Sourcing Eco-Epidemiological Field Parameters to Describe a Slum Household-Based Pathogenic Leptospira Population Dynamics Simulation Model in Kenya: A Pseudo-Longitudinal Study Protocol
Sourcing Eco-Epidemiological Field Parameters to Describe a Slum Household-Based Pathogenic Leptospira Population Dynamics Simulation Model in Kenya: A Pseudo-Longitudinal Study Protocol
John Gachohi1*, Simon Karanja1, Salome Wanyoike2, Nduhiu Gitahi3, Salome Bukachi4, Kenneth Ngure1
1School of Public Health, College of Health Sciences, Jomo Kenyatta University of Agriculture and Technology, P.O. Box 62000, 00200 Nairobi; 2Ministry of Agriculture, Livestock and Fisheries, State Department of Livestock, Directorate of Veterinary Services, P.O. Kangemi Post Code 00625 Nairobi; 3Department of Public Health, Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Nairobi, P.O. Box 29053 Nairobi; 4Institute of Anthropology, Gender and African Studies, University of Nairobi, P.O. Box 30197, 00100, Nairobi.
Abstract | Leptospirosis is a neglected zoonosis and an emerging urban slum health problem. There is a scarcity of population-based information on the distribution of the infection and transmission determinants for urban Leptospirosis in developing countries. This study aims to source dynamic ecological and epidemiological field parameters to describe a slum household-based pathogenic Leptospira population dynamics simulation model. The model will be analyzed to assess the role of slum-based biotic (human and animal) and abiotic (soil, water and weather) habitats in Leptospira propagation. Influential habitats would serve as key targets for interventions. To achieve this aim, we have designed a pseudo-longitudinal survey to be undertaken for two years in Kibera slums in Nairobi city Kenya. Human and animal blood and urine samples will be collected at the household level whereas soil and water samples will be collected at the community level. Attempts will be made to isolate Leptospira bacteria from urine, soil and water samples. Antibody detection in animal and human serum samples will utilize Microscopic Agglutination Test (MAT). Study outcomes will include (i) geo-referencing of adverse environmental attributes that support Leptospira growth, (ii) animal and human seasonal public health burden of Leptospirosis, (iii) seasonal variation in the distribution of Leptospira determinants in a slum setting, and (iv) a simulation model based on an ecological metapopulation framework to track Leptospira bacterial population dynamics in biotic and abiotic habitats in a slum setting. This is the first study in Kenya to describe the role of temporal dynamics of multihost community and adverse environmental attributes in Leptospira propagation in a slum setting. The study is strengthened by the pseudo-longitudinal design whose findings are expected to inform public health decision-making. Going forward, it will be possible to parameterize complex realistic Leptospira transmission models for understanding urban Leptospirosis dynamics.
Editor | Muhammad Abubakar, National Veterinary Laboratories, Park Road, Islamabad, Pakistan.
Received | October 15, 2016; Accepted | October 17, 2016; Published | October 20, 2016
*Correspondence | John Gachohi, School of Public Health, College of Health Sciences, Jomo Kenyatta University of Agriculture and Technology, P.O. Box 62000, 00200 Nairobi; Email: mwangigachohi@gmail.com; jgachohi@jkuat.ac.ke
Citation | Gachohi, J., S. Karanja, S. Wanyoike, N. Gitahi, S. Bukachi and K. Ngure. 2016. Sourcing eco-epidemiological field parameters to describe a slum household-based pathogenic Leptospira population dynamics simulation model in Kenya: A pseudo-longitudinal study protocol. Veterinary Sciences: Research and Reviews, 2(3): 66-75.
