Study of Postharvest Weed Population in Paddy Fields
Study of Postharvest Weed Population in Paddy Fields
Mohammad Javad Golmohammadi1, Hamid Reza Mohammaddoust Chamanabad1*, Bijan Yaghoubi2 and Mostafa Oveisi3
1Department of Agronomy and Plant Breeding, Faculty of Agriculture and Natural Resource University of Mohaghegh Ardabili, Ardabil, Iran; 2Associate Professor. Rice Research Institute, Rasht, Iran; 3Department of Agronomy and Plant Breeding, Faculty of Agriculture University of Tehran; Karaj, Iran.
Abstract | The emergence of weeds in agricultural fields is one of the most challenging problems of humankind since the beginning of agriculture. An important part of the proper management of weeds in the paddy fields is rice postharvest weed management until land preparation for re-cultivation. In order to determine the frequency and relative cover (RC), the samples were collected from 481 fields during the growing season and 255 fields after harvesting rice in Guilan province (Iran) from 2014 to 2016 (from April to March of each year) based on the method of Thomas by throwing 0.5m × 0.5m quadrat to count existing genus and species of weeds. According to the results, the highest frequency was related to weed species of Echinochloa crusgalli (96.9%), Paspalum distichum (78.8%) and Eclipta prostrate (51.8%). Cyperaceae and Poaceae families showed the highest frequency with 13 species (20%) and 8 species (12%), respectively. The RC of E. crusgalli had the highest dominance (51.4%) after rice harvesting in the fields of Guilan province. Of the 5 predominant species with the highest frequency in after harvesting rice, 4 species had the highest frequency and dominance during the growing season of rice.
Received | October 31, 2017; Accepted | January 10, 2018; Published | May 24, 2018
*Correspondence | Hamid Reza Mohammaddoust Chamanabad, Department of Agronomy and Plant Breeding, Faculty of Agriculture and Natural Resource University of Mohaghegh Ardabili, Ardabil, Iran; Email: hr_chamanabad@yahoo.com
Citation | Golmohammadi, M.J., H.R.M. Chamanabad, B. Yaghoubi and M. Oveisi. 2017. Study of postharvest weed population in paddy fields . Sarhad Journal of Agriculture, 34(2): 395-399.
DOI | http://dx.doi.org/10.17582/journal.sja/2018/34.2.395.399
Keywords | Species diversity, Weed frequency, Sustainable management, Rice, Guilan
Introduction
Weeds are a serious challenge in the rice production (Tshewang et al., 2016). Knowing the weeds of a region is an important issue in terms of domestic and foreign quarantine (Aghabeigi, 1992). Due to early harvesting of rice in many regions of Guilan province and favorable conditions for weed growth, weed management in fall is one of the important strategies in reducing seed production. One of the consequences of the critical period hypothesis is that farmers are often unaware of late-season weed control and preventing seed production. The postharvest period is a great time to manage winter annual, biennial and perennial weeds (Rajcan et al., 2004). The late-season weed, seed bank and weed seed bank cover the soil again and perpetuate the weed infestation (Minbashi et al., 2011). The postharvest weed management has a direct impact on the dynamics of weed population and control at the time of crop production. After harvesting the crops, the weeds can germinate and grow, causing weed management problems in the coming season (Rodenburg et al., 2011).
In the absence of proper weed management, including the prevention of seed production after harvesting the rice, many species of weeds such as E. crusgalli, Carex and Polygonum produce large amounts of seeds and buds in non-seasonal crops, increase the seed and bud bank, ultimately become the main source of contamination in subsequent cultivation and hence result in the difficulty in the integrated management of next season in the paddy fields. The prevention can be reintegrated into all aspects of agricultural production and can always be the first line of defense (Minbashi et al., 2011).
The postharvest weed management can reduce weed pressure, manage herbicide-resistant weeds and reduce the costs of agricultural operations for the next growing season. Information on the frequency and composition of seeds in the weed seed bank is very important for identifying the weed dynamics. It is also very useful to apply the seed bank to predict the future weed population (Ball and Miller, 1989).
Knowing the frequency and the RC of rice postharvest weeds can be helpful in managing the species from the persistent and troublesome weeds in the rice-growing season.
