The Effect of Dietary Supplementation of 17α -Methyltestosterone and 17β-Estradiol Hormones on Growth, Sex Conversion and Reproduction in Electric Yellow Cichlid (Labidochromis caeruleus)
The Effect of Dietary Supplementation of 17α -Methyltestosterone and 17β-Estradiol Hormones on Growth, Sex Conversion and Reproduction in Electric Yellow Cichlid (Labidochromis caeruleus)
Zafer Karslı1,*, Dilek Şahin1, Meryem Öz2, Ünal Öz2 and Orhan Aral2
1Vocational School, Sinop University, 57000, Sinop, Turkey
2Fisheries Faculty, Sinop University, 57000, Sinop, Turkey
ABSTRACT
In this study, effects of dietary supplementation of two different hormones on growth, sex conversion and reproduction of electric yellow cichlid, Labidochromis caeruleus were investigated. Moreover, possible negative (sterilization, mortality, reproduction performance etc.) and positive (growth, sex conversation etc.) impacts of hormone administration were also assessed. This study had two experiments. In the first experiment, six experimental feeds were prepared by adding two different hormones (17α-Methyltestosterone (Mt), 17β-Estradiol (Es) at three different doses (20, 40, 60 mg kg-1) and control feed was without hormone. All six experiment groups receiving experimental diets and control were conducted in 3 replicates in 21 aquariums for 3 months in the first experiment. L. caeruleus were fed orally with these hormone-supplemented feeds and growth parameters, sex conversion and survival rate were examined. In the second experiment, fish from the first experiment were fed with commercial cichlid feed containing no hormones and numbers of fry produced was recorded. At the end of 90-day feeding period in the first experiment, the best growth was obtained in 60 mg kg-1 Mt treated group (1.97 ±0.17 g). Sex conversion to all male population (100% male) was observed in 17α-Mt treated groups, while, conversion to 82.22, 86.67 and 86.67% females was observed in 17β-Es treated (20, 40 and 60 mg kg-1) groups, respectively. In the second experiment, after feeding the fish with a commercial feed for 3 months, fish fry was released from fish mouth and numbers of fry were counted. The present findings revealed that 17α-Mt hormone was more effective in growth and sex conversion, while the survival rate and number of fry produced were negatively affected by the increased dose of both hormones.
Article Information
Received 05 January 2021
Revised 24 February 2021
Accepted 05 March 2021
Available online 12 August 2021
(early access)
Published 03 December 2021
Authors’ Contribution
ZK planned the experiments, performed the statistical analyses, wrote and directed this project. DŞ collected the data and wrote this manuscript. MÖ collected data. ÜÖ performed the statistical analyses. OA revised the manuscript.
Key words
Development, Electric yellow cichlid, Hormone treatment, Reproductive performance, Sex conversion.
DOI: https://dx.doi.org/10.17582/journal.pjz/20210105110138
* Corresponding author: zakarsli@sinop.edu.tr
0030-9923/2022/0001-0213 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
Inadequate and interrupted supply of feed raw materials increase price of raw materials making serious burdens on feed supply to fish producing industry. In ornamental fish production, the time required to reach a market size is also an important issue. High-protein feeds accelerate and increase growth rate, simultaneously also increase feed cost. Low-protein feeds may reduce feed costs, but they recess growth and development of the fish (Vandenberg and Moccia, 1998).
Growth promoting agents such as hormones are commonly used in animal raising to accelerate growth and development. 17β-Es (Estradiol) and 17α-Mt (Methyltestosterone) are one of the most effective among anabolic steroids. Such growth promoters supplemented into feeds increase appetite of animals, feed conversion ratio and consequently enhance reproductive performance and number of fry production. Incorporation of these compounds generally increase protein synthesis, improve proteolytic enzyme activity in digestive tract, raise amino acid absorption from intestines and proteolytic enzyme activity of muscles. The primary target in fish rearing is to get desired growth with a minimum expense as feed cost. Several studies have revealed that in aquarium fish rearing, it was possible to achieve marketable size in a short time with the use of growth-promoting hormones and these fishes provided greater economic return. Using these hormones, it is possible to raise fish more economically with less feed consumption. Growth, reproduction and survival rate in fish already undergone sex change are important issues. The primary target in sex change studies in fish is to get higher growth rate. Moreover, reproductive activity may be ceased, thus no fish are produced through breeding. However, limiting the reproduction of ornamental fish may create problems in marketing of aquarium fish species. Similar to various animal species, juvenile production is a significant issue in aquarium fish raising and it is impossible to get economic yield without reproduction (Buttery and Sweet, 1993; Ergun et al., 2010; Gannam and Lovell, 1991; Rendón von Osten et al., 2019; Santandreu and Diaz, 1994; Turan et al., 2005).
