agglutination Test and Polymerase chain reaction (PCR) for the detection of brucellosis in milk and serum samples of bovines, collected from private farms located in the district Peshawar of Khyber PakhtunKhwa region of Pakistan.
MATERIALS AND METHODS
In this study, 142 milk and blood samples from bovines were collected (70 cattle and 72 buffaloes) using the simple random sampling from different private farms in district Peshawar of Khyber PakhtunKhwa region in Pakistan.
Initial Screening Tests on Milk and Serum Samples of Bovines
The serum samples were subjected to SPAT for screening Brucella antibodies as described by (Alton et al., 1975). The results of agglutination were recorded. A titer of 1:80 or above was considered positive for brucella infection. Milk ring test was conducted on milk samples as described by (Alton et al., 1988). The positive samples were differentiated on the basis of blue ring present on the top of milk after overnight reaction.
DNA Extraction and PCR
DNA was extracted from same serum and milk samples by using DNA isolation kit (Shanghai ZJ Bio– Tech Co., Ltd. China), then PCR assay was performed in a total reaction volume of 50μl (Shanghai ZJ Bio– Tech Co., Ltd. China), according to supplier’s manual. This kit contains a reaction mix for the specific amplification of Brucella DNA. The amplification was performed in a DNA thermal cycler (Multi–gene Labnet international Inc.USA). Initial denaturation was carried out at 94 °C for 2 minutes, and then for 35 cycles the sample DNA was denatured at 93 °C for 15 seconds, annealed at 55°C for 30 seconds, and extended at 72°C for 30 seconds. The final incubation was done at 72 °C for 10 minutes. For positive controls, DNA extracted from Brucella abortus and Brucella melitensis strains, obtained from veterinary research institute, Peshawar was used. However, for negative control, distilled water was used. The PCR products were resolved and analyzed by using 1.5% of agarose gel electrophoresis and photographed on UV photo–documentation system (Multi–gene Labnet international Inc.USA) .The clear bands of Brucella species DNA were considered as positive results.
STATISTICAL ANALYSIS
Considering PCR as a gold standard diagnostic test, relative sensitivity, specificity, positive and negative predictive values of RBPT and STAT were calculated by using the MedCalc Software (Version 12.3.0).
RESULTS
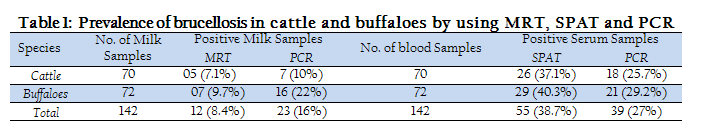
The prevalence rate of Brucellosis detected in cattle and buffaloes in district Peshawar, using MRT, SPAT and PCR are given in Table 1. The overall prevalence rate detected by SPAT (38.7%) was greater than MRT (8.4%) when a total of 142 milk and serum samples of bovines were tested by MRT and SPAT. Moreover, serum samples were found more positive by PCR than milk samples. But PCR gave more positive results than MRT and less positive results than SPAT (Table 1).
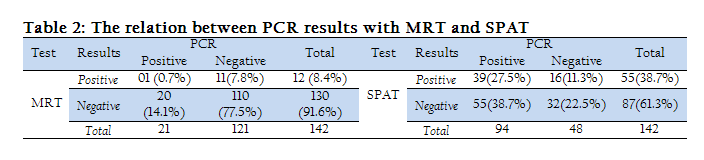
It was also found that 0.7 % milk samples were positive to both MRT and PCR and 77.5 % milk samples were negative to both tests. Similarly, 27.5% serum samples were positive to both SPAT and PCR and 22.5 % serum samples were negative to both tests (Table 2).
Statistically, MRT and SPAT were found less sensitive and more specific in detecting Brucella species antibodies. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value of MRT and SPAT, when compared with PCR are given in Table 3. When PCR was used to detect Brucella species DNA in milk and serum samples of bovines, it gave much positive results than MRT and SPAT. PCR–amplified products of 306bp and 312bp were clearly visualized on agarose gel electrophoresis for Brucella abortus and Brucella Melitensis respectively (Figure 1).