Materials and Methods
The present experiment was conducted from 2014 to 2016 for three years in the paddy fields of Guilan province and after harvesting the rice from April to March of each year. The sampling was performed in 16 counties of the province from 481 fields during the growing season and 255 fields after harvesting rice. According to the determination of RC, sampling was done by the 0.25 m2 quadrat and divided into 10 × 10 cm plots in a sampling unit according to W pattern. The weeds were counted in each plot and the genus and species were identified. The number of plots covered by the weed species represented the percentage of weed coverage.
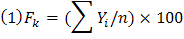
Where;
Fk: Frequency of species; Yi: Presence or absence of species; n: Number of fields visited (Nkoa et al., 2015; Minbashi et al., 2011).
Results and Discussion
The results presented in Table 1 show that E. crusgalli accounted for the highest frequency (96.9%) and RC (51.4%) of weeds after rice harvesting, and 65 weed species belonging to 27 plant families were recorded in the paddy fields after harvest. The Cyperaceae and Poaceae families showed the highest frequency with 13 species (20%) and 8 species (12%), respectively. The identified species (n=65) included monocotyledons (n=29), dicotyledons (n=30), pteridophytes (n=2), ferns (n=3) and algae (n=1). Ten species of plants were found floating and submerged in water. Weed flora composition in rice fields during growing season of 16 counties in Guilan Province includes 66 species belonging to 29 families. 52 out of 65 (80%) weed species identified after rice harvest were the same species recorded in the paddy fields during crop season in the Guilan province. In addition 4 out of 5 main dominant weeds with highest frequency after rice harvest were the same species most prevalent in cropping season. Echinochloa spp is one of the significant weeds in the paddy fields, which has two important and damaging species (E. crus-galli, E. Oryzoides) in the crop season. E. oryzoides is a weed that mimics the rice, has 70% less frequency than in E. crusgalli after harvesting the rice, and often appears during the growing season of the rice (Table 1).
Table 1: Species of postharvest weeds in the paddy fields (from August to March of each year), 2014-2016.
| No | Species | Family | frequency | relative cover (RC) |
| 1 | Echinochloa cruss galli | Poaceae | 98.86 | 51.36 |
| 2 | Paspalum distichum | Poaceae | 78.82 | 30.68 |
| 3 | Eclipta alba | Asteraceae | 51.76 | 7.76 |
| 4 | Algue blue-green | Cyanophyceae | 32.94 | 14.35 |
| 5 | Cyperus difformis | Cyperaceae | 43.14 | 12.08 |
|
6 |
Cyperus serotinus | Cyperaceae | 41.57 | 7.33 |
| 7 | Cyperus strigosus | Cyperaceae | 35.29 | 2.07 |
| 8 | Ammania multiflorum | Lythraceae | 32.16 | 4.60 |
| 9 |
Echinochloa oryzoides |
Graminae | 28.24 | 5.68 |
| 10 | Xanthium strumarium | Asteraceae | 26.27 | 3.33 |
| 11 | Azolla filiculoides | Salviniaceae | 25.88 | 17.08 |
| 12 | Alternathra sessilis | Amaranthaceae | 25.49 | 3.67 |
| 13 | Alisma plantago-aquatica | Alismaceae | 24.31 | 3.89 |
| 14 | Cyperus longus | Cyperaceae | 23.14 | 2.46 |
| 15 | Lemna minor | Lemnaceae | 21.18 | 4.58 |
| 16 | Cyperus esculenthus | Cyperaceae | 20.39 | 9.76 |
| 17 | Polygonum persicaria | Polygonaceae | 19.61 | 1.84 |