Electric yellow cichlid (Labidochromis caeruleus Fryer, 1956) is a native from the Lake Malawi of East Africa, which is the third largest lake of Africa. Shallow costs of Lake Malawi constitute the natural habitat of this species. Due to its easy reproduction capability, it has a significant place in aquarium fish trade worldwide. Electric yellow cichlid belongs to Cichlidae family under Perciformes order. Males are larger with brighter colors than the females. Under natural condition, males can grow up to 15 cm. Black color is more intense and wider in fins of male fish, but females have fainted and thinner black bands (Lewis, 1982; Morioka and Matsumoto, 2008).
In this study, effects of dietary supplementation of two different hormones (17β-Es (Estradiol) and 17α-Mt (Methyltestosterone)) on growth, sex change and reproductive performance of electric yellow cichlid (L. caeruleus) were investigated and possible negative impact of hormone treatment, like sterilization was tried to be identified.
Materials and methods
In the first trial of study, experimental fish were selected from a stock of 450 fish with an average body weight of 0.64 ±0.01 g. The selected fish were distributed to 7 groups containing 15 fish and 30 liters water in each and placed in 45 liters (20×45×50 cm) aquariums containing 30 liters water with 3 replicates per group. Commercial cichlid feed in crumbled form fitting to fish mouth was used. The feed composed of 47.5% crude protein, 6.5% crude lipid and 2% crude cellulose. Control feed was not supplemented with hormone, however, other six feeds were supplemented with three doses (20, 40, 60 mg kg-1) of 17β-Es (Estradiol) and 17α-Mt (Methyltestosterone) hormones and fish were fed with these diets for 3 months (Table I). In the experiment, sex conversation of fish were made by looking at first and second sex characteristics. In the second trial of study, previously non-reproduced 1 male (2.59 ±0.03 g, 6.08 ±0.04 cm) and previously non-ovulated and to be ovulated for the first time, 4 females (2.24 ±0.07 g, 5.13 ±0.02 cm) were selected (sex ratio 4F:1M) from each group at the end of 3-month feeding. They were placed into 12 aquariums (30-liters, 20×45×50 cm) randomly divided into 4 groups containing 3 replicates in each. Also, in the second trial of study, 60 mature fish (48 females and 12 males) were used. Stock aquariums were also arranged for each group. Apart from the control group, the fish fed with 20 mg kg-1 17β-Es and 17α-Mt were placed into the first group (group A), the fish fed with 40 mg kg-1 17β-Es and 17α-Mt were placed into the second group (group B) and the fish fed with 60 mg kg-1 17β-Es and 17α-Mt were placed into the third group (group C). Then, all fish groups were fed with commercial cichlid feed without hormone for 90 days and number of fry produced from each group were observed. The fish with fry in mouth was taken to a separate aquarium, fry were released from the mouth by regurgitation and their number was counted. A daylight photoperiod (12 h light–12 h dark) was applied throughout the experiments. Water temperature, pH, dissolved oxygen and NH4+ values were measured at certain intervals and maintained at constant levels within optimum ranges.
Table I.- Chemical composition of the experimental diets used in feeding trial involving the electric yellow cichlid and the added hormone doses to the feed.