DOI | http://dx.doi.org/10.17582/journal.vsrr/2016.2.3.66.75
Introduction
Leptospirosis is an emerging neglected zoonosis caused by infection with different serovars of bacteria of the genus Leptospira (Bharti et al., 2013). The bacteria are found in a wide range of animal reservoir hosts including rats and other peri-domestic rodents, livestock and domestic pets worldwide (Hartskeerl et al., 2011). Humans and animals acquire the infection through direct contact with affected animals or indirectly through certain environmental indices (food, water and/or soil) contaminated by animal urine as pathogenic leptospires live in the kidneys of their natural hosts (de Vries et al., 2014). The introduction and persistence of the bacteria in an environment are highly dependent on a set of interacting dynamic variables – increased rainfall that leads to generalized damp conditions, animal ecology, socio-ecology, socio-economic, and environmental sanitation (Reis et al., 2008; Lau et al., 2010). Human infection is characterized by a non-specific clinical syndrome that ranges from mild or asymptomatic febrile infections to severe, life-threatening illnesses (de Vries et al., 2014). As a neglected infectious disease, the actual global burden of Leptospirosis is unknown though estimates are becoming available (Costa et al., 2015). Globally, 1.03 million cases (95% confidence interval 434,000–1,750,000) and 58,900 deaths (95% CI 23,800–95,900) due to leptospirosis were estimated (Costa et al., 2015). The Global Burden of Disease (GBD) and World Health Organization (WHO) regions that had the highest estimates of leptospirosis morbidity and mortality included East Sub-Saharan Africa (where Kenya is located) among other regions (Costa et al., 2015). Previously, the global burden had been hypothesized to be similar to that of dengue fever (Hartskeerl et al., 2011) whose global Disability Adjusted Life Years (DALYs) lost has been estimated at 700,000 per year (Hotez et al., 2009).
System dynamics modelling, implemented in a mathematical framework, allows for the conceptualization, understanding and communication of the behaviour and processes of a particular system. In a disease system context, mathematical models are critical as they can greatly advance the understanding of pathogen transmission ecology (Rudge et al., 2013) and subsequently support the decision-making process through public health policy in its control (Lee et al., 2010). In particular, models can be used predictively by taking into account the known behaviour and attempt to determine the future behaviour (Githeko et al., 2001). More importantly, models can be used experimentally to test, in a rapid manner, a wide range of preventative and control strategies and outbreak scenarios without any of the risks associated with testing during a real outbreak (Gachohi et al., 2016). A major challenge in the evaluation of the burden of zoonoses, including leptospirosis, is the lack of models and their parameters that may enable the understanding about the disease ecology (Lloyd-Smith et al., 2009). Current leptospirosis models involve aspects of the spread of the infection in Thailand (Triampo et al., 2007), the infection dynamics of rodents in Tanzania to determine risk in humans (Holt et al., 2006) and responses to some environmental drivers (Codeco et al., 2008). This concern provides the motivation for the current project.
This project aims to bridge this gap by developing a slum household-based Leptospira population dynamics model in a metapopulation framework accounting for biotic and abiotic environments in a slum settlement in Kenya. The project concentrates on a slum settlement as a study area for two reasons. Firstly, leptospirosis is an emerging urban health problem in slum settlements worldwide (Reis et al., 2008). Although, the term “slum” is applied to a great variety of settlement types, the United Nations (UN) describes a slum household as a group of individuals living under the same roof where the following are either absent or deficient: access to safe water, access to sanitation infrastructure, satisfactory structural quality of housing and house space (UN-Habitat, 2003). In South America, and Brazil in particular, emerging evidence on the risk factors of Leptospira infection include these slum conditions in addition to favourable climatic, environmental and socio-economic factors. The latter include seasonal heavy rainfall and residence in attendant flood-risk sites with open sewers and proximity to accumulated refuse and presence of rodents (Sarkar et al., 2002; Maciel et al., 2008; Reis et al., 2008). The Kenyan slums are particularly characterized by seasonal flooding due to poor drainage systems in cities. Secondly, a recent cross-sectional survey of Leptospira infection in rodents in the Kibera slum settlement in Nairobi, Kenya, reported a high prevalence (18%) of pathogenic leptospires in rodents and frequent contact between humans and the rodents (Halliday et al., 2013). These observations point to possible increased risk of the infection among slum dwellers in Kenya.
In order to fully describe the model, we are conducting four seasonally repeated cross-sectional surveys to source field ecological and epidemiological parameters in the Kibera slum settlement in Nairobi city in Kenya. Although the burden of leptospirosis is not known in the area, previous studies in Egypt and Tanzania have reported that the infection can account for a considerable proportion of febrile illnesses (Ismail et al., 2006; Biggs et al., 2011). In the Kibera slums, acute febrile illness is frequently reported with an average of 2.7 occurrences per person per year for children less than 5 years of age and 0.58 occurrences for persons older than 5 years of age based on household visit data (Feikin et al., 2011).