| 18 | Alopecurus myosorides |
Poaceae |
18.82 | 3.55 |
|
19 |
Hydrocotyle heteomeria | Apiaceae | 17.65 | 1.40 |
| 20 | Polygonum hydropiper | Polygonaceae | 14.12 | 0.86 |
| 21 | Sagittaria trifolia | Alismaceae | 14.12 | 2.31 |
| 22 | Schoenoplectus juncoides | Juncaceae | 12.16 | 0.67 |
| 23 | Pycreus flavescense | Cyperaceae | 11.76 | 1.04 |
| 24 | Ludwigia epilobioides | Onagraceae | 10.59 | 2.67 |
| 25 | Monochoria vaginalis | Pontederiaceae | 10.59 | 5.87 |
| 26 | Bidens tripartita | Asteraceae | 9.41 | 0.45 |
| 27 | Echinochloa colona | Graminae | 7.84 | 0.77 |
| 28 | Scirpus maritimus | Cyperaceae | 7.84 | 3.37 |
|
29 |
Ammannia gracilis | Lythraceae | 7.45 | 0.91 |
| 30 | Ammannia baccifera | Lythraceae | 7.06 | 0.42 |
| 31 | Ludwigia palustris | Onagraceae | 6.67 | 1.90 |
| 32 | Najas marina | Hydrocharitaceae | 6.67 | 1.22 |
| 33 | Cardamine pensylvanica | Brassicaceae | 6.27 | 0.78 |
| 34 | Cyperus rotundus | Cyperaceae | 6.27 | 0.52 |
| 35 | Pycreus lanceolatus | Cyperaceae | 5.10 | 0.44 |
| 36 | Riccia glauca | Ricciaceae | 5.10 | 0.66 |
| 37 | Rotala indica | Lythraceae | 5.10 | 0.52 |
| 38 | Scirpus mucronatus | Cyperaceae | 5.10 | 0.36 |
|
39 |
Potamogeton nodosus | Potamogetonaceae | 4.71 | 0.80 |
| 40 | Cyperus glomeratus | Cyperaceae | 4.31 | 0.25 |
| 41 | Polygonum hydropiperoides | Polygonaceae | 3.92 | 0.22 |
| 42 | Ranunculus aquatilis | Ranunculaceae | 3.14 | 0.44 |
| 43 | Acalypha australis | Euphorbiaceae | 2.75 | 0.13 |
| 44 | Amaranthus retroflexus | Amaranthaceae | 2.75 | 0.11 |
| 45 | Nasturtium officinale | Brassicaceae | 2.35 | 0.41 |
| 46 | Coix lacryma-jobi | Graminae | 1.96 | 0.09 |
| 47 | Equisetum palustre | Equisetaceae | 1.96 | 0.19 |
| 48 | Najas minor | Hydrocharitaceae | 1.96 | 0.63 |
|
19 |
Rumex crispus | Polygonaceae | 1.96 | 0.08 |
| 50 | Digitaria sanguinalis | Graminae | 1.57 | 0.06 |
| 51 | Abutilon theophrasti | Malvaceae | 1.57 | 0.06 |
| 52 | Marsilea quadrifolia | Marsileaceae | 1.57 | 0.30 |
| 53 | Bergia capensis | Elatinaceae | 0.18 | 0.08 |
| 54 | Cyperus odoratus | Cyperaceae | 1.18 | 0.13 |
| 55 | Equisetum arvensis | Equisetaceae | 0.78 | 0.03 |
| 56 | Solanum nigrum | Solanaceae | 0.78 | 0.03 |
| 57 | Azolla pinnata | Salviniaceae | 0.39 | 0.16 |
| 58 | Butomus umbelatus | Butomaceae | 0.39 | 0.02 |
|
59 |
Fimbristylis miliacea | Cyperaceae | 0.39 | 0.02 |
| 60 | Phragmites australis | Graminae | 0.39 | 0.02 |
| 61 | Physalis peruviana | Solanaceae | 0.39 | 0.08 |
| 62 | Potamogeton crispus | Potamogetonaceae | 0.39 | 0.02 |
| 63 | Sonchus arvensis | Asteraceae | 0.39 | 0.02 |
| 64 | Typha latifolia | Typhaceae | 0.39 | 0.05 |
| 65 | Typha minima | Typhaceae | 0.39 |
0.02 |
The most predominant weeds after harvesting the rice based on the RC were different species of Echinochloa, Cyperus, Azolla and Algue blue-green, as 57.8%, 39.6%, 17.2% and 14.4%, respectively. The relative dominance of different species of Echinochloa and Cyperus during and after the crop season has a greater role in absorbing light due to the height and vertical distribution of the aerial parts and as a result occupies more ecological niches. One of the characteristics of the RC is to measure the weed frequency and biomass. Since the layers of the plants mingle with each other, the total coverage may be higher than 100% (Mohammaddoust chamanabad, 2011).