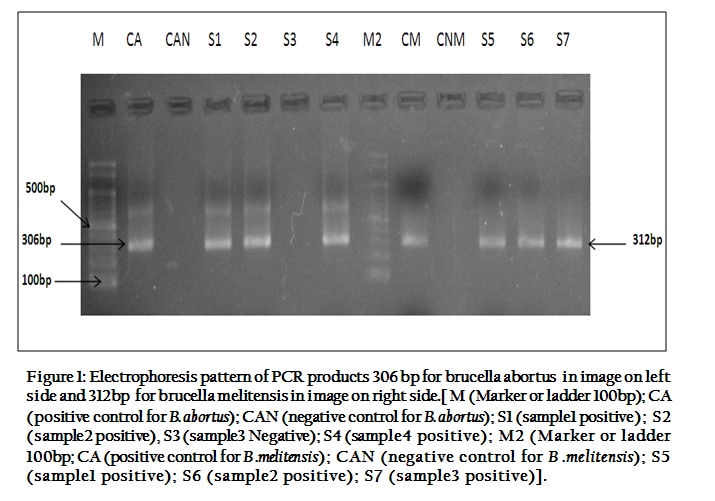
Figure 1: Electrophoresis pattern of PCR products 306 bp for brucella abortus in image on left side and 312bp for brucella melitensis in image on right side.
DISCUSSION
Brucellosis has been recognized as an important zoonotic disease as it concerned with both animal and human health. As this disease make vulnerable economic losses to the country particularly to livestock industry, therefore, definitive diagnosis means (conventional and molecular techniques) are required for its effective eradication.
Currently, veterinary diagnostic laboratories utilize Milk Ring Test for diagnosis of brucellosis in bovine milk samples, which indirectly identifies Brucella species in the host (Chimana et al., 2010). In present study, all collected milk samples from bovines were tested by MRT and PCR for diagnosis of brucellosis. The prevalence rate of Brucellosis was 8.4% when using MRT lower than the rate obtained when PCR was used as 16% reacted positively. This difference could be attributed to the fact that the MRT test results may be false–negative when the milk samples contain small quantities of IgM and IgA antibodies, or due to deficiency of the fat clustering factors (O’Leary et al., 2006). In contrast, sensitivity of PCR for detection of Brucella genome is extremely high as it could detect Brucella genome from 30fg of total DNA (Rabab et al., 2000). In a study PCR was used for diagnosis of Brucellosis in bovines and showed considerable increase sensitivity as compared to MRT (Al–Mariri and Haj–Mahmoud, 2010). Similarly, in another study (Akhtar et al., 2010) diagnostic efficacy of RBPT, MRT and PCR for brucellosis infection was evaluated in cattle and buffaloes. The antigenic detection of Brucella using PCR gave more positive results than conventional RBPT and MRT. However, in the present study when the two tests MRT and PCR were used together to evaluate the prevalence, only 0.7 % of the milk samples were positive and 77.5% were negative to both tests.
The SPAT is one of standard serological screening tests, used for the diagnosis of brucellosis (Memish and Balkhy, 2004). During this study, the prevalence rate of Brucellosis was 38.7% when using SPAT higher than the rate obtained when PCR was used, 27% reacted positively, which was contrary to that reported previously (Asif et al., 2009). Moreover, serological tests like RBPT and SPAT give false–positive results due to cross–reactions with other gram negative bacteria including Salmonella, E. coli, Yersinia enterocolitica, Vibrio cholerae etc. (Al–Attas et al., 2000). When the two tests SPAT and PCR were used together to evaluate the prevalence, 27.5% of the blood samples were positive in both SPAT and PCR and 22.5% were negative to both tests.