The ultimate goal of this study is to empower the urban slum people through mitigated adverse public health burdens arising from leptospirosis. The specific objectives include 1) to identify and characterize seasonal Leptospira bacterial putative exposure in high frequency animal-human contact sites in slum household environments, 2) to determine seasonal public health burden of leptospirosis in both slum livestock and dwellers, 3) to determine the effects of water, sanitation and hygiene and socioeconomic and behavioural characteristics of the slum dwellers on Leptospira bacterial transmission in humans, and 4) to develop and analyze Leptospira regression and population dynamics simulation models in a slum household setting and apply them as tools to generate policy-relevant inference in support of informed decision-making of targeting resources by (i) assessing the contribution that biotic and abiotic slum-based habitats play in the observed Leptospira bacteria dynamics, (ii) identify vulnerable slum-based habitats to focus interventions to effectively reduce the Leptospira bacterial loads and (iii) assessing effectiveness of relative impacts of preventative and control strategies targeting these habitats.
Materials and Methods
Study Site
Kibera slum is situated on a 2.5 square kilometres and is approximately five kilometres away from the Nairobi city centre. The slum is a densely populated with over 800,000 inhabitants. There are more than 30,000 house structures in Kibera slums which are mud-walled and thatched with corrugated iron sheets. A household in the slums comprises of five to seven members on average. The study site is an area in the slum where a previous study on Leptospira infection in rodents was conducted (Halliday et al., 2013) (Figure 1). The site has an area of approximately 0.38km2 with a human population density of approximately 77,000 persons/km2 (Feikin et al., 2011). The site experiences long rains between March and May and short rains between October and November. The site is characterized by poor quality housing, poor sanitation, and limited access to clean water (Halliday et al., 2013). The site constitutes the Soweto administrative area (Halliday et al., 2013).
Study Design
Four repeated seasonally-based cross-sectional surveys (pseudo-longitudinal study) will be carried out, i.e. at the end of selected and specific (prolonged) dry and wet seasons for two years. In a calendar year, this will coincide with March, June, September and December.
Sample Size
This will be a community-based survey whose sampling unit will be a plot. During a reconnaissance visit by the research team in May 2016, it was established that the study area is divided into four zones labelled A, B, C and D (Figure 1). Each zone has numerous plots. A plot is a cluster of 5-20 households owned by a landlord who collects rent from the households. Zone A will be excluded from the survey as it has already undergone substantial slum upgrading to constitute high rise residential buildings that do not fit the category of a slum. Zone B has 1050 plots, Zone C has 699 plots and Zone D has 990 plots. Assuming a mean average of 10 households per plot, the number of households in the study site is estimated to be 27,390 and approximately 136,950 dwellers. We use the following formula to calculate the number of households for sampling (Cochran, 1963):
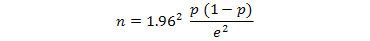
Where;
n is the sample size, 1.96 is the z value for the desired confidence level (95%), p is an estimate of the probable prevalence of Leptospira antibodies, and e is the level of precision (tolerable error) (distance of the sample estimate in either direction from the true population proportion considered acceptable). The overall prevalence of Leptospira antibodies in a slum setting in Brazil was 15.4% (Reis et al., 2008) and we use the latter to calculate the human sample size. A 5% tolerable error is assumed. This gives a sample size of 201 households. For livestock sample size, the overall national prevalence of Leptospira antibodies in livestock was 16% in Kenya (Wanyangu et al., 1993). Assuming a 5% tolerable error, this gives a sample size of 207 animals. To harmonize the sample sizes, we will sample 210 households and 210 animals. Therefore, approximately a total of 21 plots will be sampled.