There is a direct relationship between the frequency of postharvest weed and weed growth during rice production. Accordingly, improper management of the persistent and troublesome rice postharvest weeds doubles the problems, increases the cost of management in the crop season, and affects the quantitative and qualitative yield of the product. Favorable climatic conditions after rice harvest in late August and early September, especially suitable temperatures and good rainfall provides desirable condition for maturing the weeds beneath rice canopy or some weeds that germinate and complete their life cycle after rice crop season. The weeds that germinate later in the growing season have less competition, biomass and seed production compared to those that germinate earlier (Hartzler et al., 2004; Chauhan and Johnson, 2010).
Many of the weeds in the rice-growing areas have annual growth period. The annual weed seeds are capable of germination under unsuitable conditions and able to complete their life cycle from seed to seed during the growing season (Singh et al., 2008). Studies have shown that the release of arable land after harvesting the rice (seen in more than 90% of the paddy fields of Guilan province) leads to re-growth of weeds, which in turn increases seed banks and buds in the soil and begins to grow in the subsequent crop seasons. Our results showed that annual plants such as Echinochloa spp, E. prostrata and C. difformis complete their life cycle after rice harvest and produced a lot of seeds, which can result to contamination of irrigation canals, transfer to other fields by birds and animals, and eventually increase seed bank in next crop season. The main source of weed infestation in the crops is their seed bank, which is emptied due to germination, physiological age, rottenness and hunting. The changes in weed seed bank have a significant impact on the population of weed species (Singh et al., 2017). Table 2 shows the frequency percentage of ten weed species in the next season after harvesting the rice and during crop season. In the growing season, among the narrow-leaf species, the most prevalent and most frequent species (89.8%) was E. cruss galli weeds.
The frequencies of more than 50% of the species belonged to Polygonum and E. oryzoides. Among Carex, the most frequent species was C.difformis (56.5%); as well as C. serotinus and C. esculenthus had a frequency of 34.5% and 31.6%, respectively. Among broad-leaf species, the most frequent species were respectively E. prostrate (49.5%) and S. trifolia (31.6%) and A. plantago-aquatica (28.7%) in the paddy fields. Among the floating and submerged species in water, A. filiculoides, L. minor and A. blue-green had the highest frequency of 34.1%, 28.3% and 17.3%, respectively (Table 2).
Table 2: Frequency of the most important weeds after harvesting and during growing season of rice in the fields of Guilan province.
| No | Species | Postharvest frequency percentage | Growing-season frequency percentage |
| 1 | Echinochloa cruss galli | 96.7 | 89.8 |
| 2 | Paspalum distichum | 78.8 | 78.8 |
| 3 | Eclipta alba | 51.8 | 49.5 |
| 4 | Cyperus difformis | 43.1 | 56.5 |
| 5 | Cyperus serotinus | 41.6 | 34.5 |
| 6 | Cyperus strigosus | 35.3 | 2.5 |
| 7 | Algue blue-green | 33 | 17.3 |
| 8 | Ammania multiflorum | 32.1 | 16.8 |
| 9 | Echinochloa oryzoides | 28.2 | 60.1 |
| 10 | Xanthium strumarium | 26.3 | 13.5 |
| 11 | Azolla filiculoides | 25.9 |
34.1 |
The survival of many plants, especially annual plants, depends on the production of a large number of seeds, and this is the key to solve the resulting problems because the weeds can be controlled by preventing their seed production. Failure to prevent their seed production increases the number of seeds in the soil and thus enhances the severity of crop infestation with weeds. This issue should be considered seriously in weed management programs and weed management should be done before flowering (Mohammaddoust chamanabad, 2011). In this study, the annual plants such as E. crusgalli, E. prostrate and C. difformis produced a lot of seeds after harvesting the rice, thereby contaminating irrigation canals, transferring to other fields by birds and animals, and ultimately increasing the seeds and buds in the field during the new growing season. One of the factors in reducing seed banks is to decrease the entry of weeds into the soil. This will help reduce weed populations in the coming years (Lindstrom and Kokko, 2000). X. strumarium with a frequency of 26% is a species whose seed releasing contributes to the spread of contamination and many prickles on seeds cause a lot of trouble for workers when transplanting in the paddy fields during the crop season. The weeds should be removed before flowering and fruiting due to multiplying contamination by seed production and increasing seed banks in the soil. The weeds produce many seed, may remain in the soil and increase the weed seed bank for the next growing season (Nithya and Ramamoorthy, 2015). The appropriate identification and management of late- and postharvest of the rice can play an important role in reducing the seed bank and buds in the soil, which is one of the important strategies for postharvest weed management (Eubank et al., 2012).