In this study, the antibodies detection of Brucella using MRT (8.4%) and SPAT (38.7%) was compared in milk and serum samples respectively. The SPAT test showed more positive results in serum samples as compared MRT in milk samples. This difference may be due to the fact that milk proteins hamper the Brucella antibodies isolation (Akhtar et al., 2010).
Statistically, the MRT showed very low sensitivity (4.8%), while its specificity was 90.9 % as compared to 70% sensitivity and 80% specificity in bovines reported earlier (Al–Mariri and Haj–Mahmoud, 2010). The SPAT also showed low sensitivity (41 %), while its specificity was 66.7% in bovines. High specificities and low sensitivities of MRT and SPAT in bovines suggested the poor efficacy of both tests used individually as compare to PCR. The PCR is currently used for the diagnosis of many diseases including Brucellosis, as PCR is more reliable and sensitive due to its ability for antigenic detection than antibody detection (Akhtar et al., 2010). In present study, the calculated positive and negative predictive values of MRT and SPAT in bovines were in correspondence with their specificity and sensitivity values respectively. However, when PCR was evaluated for its diagnostic efficacy for detection of Brucella species in milk and serum samples, PCR gave more positive results on serum samples than on milk samples. These findings are consistent with the results and suggestions given in another study (Akhtar et al., 2010). Therefore, the combination of conventional routine screening tests (MRT and SPAT) along with serum–PCR may be recommended in livestock diagnostic laboratories.
The inconsistent results in terms of the sensitivity and specificity of MRT and SPAT in bovines suggested that these tests may be used for routine screening of herds but not for confirmatory diagnosis of brucellosis in individual animals. Therefore, after the initial screening by MRT and SPAT a further confirmation through PCR is needed for accurate diagnosis of Brucellosis in livestock diagnostic laboratories. Furthermore, As PCR is more reliable test, when its sensitivity is considered for antigen detection in clinical samples than antibody detection, it may be included as a routine screening test in clinical practice in farms animals irrespective of high cost as compared to conventional tests in order to reduce economic losses in Pakistan.
ACKNOWLEDGEMENT
We would like to thank Ms. Mussarat Jabeen (Lecturer in English, Frontier Education Foundation College for Girls, Kohat) for her valuable input in writing manuscript in English.
REFERENCES
Mansoor M and Arshed MJ (2011). Bovine Brucellosis. Old and new concepts with Pakistan perspective. Pak.Vet. J. 32(2): 1–9.
Abubakar M, Arshed MJ, Hussain M, Ehtisham–ul–Haq and Ali Q (2010). Serological evidence of Brucella abortus prevalence in Punjab province, Pakistan– a cross–sectional study. Transbound. Emerg. Dis. 57(6): 443–447.
http://dx.doi.org/10.1111/j.1865-1682.2010.01171.x
PMid:21117286
Afzal M and Naqvi AN (2004). Livestock Genetic Resources of Pakistan. Scie. Vision 9(3–4): 185–198.
Akhtar R, Chaudhry ZI, Shakoori AR, Ahmad M and Aslam A (2010). Comparative efficacy of conventional diagnostic methods and evaluation of polymerase chain reaction for the diagnosis of bovine brucellosis. Vet. World 3(2): 53–56.
Al–Attas, RA, lkhalifa MA and Alqurashi AR (2000). Evaluation of PCR, culture and serology for the diagnosis of acute human brucellosis. Ann. Saudi Med. 20(3–4): 224–228.
PMid:17322662
Al–Mariri A and Haj–Mahmoud N (2010). Detection of Brucella abortus in Bovine Milk by Polymerase Chain Reaction. ACTA VET. BRNO. 79(2): 277–280.
http://dx.doi.org/10.2754/avb201079020277
Alton GG, Jones LM and Pietz DE (1975). Laboratory techniques in brucellosis.In:World Health Organization Monograph Series Nº 55, Geneva, Switzerland. Volume 2: 1–163.
Alton GG, Jones LM, Angus RD and Verger JM (1988). Techniques for the Brucellosis Laboratory. Institute National de la Recherche Agronomique, Paris, France. 13–61.