Sampling
The 21 plots will be proportionately allocated to the three zones as follows: the sample size of each zone will be proportionate to the plots population size of the zone. Zonal plot sample sizes are determined by the following equation:
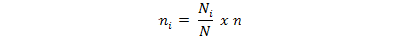
Where;
ni is the sample size for a zone i, Ni is the plot population size for zone i, N is total number of plots in the study site, and n is the calculated sample size.
Thus 9 plots, 6 plots and 8 plots will be respectively sampled in zones B, C and D. To obtain the plots for each zone, a map of a zone will be constructed and the center identified. Four transects corresponding to the four directional cardinal points will generated. One transect out of the four will be randomly selected. The first plot falling on the path will become the first randomly selected plot. Subsequently, the selected path will be used for systematic random sampling of plots. A transect will be assumed to have a quarter of the total number of plots in a zone. To compute the skipping interval, the total number of plots in a zone will be divided by four and the figure obtained divided by the sample size of plots in that zone. The skipping interval in each zone is computed and illustrated in Table 1. Cluster sampling of households, i.e. all households within each selected plot will be included in the sample. This exercise will be undertaken until the respective sample size of households and animals in a given zone is accomplished. This task will be implemented during each of the four repeated seasonally-based cross sectional surveys.
Table 1: Skipping interval computed from approximate number of plots per zone, approximate number of plots per transect and zonal plot sample sizes
| Zone | Approximate number of plots per zone | Approximate number of plots per transect | Zonal plot sample size | Zonal skipping interval |
| B | 1050 | 263 | 9 | 33 |
| C | 699 | 175 | 6 | 21 |
| D | 990 | 248 | 8 | 30 |
Animals reared in the area include pigs and goats and sheep to a lesser extent. Household owned dogs are plentiful. This study will sample pigs and dogs owing to their roaming nature in the slums in search of food and, therefore, could be potential sources of introducing and spreading Leptospira bacteria in the environment. The 210 animal samples will be shared in a ratio of 1:1 between the two species.
The specific research activities under each objective are listed below:
Objective 1
To identify and characterize seasonal Leptospira bacterial putative exposure in high frequency animal-human contact sites in slum household environments.
Rationale: Inadequate sanitation infrastructure in slum informal settlements has been reported as key environmental sources of Leptospira transmission.
Tasks under this objective: 1) Select 210 households for human sampling and 210 animals of the following species–pigs and or dogs from previously selected households or additional households. Geospatially and ecologically characterize households owning a pig and or a dog. 2) Spatial proximity of the identified households to specific adverse environmental exposures will be undertaken. The exposures will include open sewers, accumulated refuse and garbage sites. We intend to use simple straight line/Euclidean distances to estimate proximity to the environmental exposures.
Objective 2
To determine seasonal public health burden of leptospirosis in slum dwellers and animals.
Rationale: Identification of prevention and control measures of urban leptospirosis has been hindered by the scarcity of population-based evidence on Leptospira transmission determinants. This objective is designed to estimate seasonally-based seroprevalence of Leptospira antibodies in humans and animals in the urban slum setting.
Tasks under this objective: 1) The field team accomplishing this objective will be a veterinarian, an animal health technician, a medical laboratory technician and a local community health worker. 2) From the identified households, the household head will be consented and a blood sample collected from a randomly selected individual in the household. The individual will be picked randomly from among those present in the home at the time of sampling upon consent. Similarly, either a pig or a dog will be picked randomly from among livestock and pets present in the home at the time of sampling. 3) From the randomly identified human and animal, the field team will obtain a blood sample. In each cross-sectional survey, we intend to, therefore, collect 210 human blood samples and 210 animal blood samples. The blood samples will be processed for serum preparation. In addition, we intend to collect urine from humans and animals in a household that presents the following – occurrence of a clinical description in humans consistent with the WHO case definition of Leptospirosis in the preceding 3 months to the field study to a maximum of 20 samples each for human and animals. In case we will miss a household with a clinical description, we will sample every tenth household to give an arbitrary sample size of 25 per season. For 4 seasons, we will collect 100 human and 100 animal urine samples.