|
Chemical composition |
Diet |
||||||
|
1 (control) |
2 (17α-MT) |
3 (17α-MT) |
4 (17α-MT) |
5 (17β-ES) |
6 (17β-ES) |
7(17β-ES) |
|
|
Crude protein (%) |
47.5 |
47.5 |
47.5 |
47.5 |
47.5 |
47.5 |
47.5 |
|
Crude lipid (%) |
6.5 |
6.5 |
6.5 |
6.5 |
6.5 |
6.5 |
6.5 |
|
Crude fibre (%) |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
|
Moisture content (%) |
6.0 |
6.0 |
6.0 |
6.0 |
6.0 |
6.0 |
6.0 |
|
Ash |
10.5 |
10.5 |
10.5 |
10.5 |
10.5 |
10.5 |
10.5 |
|
NFEa |
27.5 |
27.5 |
27.5 |
27.5 |
27.5 |
27.5 |
27.5 |
|
17α-Methyltestosterone mg kg-1 |
- |
20 |
40 |
60 |
- |
- |
- |
|
17β-Estradiol mg kg-1 |
- |
- |
- |
- |
20 |
40 |
60 |
|
Proximate analysis |
|||||||
|
GEb, kj g-1 |
18.53 |
18.53 |
18.53 |
18.53 |
18.53 |
18.53 |
18.53 |
Vitamin A, 29770 IU/kg; Vitamin D, 1860 IU/kg; E5 Mangenese, 67 mg kg-1; E6 Zinc, 40 mg kg-1; E1 Iron, 26 mg kg-1.a NFE, nitrogen free extract = 100- (% Moisture + % Crude Protein + % Crude Lipid + % ash + % Crude fiber). b GE, gross energy = (% Crude protein × 23.6) + (% Crude lipid × 39.5) + (% carbonhydrates × 17.3) (Koshio et al., 1993).
YSI Professional Plus meter was used to measure daily temperature, dissolved oxygen, pH and NH4+ values. The average water temperature, dissolved oxygen, pH and NH4+ values were determined as 27.3 ±0.02°C, 6.89 ±0.01 ppm, 8.28 ±0.06 and 3.1 ±0.05 mgl-1, respectively.
Fish were fed twice a day manually (at 09:00 and 16:00 h) until satiation. Waste materials were syphoned out every day.
Growth parameters
Fish growth performances were calculated according to the following formulas:

Where, SGR is specific growth rate, ln is natural logarithm, W0 is initial weight (g), Wt is final weight (g) and t is time in days from stocking to harvesting.
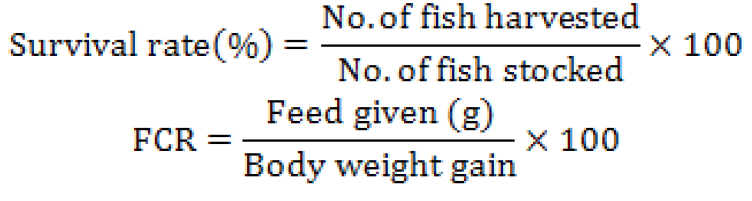
The condition factor (CF) of the experimental fish was estimated from the relationship:
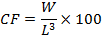
Where; W is weight of the fish in g, and L is the total length of the fish in cm.
Experimental data were subjected to statistical analyses with the aid of “Minitab Release 17 for Windows” software. One-way analysis of variance (ANOVA) was conducted. Group means were expressed in mean ± standard error. Significant differences in means were compared using Fisher’s test at 0.05 significance level.
Ethical note
This research was approved by the Ethics Committee of Sinop University with Reference No. MYO-1901-16-22. All the procedures applied in this study took into account the importance of preventing, or at least minimizing, any kind of animal discomfort or suffering.
Results
Growth efficiency, survival and sex
Considering the growth parameters, the highest growth and feed conversion ratios were obtained from 60 mg kg-1 17α-Mt group and the lowest values were obtained from 17β-Es (20, 40, 60 mg kg-1) hormone administered groups (p<0.05). Besides, 17α-Mt hormone groups had better feed conversion ratios than that of other groups (Table II). At the end of 90 days feeding trial, the highest survival rates were obtained from 20 mg kg-1 17α-Mt (84.44%) and control (80%) groups. Survival rate in the treatment groups decreased with increasing hormone dose and especially, 17β-Es hormone groups had significantly lower survival rates than the other groups (Table II). The 17α-Mt hormone was more effective on sex conversion and the fish fed with this hormone were all turned into male fish (100% male) (Table II). On the other hand, in 17β-Es hormone groups, a slight increase was observed in female numbers with increasing hormone dose, but that difference was not found to be significant (p > 0.05).