Asif M, Awan AR, Babar ME, Ali A, Firyal S and Khan QM (2009). Development of genetic marker for molecular detection of Brucella abortus. Pak. J. Zool. Suppl. Ser. 9: 267–271.
Chimana HM, Muma JB, Samui KL, Hangombe BM, Munyeme M, Matope G, Phiri AM, Skjerve E and Tryland M (2010). A comparative study of the seroprevalence of brucellosis in commercial and small–scale mixed dairy beef cattle enterprises of Lusaka province and Chibombo district, Zambia. Trop. Anim. Health Prod. 42(7): 1541–1545.
http://dx.doi.org/10.1007/s11250-010-9604-4
PMid:20517646
Gul ST and Khan A (2007). Epidemiology and epizootology of brucellosis. A review. Pak. Vet. J. 27(3): 145–151.
Hamidullah M, Khan R and Khan I (2009). Seroprevalence of brucellosis in animals in district Kohat NWFP and comparison of two serological tests. Pak. Jol of Sci. 61(4):242–243.
Iftikhar H, Arshad MI, Mahmood MS and Akhtar M (2008). Seroprevalence of Brucellosis in Human, Cattle, and Buffalo Populations in Pakistan. Turk. J. Vet. Anim. Sci. 32(4): 315–318.
Karaca M, abur CB, Uelebi B, Akkan HA, Tutuncu M, Keleu II, Atalay UB and Kiliu S (2007). Investigation on the seroprevalence of toxoplasmosis, listeriosis and brucellosis in goats living in the region of Van, Turkey. Yuzuncu Yil universitesi Veteriner Fakultesi Dergisi. 18(1): 45–49.
Maadi H, Moharamnejad M and Haghi M (2011). Prevalence of Brucellosis in Cattle in Urmia, Iran. Pak. Vet. 31(1): 81–82.
Memish AZ and Balkhy HH (2004). Brucellosis and international travel. J. Travel Med. 11 (1): 49–55.
http://dx.doi.org/10.2310/7060.2004.13551
PMid:14769288
Mukhtar F and Kokab F (2008). Brucella serology in abattoir workers. J. Ayub Med. Coll. 20(3): 57–61.
Munir R, Afzal M, Hussain M, Naqvi SMS and Khanum A (2010). Outer membrane proteins of B. abortus vicinal and field strains and their immune response in buffaloes. Pak. Vet. J. 30(2): 110–114.
O'Leary S, Sheahan M and Sweeney T (2006). Brucella abortus detection by PCR assay in blood, milk and lymph tissue of serologically positive cows. Res. Vet. Sci. 81(2): 170–176.
http://dx.doi.org/10.1016/j.rvsc.2005.12.001
PMid:16545848
Omer MM, Mussa MT, Bakhiet MR and Perrett L (2010). Brucellosis in camels, cattle and humans: associations and evaluation of serological tests used for diagnosis of the disease in certain nomadic localities in Sudan. Rev. Sci. Tech. 29(3): 663–669.
PMid:21309464
Poester FP, Nielsen K, Samartino LE and Yu WL (2010). Diagnosis of Brucellosis. The Open Vet. Sci. J. 4: 46–60.
Rabab A, Attas A, Khalifa MA, Al Qurashi AR, Badawy M and Al Gulay N (2000). Evaluation of PCR, culture and serology for the diagnosis of acute human Brucellosis. Ann. Saudi Med. 20(3–4): 224–228.
Radostits OM, Gay CC, Blood DC and Hinchcliff KW (2000). Veterinary Medicine ELBS. Bailliere Tindall, London, UK. Edition 9: 870–871.
Shafee M, Rabbani M, Sheikh AA, Ahmad MD, Razzaq A (2011). Prevalence of bovine brucellosis in organized dairy farms, using milk ELISA, in Quetta City, Balochistan, Pakistan. Vet. Medic. Int. 1–3