Serology: Microscopic Agglutination Test (MAT) serological testing of previous exposure will be carried out. A panel of five reference strains (WHO Collaborative Laboratory for Leptospirosis, Royal Tropical Institute, Holland) will be used. These will include L. interrogans serovars Autumnalis, Canicola and Copenhageni, L. borgspetersenii serovar Ballum, and L. kirschneri serovar Grippotyphosa. Previous Leptospira sequence studies in the selected study site identified L. interrogans and L. kirschneri in the rodent population (Halliday et al., 2013). Screening will be performed with serum dilutions of 1:25, 1:50 and 1:100. When agglutination is observed at a dilution of 1:100, the sample will be titrated to determine the highest titer.
Leptospira isolation from urine: Human and animal urine samples will be passed through sterile micro-pore size membrane filter. Five to 10 drops of the samples will be inoculated into the modified semisolid Ellinghausen McCullough Johnson Harris (EMJH) medium. The media will be incorporated with 5-fluorouracil (5-FU) to minimize bacterial contamination. The enrichment cultures will be incubated aerobically at room temperature for 30 days and examined for the presence of Leptospira by dark-field microscope. If the bacteria are not detectable after 30 days of incubation, the sample will be considered to be negative. Presence of at least four Leptospira present in a microscopic field is considered as a positive result. The pathogenic leptospires will be differentiated from saprophytic (free-living) leptospires by 8-Azaguanine Inhibition Test. The leptospiral isolates together with reference controls for pathogenic and saprophytic (L. patoc) will be maintained in liquid media containing 225μg/ml concentration of 8-Azaguanine. The cultures will be incubated at 30ºC and observed under dark-field microscope for growth rate by comparing them to the controls made up of the same media but without 8-azaguanine. Pathogenic leptospires are not expected to grow in 8-azaguanine media compared to saprophytic leptospires which are resistant to the compound.
Objective 3
To determine the effects of water, sanitation and hygiene and socioeconomic and behavioural characteristics of the slum dwellers on Leptospira bacterial transmission in humans.
Rationale: Certain societal infrastructures link with certain individual socioeconomic and behavioural determinants in slum settlements to become key environmental sources of Leptospira transmission.
Tasks under this objective: 1) The field team accomplishing this objective will be two research assistants. 2) Geo-codify data on flooding, sewerage systems, accumulated refuse and garbage sites near selected households. 3) A reconnaissance visit to Kibera by the study team identified water tanks, tap water and rainwater as the major water sources in Kibera. For this reason, we will sample openly draining sewer water and pools from 25 sites close to households reporting occurrence of a clinical description in humans consistent with the WHO case definition of Leptospirosis in the preceding 3 months to the study. In case we will miss a clinical description, we will sample every tenth household to give an arbitrary sample size of 25 per season. For 4 seasons, we will collect 100 water samples. 4) Soil samples will be collected from damp sites inside (n = 10) or the immediate outside (n = 10) and in footwear (n = 5) of households reporting occurrence of a clinical description in humans consistent with the WHO case definition of Leptospirosis in the preceding 3 months to the study. In case we will miss a clinical description, we will sample every tenth household. An additional 25 sites will include frequented social places in the community such as school play fields, class rooms, churches, social halls, eating places etc. For 4 seasons, we will collect 200 samples.
Leptospira isolation in soil and water samples: a) Water samples will be processed similar to urine samples. b) The soil samples will be passed through sterile micro-pore size membrane filter. Soil samples will be placed in a sterile 50 ml falcon tubes and soaked in Leptospire-free distilled water and mixed by vigorous shaking. Upon settling for 5 to 7 minutes, the samples will be filtered using 0.22-μm membranes in a similar fashion as water and urine samples. c) Isolation will follow the same protocol as for urine samples
Household survey: A standardized questionnaire will be administered at the household level to obtain information on demographic and socioeconomic indicators. This will include employment and occupation. In addition, we will seek information on exposures to sources of water, sanitation and hygiene including access to piped water, reported sightings of rats, rearing of chickens and presence of uncollected garbage or flooding around the household.
Collection of seasonal weather data: Collect weather data from Kenyan meteorological department in regard to rainfall following each of the cross-sectional surveys.
Objective 4
To develop and analyze Leptospira regression and population dynamics simulation models in a slum household.