Reproductive performance
Experimental fish were fed with hormone-containing feeds for 90 days in the first stage of the study and then, all fish groups were fed with commercial feed without hormone supplementation for 90 days to identify negative impacts of hormone treatment, like infertility. At the end of the experiments, the highest number of fry was obtained from the control group (268 fry) and the lowest fry yields were obtained from group B (40 fry) and group C (31 fry). The number of fry from control group without hormone treatment in the first stage was significantly different from the fry yields of hormone-treated groups A, B and C (p < 0.05). The number of fry from hormone treated group A was also significantly different from that of the hormone-treated groups B and C (Table III). This result indicated a decrease in number of fry with increasing hormone doses.
Discussion
In several studies, besides positive impacts of hormone treatments on growth, coloration and desired gender fish production, various negative impacts such as increased death rate, low level of sex hormones and infertility were also reported. Such negative impacts of hormones mostly varied based on fish species, hormone type, dose and application mode (injection, supplementation with feed or water) and duration of administration, physical and chemical characteristics of aquarium water.
This study was conducted in two stages and significant differences were observed in growth parameters (survival rate, condition factor, sex ratio) in the first stage (p < 0.05) (Table II).
In a study conducted with Colisa lalia (Dwarf gourami) species, Biswas et al. (2014) applied three different doses (5, 10, 15 mg kg-1) of 17α-Mt hormone and reported the best growth in 10 mg kg-1 hormone-treated group. In another study conducted with Oreochromis niloticus (Nile tilapia),
Table II.- Growth parameters, feed conversion ratio (FCR), survival rate, condition factor and sex ratio of electric yellow cichlid after 3 months of feeding.
|
Groups |
Control |
17α-MT |
17β-ES |
|||||
|
20 mg |
40 mg |
60 mg |
20 mg |
40 mg |
60 mg |
|||
|
IBW (g) |
0.64 ±0.01a |
0.64 ±0.01a |
0.64 ±0.01a |
0.64 ±0.01a |
0.64 ±0.01a |
0.64 ±0.01a |
0.64 ±0.01a |
|
|
FBW (g) |
1.61 ±0.19c |
1.88 ±0.12ab |
1.80 ±0.21b |
1.97 ±0.17a |
1.49 ±0.15d |
1.44 ±0.28d |
1.44 ±0.27d |
|
|
Weight gain (g) |
0.96 ±0.11c |
1.23 ±0.14ab |
1.15 ±0.12b |
1.32 ±0.08a |
0.84 ±0.12d |
0.79 ±0.06d |
0.79 ±0.05d |
|
|
WGP (%) |
148.11 ±16.9c |
189.72 ±16.21ab |
177.39 ±14.3b |
203.59 ±27.12a |
129.62 ±11.4d |
121.91 ±9.83d |
121.91 ±10.71d |
|
|
SGR (%/ day) |
1.01 ±0.09c |
1.18 ±0.19ab |
1.13 ±0.22b |
1.23 ±0.27a |
0.92 ±0.14d |
0.88 ±0.13d |
0.88 ±0.18d |
|
|
FCR |
3.16 ±0.48a |
2.61 ±0.88b |
2.67 ±0.92b |
2.21 ±0.56c |
3.04 ±0.77a |
2.53 ±0.51b |
3.24 ±0.89a |
|
|
Survival rate (%) |
80.00 ±6.65a |
84.44 ±3.85a |
62.20 ±6.65b |
62.20 ±10.18b |
68.8 ±13.3b |
64.44 ±7.47b |
53.3 ±17.7c |
|
|
Condition factor |
1.19 ±0.02c |
1.18 ±0.05c |
1.22 ±0.07bc |
1.22 ±0.03bc |
1.32 ±0.11a |
1.23 ±0.06b |
1.23 ±0.05b |
|
|
Sex conversion (%) |
||||||||
|
Male |
28.89b |
100a |
100a |
100a |
17.78bc |
13.33c |
13.33c |
|
|
Female |
71.11b |
0a |
0a |
0a |
82.22bc |
86.67c |
86.67c |
|
Values (mean ± standard error of means for triplicates) with different superscripts in the same row are significantly different (one-way ANOVA and Fisher’s multiple-range test, p < 0.05). IBW, initial body weight; FBW, final body weight; FCR, feed conversion ratio; SGR, specific growth rate; WGP, weight gain percentage.