During three years, from 736 rice fields of Guilan province during the growing season and after harvest, until the land preparation stage, sampling was done. The weeds of E. cruss galli, P. distichum, E. prostrate, C. difformis and C. serotinus were highest in rice fields, respectively. E. crussgalli, P. distichum, E. oryziodes, C. difformis, E. prostrate and C. serotinus were the highest during the growing season of rice (Table 2). Considering the favorable temperature and rainfall conditions after rice harvesting and re-growth of weeds, preventing sprouting and weeding of seeds after harvest, greatly prevent the spread of contamination.
Author’s Contribution
All the authors contributed equally for this researcha nd the manuscript.
References
Ball, D.A. and S.D. Miller. 1989. A comparison of techniques for estimation of arable soil seed banks and their relationship to weed flora. Weed Res. 29:365-373. https://doi.org/10.1111/j.1365-3180.1989.tb01307.x
Chauhan, B.S. and Johnson, D.E. 2010. Implications of narrow crop row spacing and delayed Echinochloa colonum and Echinochloa crussgalli emergence for weed growth and crop yield loss in aerobic rice. Field Crop. Res. 117: 177-182. https://doi.org/10.1016/j.fcr.2010.02.014
Eubank, T. and J. Bond. 2012. Post-harvest weed control options. Mississippi State University Cooperative Extension Service. http://www.mississippi-crops.com
Hartzler, R.G., Battles, B.A. and Nordby, D. 2004. Effect of water hemp (Amaranthus rudis) emergence data on growth and fecundity in soybean. Weed Sci. 52: 242-245. https://doi.org/10.1614/WS-03-004R
Lindstrom, J. and Kokko, H. 2000. Cohorts effects and population dynamics. Ecol. Lett. 5: 338-344. https://doi.org/10.1046/j.1461-0248.2002.00317.x
Mohammaddoust Chamanabad, H.R. 2011. An introduction to the Scientific and Practical Principles of Weed Control. Jahad Daneshgahi of Ardabil. 238 pages.
Nithya, J. and Ramamoorthy, D. 2015. Floristic composition and weed diversity in rice fields. Indian J. Weed Sci. 47(4):417-421.
Nkoa R., Owen, M.D.K. and Swanton, C.J. 2015. Weed Abundance, distribution, diversity, and community analyses. Weed Sci. 63(sp1):64-90. https://doi.org/10.1614/WS-D-13-00075.1
Rodenburg, J., H. Meinke and D.E. Johnson. 2011. Challenges for weed management in African rice systems in a changing climate. J. Agric. Sci. 149: 427–435. https://doi.org/10.1017/S0021859611000207
Rodenburg, J. and D.E. Johnson. 2013. 16 managing weeds of rice in Africa. Realizing Africa’s Rice Promise. Crop Prot. 29: 204-212. https://doi.org/10.1079/9781845938123.0204
Rajcan, I., K.J. Chandler and C.J. Swanton. 2004. Red-far-red ratio of reflected light: a hypothesis of why early-season weed control is important in corn. Weed Sci. 52:774-778. https://doi.org/10.1614/WS-03-158R
Singh, A., G.P. Sharma and A.S. Raghuban shi. 2008. Dynamics of the functional groups in the weed flora of dryland and irrigated agro ecosystems in the Gangetic plains of India. Weed Biol. Manage. 8: 250–259. https://doi.org/10.1111/j.1445-6664.2008.00305.x
Singh, M., Bhullar, M.S. and Chauhan, B.S. 2017. Relative time of weed and crop emergence is crucial for managing weed seed production. Crop prot.99: 33-38. https://doi.org/10.1016/j.cropro.2017.05.013
Tshewang, S., Sindel, B.M., Ghimiray, M. and Chauhan, B.S. 2016. Weed management challenges in rice (Oryza sativa L.) for food security in Bhutan. Crop prot. 90: 117-124. https://doi.org/10.1016/j.cropro.2016.08.031
To share on other social networks, click on any share button. What are these?