Rationale: The multihost ecology of zoonoses, such as Leptospirosis, leads to complex dynamics. Analytical tools, such as mathematical modeling can be useful for advancing ecological understanding of multihost ecology of zoonoses, development of effective control policies and research agenda.
Tasks under this objective: 1) Build regression models to identify the determinants for infection in the urban slum setting. The models will explore the relationship between the presence of Leptospira antibodies identified in humans and animals and attributes of water, sanitation and hygiene and indicators of socioeconomic status. 2) Develop a system dynamics model based on an ecological metapopulation framework to track Leptospira population dynamics inside the slum dwellers and livestock and in the slum environment. 3) Established methods will be followed based on an ecological metapopulation framework (Hanski and Gilpin, 1997). A metapopulation is defined in ecology as a population of subpopulations interconnected by immigration (Hanski and Gilpin, 1997) as shown in Figure 2. a) Metapopulation biology is concerned with the dynamic consequences of migration among local populations and the conditions of persistence in each subpopulation. This framework will allow for the study of Leptospira bacterial populations capable of moving between spatially segregated habitats including livestock, humans, water and the physical environment. The framework is illustrated in Figure 3. b) Model description: A system of coupled ordinary differential equations will be parameterized to represent population dynamics for Leptospira bacteria while allowing for transfer between habitats. These parameters will include the rate of movement between habitat i and habitat j. If Leptospira growth in a habitat is assumed, as for example in livestock and humans, an additional intra-habitat growth rate will be included. Others include non–density dependent mortality (population-level decay) and density-dependent mortality through, for example, a logistic function dependent on the habitat’s carrying capacity. All habitats receive the bacteria from and contaminate all other states. An exception is rodents, livestock and dogs that are assumed not to acquire the bacteria from humans (NB: Rodent data will be sourced from the literature). Rainfall data will be used as a forcing function that influences bacterial growth in the environment.
Data Management
All data will be gathered via an electronic data gathering system using Open Data Kit (ODK) on handheld tablets. Each visited household will have a unique identifying study code. In this manner, samples and documents will not bear any information that may identify the household or study participants. Electronic data entry tablets will be password protected and will be accessible only by authorized users. Papers will be used in the event of technical difficulties which, currently, are unforeseen. A central database is being developed to ensure secure and confidential data management.
Ethical Approval and Ethical Considerations
Ethical approval for this study has been obtained from the Kenyatta University’s Ethical Review Committee (KUERC) (KU/R/COMM/51/797). Written informed consent will be sought from study participants at the time of recruitment for both participation as well as storage and future use of human, veterinary and environmental samples. The consent will be sought from study participants following provision of information on the purpose, methods, risks and benefits of the study so as to make a voluntary and un-coerced decision on whether to participate. The probability of harm or injury (physical and/or psychological) occurring as a result of participation in the study will be negligent as the risk will not be greater than that attached to routine medical practice. Participants will be assured that in all forms of dissemination, they will not be identified by name, household, administrative area or any other identifier. The study participants will be assured that all information generated in this study will remain confidential. The right to service of study participants will be considered, i.e. if at any point during household visits the study team encounters a case that needs urgent medical attention, the study team will advise on the appropriate hospital that can attend to the case.
Discussion
We have described a repeated cross-sectional (pseudo-longitudinal) study intended to obtain data necessary for parameterizing a model that tracks the movement of pathogenic Leptospira bacteria between humans, animals and the physical environment in a slum setting. Repeated cross-sectional data consist of observations on individual survey samples selected from the same context at different time-points and can, therefore, be assumed as clustered within time-points (Firebaugh, 1997). This study will intensively obtain an estimated seasonally-based 840 human sera samples, 840 animal sera samples, 100 human urine samples, 100 animal urine samples, 100 water samples and 200 soil samples from the slum households and community within two years. We aim not only to estimate the seasonal-based prevalence of Leptospira antibodies in humans and animals but also isolating circulating Leptospira in the Kibera slums from the biotic (human and animal urine) and abiotic (soil and water) samples. Samples, data and findings from this work are hoped to be a valuable resource to local and international medical and public health communities in advancing the ecological understanding of distribution of the zoonosis and their determinants in a slum setting.