17α-Mt hormone was reported to increase appetite, thus increased feeding rate and accelerated growth (Ajiboye et al., 2015). Greisy and Gamal (2012) reported the best growth of O. niloticus at 60 mg kg-1 17α-Mt treatment. Contrarily to these studies, hormone treatment caused lower growth and development than in untreated group in Betta splendens (Kirankumar and Pandian, 2002). Similarly, Sreenivasa and Prabhadevi (2018) observed a recess in growth and development with increasing hormone doses. George and Pandian (1996) demonstrated that high doses of 17α-Mt and 17β-Es hormones negatively influenced growth and development of zebra cichlids. Carvalho et al. (2014) observed slower growth rates of Centropomus undecimalis treated with 17β-Es hormone compared to that of untreated group.
Table III.- Total number of fry produced from electric yellow cichlid (Labidochromis caeruleus) fed commercial cichlid feed for 90 days.
|
Time (days) |
Groups |
|||
|
Control |
A |
B |
C |
|
|
30 |
31a |
0b |
0b |
0b |
|
60 |
71a |
46b |
11c |
0d |
|
90 |
166a |
28b |
29b |
31b |
|
Total |
268a |
74b |
40c |
31c |
Values (mean ± standard error of means for triplicates) with different superscripts in the same row are significantly different (one-way ANOVA and Fisher’s multiple-range test, p < 0.05).
Several researchers administered 17α-Mt hormone to different fish species and reported increased male ratios with increasing hormone doses and all fish were reported to be male, especially at high doses (Sreenivasa and Prabhadevi, 2018; Olufeagba et al., 2017; Greisy and Gamal, 2012; Shamsuddin et al., 2012; Kirankumar and Pandian, 2002). Tamaru et al. (2009) applied different doses of 17β-ES hormone to swordtail fish and reported the most appropriate dose for feminization as 400 mg kg-1 (97%).
Kirankumar and Pandian (2002) determined different hormone doses to Betta splendens and George and Pandian (1998) to Poecilia sphenops species and reported significant decrease in survival rates with increasing hormone doses. Besides, Greisy and Gamal (2012) found the best survival rate at 60 mg kg-1 17α-Mt and Ajiboye et al. (2015) obtained 100% survival in all treated groups.
In the second phase of study, number of fry produced was investigated. Decreasing fry quantity was observed with increase in hormone doses, however, significant increase in number of fry was observed in the initial months as compared to later months (Table III). Similarly, Greisy and Gamal (2012) observed a reduced testicular development at hormone doses above 40 mg kg-1. Moreover, 17α-Mt hormone reduced sperm production and natural or synthetic estrogen significantly decreased fecundity and fry output of fish (Pandian and Kirankumar, 2003). Again, 17α-Mt or natural androgen was applied to black molly (P. sphenops) at different doses and reduced sperm counts and courting frequencies were observed with increasing hormone doses. Reduced fertilization rate of eggs was found in black molly with estradiol hormone treatment and hormone-treated groups had lower fry output than that of untreated groups (George and Pandian, 1998). Kirankumar and Pandian (2002) applied 17α-Mt hormone to B. splendens and described functional disorders like low number of sperms, sperm intensity, motility and fertilization rate in all male fish. There was no reproductive activity in 17α-Mt-treated O. niloticus (Olufeagba et al., 2017). Moreover, high androgen doses caused sterility in Cyprinus carpio (Das et al., 1990), and salmon and carp (Pandian and Sheela, 1995).
In several experiments, gonadal development recessed, ovulation period was negatively influenced, and fecundity and fry production decreased in fish fed with hormone-supplemented diets. George and Pandian (1996) observed that 17α-Mt and 17β-Es treatments reduced gonado-somatic index (GSI) values and fry output of Cichlasoma nigrofasciatum, and thus, hormone treatments negatively influenced gonadal development, ovulation period and egg production. Similarly, delayed reproduction maturity period and decreased number of eggs with increasing hormone treatments were observed in B. splendens (Pandian and Sheela,1995; Kavumpurath and Pandian, 1992) and P. sphenops (George and Pandian,1995). Contrarily, Pandian and Sheela (1995) found that hormone treatment shortened reproduction maturity period of P. sphenops. Similarly, the best GSI in C. lalia was obtained in 17α-Mt hormone-treated group (Biswas et al., 2014).
Conclusions
Present findings revealed that 17α-Mt hormone was more effective in growth and sex conversion than that of 17β-Es hormone, and survival rate and fry output were negatively influenced by increased doses of both the hormones. Moreover, 17α-Mt hormone was more effective for growth and sex conversation than 17β-Es hormone in electric yellow cichlid. In this study, it was determined that the use of 17α-Mt hormone in this fish will be advantageous, so that the fish can reach the market size in a shorter time and thereby production cost will decrease. It is also concluded that the most important negative effects of hormone applications are low survival rate, low number of fry production and sterilization when used at high doses over long period.
Acknowledgments
The authors are grateful to Sinop University Scientific Research Projects Coordinatorship for providing all facilities for this research (Project No. MYO 1901-16-22).
Statement of conflict of interest
The authors declare that there is no conflict of interest.
References
Ajiboye, O.O., Qari, R., Yakubu, A.F. and Adams, T.E., 2015. Growth performance of Nile tilapia, Oreochromis niloticus L. reared in glass aquaria tanks under different treatments and durations. Int. J. Biol. Biotechnol., 12: 203-209.
Biswas, A., Behera, S., Das, P., Meena, D.K. and Behera, B.K., 2014. Effect of methyl testosterone (17α-mt) on the phenotype, bioindices and gonads of adult male dwarf gourami (Colisa lalia). Emir. J. Fd. Agric., 26: 459-464. https://doi.org/10.9755/ejfa.v26i5.16365
Buttery, P.J. and Sweet, A., 1993. Manipulation of lean deposition in Animals. Anim. Feed Sci. Technol., 45: 97-115. https://doi.org/10.1016/0377-8401(93)90074-T
Carvalh, C.V.A., Passini, P., Costa, W.M.C., Vieira, B.N. and Cerqueira, V.R., 2014. Effect of estradiol-17β on the sex ratio, growth and survival of juvenile common snook (Centropomus undecimalis). Acta Sci. Anim. Sci., 36: 239-245. https://doi.org/10.4025/actascianimsci.v36i3.22839
Das, N.G., Mamun, F.A., Barua, P., Siddique, A.A.M. and Chowdhury, S.N., 2010. Survivality of mono-sex tilapia (Oreochromis niloticus) fry using 17α-methyltestosterone in a commercial hatchery of Chittagong, Bangladesh. J. Aquacul. Feed Sci. Nutri., 2: 16-24. https://doi.org/10.3923/joafsnu.2010.16.24
Ergün, S., Güroy, D., Tekeşoğlu, H., Güroy, B., Çelik, İ., Tekinay, A.A. and Bulut, M., 2010. Optimum dietary protein level for blue streak hap, Labidochromis caeruleus. Turkish J. Fish. aquat. Sci., 10: 27-31. https://doi.org/10.4194/trjfas.2010.0104
Gannam, A.L. and Lovell, R.T., 1991. Growth and bone development in channel catfish (Ictalurus punctatus) fed 17 α-methyltestosterone in production ponds. J. World Aquacul. Soc., 22: 95-100. https://doi.org/10.1111/j.1749-7345.1991.tb00721.x
George, T. and Pandian, T.J., 1995. Production of ZZ females in the female-heterogametic black molly, Poecilia sphenops, by endocrine sex reversal and progeny testing. Aquaculture, 136: 81-90. https://doi.org/10.1016/0044-8486(95)01035-1
George, T. and Pandian, T.J., 1996. Hormonal induction of sex reversal and progeny testing in the zebra cichlid Cichlasoma nigrofasciatum. J. exp. Zool., 275: 374–382. https://doi.org/10.1002/(SICI)1097-010X(19960801)275:5<374::AID-JEZ6>3.0.CO;2-M
George, T. and Pandian, T.J., 1998. Dietary administration of androgens induces sterility in the female-heterogametic black molly, Poecilia sphenops (Cuvier and Valenciennes, 1846). Aquacul. Res., 29: 167-175. https://doi.org/10.1111/j.1365-2109.1998.tb01121.x
Greisy, Z.A. and Gamal, A.E., 2012. Monosex production of tilapia, Oreochromis niloticus using different doses of 17a-methyltestosterone with respect to the degree of sex stability after one year of treatment. Egyptian J. aquat. Res., 38: 59–66. https://doi.org/10.1016/j.ejar.2012.08.005
Kavumpurath, S. and Pandian, T.J., 1992. Effects of induced triploidy on aggressive display in the fighting fish, Betta splendens Regan. Aquacul. Res., 23: 281–290. https://doi.org/10.1111/j.1365-2109.1992.tb00771.x
Kirankumar, S. and Pandian, T.J., 2002. Effect on growth and reproduction of hormone immersed and masculinized fighting fish, Betta splendens. J. exp. Zool., 93: 606–616. https://doi.org/10.1002/jez.10181
Koshio, S., Teshima, S., Kanazawa, A. and Watase, T., 1993. The effect of dietary protein content on growth, digestion efficiency and nitrogen excretion of juvenile kuruma prawns, Penaeus japonicus. Aquaculture, 192: 233-247.
Lewis, D.S.C., 1982. A revision of the genus Labidochromis (Teleostei: Cichlidae) from Lake Malawi. Zool. J. Linn. Soc., 75: 189-262. https://doi.org/10.1111/j.1096-3642.1982.tb01947.x
Morioka, S. and Matsumoto, S., 2008. A note on the hatching period and growth in juvenile Brycinus imberi (Pisces: Alestiidae) in the shallow habitats of Lake Malawi. Afr. J. Ecol., 46: 690-692. https://doi.org/10.1111/j.1365-2028.2007.00908.x
Olufeagba, S.O., Okomoda, V.T. and Adoga, T., 2017. Growth performance and nutrient utilization of hormonal sex-reversed male and mixed sex Oreochromis niloticus under outdoor rearing condition. Int. J. Aquacul., 7: 106-110. https://doi.org/10.5376/ija.2017.07.0016
Pandian, T.J. and Sheela, S.G., 1995. Hormonal induction of sex reversal in fish. Aquaculture, 138: 1-22. https://doi.org/10.1016/0044-8486(95)01075-0
Pandian, T.J. and Kirankumar, S., 2003. Recent advances in hormonal induction of sex reversal in fish. J. appl. Aquacul., 13: 205-230. https://doi.org/10.1300/J028v13n03_02
Rendón von Osten, J., Aguayo-Dionet, G., Dzul-Caamal, R. and Lara Flores, M., 2019. Expression of estrogenic response genes to different concentration of 17ß-estradiol in male mosquitofish (Gambusia yucatana). Iranian J. Fish. Sci., 18: 272-282.
Santandreu, J.A. and Diaz, N.F., 1994. Effects of 17 α-methyltestosterone on growth and nitrogen excretion in masu salmon (Oncorhynchus masou Brevoort). Aquaculture, 124: 321-333. https://doi.org/10.1016/0044-8486(94)90405-7
Shamsuddin, M., Belal Hossain, M., Mofizur Rahman, M., Asadujiaman, M. and Yusuf Ali, M., 2012. Performance of monosex fry production of two Nile tilapia strains: Gift and new gipu. World J. Fish. Mar. Sci., 4: 68-72.
Sreenivasa, V. and Prabhadevi, L., 2018. Optimization of hormone ınduced all male production of Oreochromis niloticus under laboratory condition at Ambo, Ethiopia. Acta Sci. Agric., 2: 16-21.
Tamaru, C.S., Yamasaki, L.S., McGovern-Hopkins, K., Malecha, S. and Vincent, D., 2009. Feminization of commercial swordtails, Xiphophorus helleri, by dietary administration of 17β - Estradiol. Aqua tips. – Center for Tropical and Subtropical Aquaculture. Available at: http://www.ctsa.org/files/notes/Feminizing_Swordtails633806780645168749.pdf
Turan, F., Akyurt, İ., Yıldırım, Y., Çek, Ş. and Turan, C., 2005. β-estradiol’ün zebra çiklit (Cichlasoma nigrofasciatum Günter,1868)’de büyüme üzerine etkisi (in Turkish). FÜ Fen ve Müh. Bil. Der., 17: 335-341.
Vandenberg, G.W. and Moccia, R.D., 1998. Growth performance and carcass composition of rainbow trout Oncorhynchus mykiss (Walbaum), fed the beta agonist ractopamine. Aquacul. Res., 29: 469-479. https://doi.org/10.1111/j.1365-2109.1998.tb01156.x
To share on other social networks, click on any share button. What are these?