Study Strengths
Accurate burden and occurrence of urban Leptospirosis is difficult to characterize in a one snapshot study. This is because of the dynamic and complex multihost ecology-environment nexus of the infection (Gaynor et al., 2007; Kawaguchi et al., 2008). In this manner, the most important strength of this study is the repeated cross-sectional nature of the study. This design will be used to determine temporal variation in the distribution of determinants of the disease and changes in exposure frequency in the slum setting. The data derived from these surveys will be appropriate to examine for dynamic associations between disease occurrence and the determinants to generate hypotheses. By determining these dynamic associations, the findings are expected to inform public health decision-making, such as when to focus surveillance and prevention for disease outbreaks. An additional strength of this study is the bacterial population dynamics modelling that integrates a broader set of Leptospira life history in humans, animals and the environment. Integration with the social scientific discipline is noted to add to the strengths of the study. The study will contribute immensely to the emerging ‘One Health’ ideology which integrates preventive disease research from the fields of human medicine, veterinary medicine, sociology and environmental science in Africa.
Limitations
Our research design does not permit the investigation of changes in the same individual over time. Statistically, in repeated cross-sectional analyses, standard errors are large whenever large variations between households (not necessarily the same households in each survey) exist, and the power to detect statistically significant differences in the estimates can be weakened. However, this disadvantage is overcome by the fact that repeated cross-sectional surveys tend to give more precise estimates of prevalence and, therefore, more precise estimates of change over time. This is because they generally have larger sample sizes. Yet, attempts will be made to build strong rapport between the study team and study participants in order to sample the same households in subsequent surveys as much as possible. In this manner, we will have a proportion of observations that are measured on the same households. Consequently, it will be possible to focus on changes occurring within households and make population inferences that are not as sensitive to between household variations.
Acknowledgments
The authors would like to acknowledge the Jomo Kenyatta University of Agriculture and Technology, Research, Production and Extension for funding this study.
Conflict of Interests
Authors declare that they have no competing interests.
Authors’ Contributions
Designed the study: JG, SK. Social and behavioural study support: SB, KN. Microbiology and laboratory support: NG, SW. Coordination of study at JKUAT and study sites: JG, SK, SW, NG, SB, KN. Wrote the manuscript: JG. All authors read and approved the final manuscript.
References
- • Bharti, A.R., Nally, J.E., Ricaldi, J.N., Matthias, M.A., Diaz, M.M., Lovett, M.A., Levett P.N., Gilman, R.H., Willig, M.R., Gotuzzo, E. and Vinetz, J.M. Peru-United States Leptospirosis Consortium. Leptospirosis: A zoonotic disease of global importance. Lancet Infect Diseases, 2003; 3: 757–771. https://doi.org/10.1016/S1473-3099(03)00830-2
- • Biggs, H.M., Bui, D.M., Galloway, R.L., Stoddard, R.A., Shadomy, S.V., Morrissey, A.B., Bartlett, J.A., Onyango, J.J., Maro, V.P., Kinabo, G.D., Saganda, W. and Crump, J.A. Leptospirosis among hospitalized febrile patients in northern Tanzania. American Journal of Tropical Medicine and Hygiene, 2011; 85: 275–281. https://doi.org/10.4269/ajtmh.2011.11-0176
- • Cochran, W.G. 1963. Sampling techniques, 2nd Edition. John Wiley and Sons, Inc., New York.
- • Codeco, C.T., Lele, S., Pascual, M., Bouma, M. and Ko, A.I. A stochastic model for ecological systems with strong nonlinear response to environmental drivers: application to two water-borne diseases. Journal of the Royal Society, 2008; 5: 247–252. https://doi.org/10.1098/rsif.2007.1135
- • Costa, F., Hagan, J.E., Calcagno, J., Kane M., Torgerson, P., Martinez-Silveira, M.S., Stein, C., Abela-Ridder, B. and Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Neglected Tropical Diseases, 2015; 9(9): e0003898. https://doi.org/10.1371/journal.pntd.0003898
- • de Vries, S.G., Visser, B.J., Nagel, I.M., Goris, M.G.A., Hartskeerl, R.A. and Grobusch, M.P. Leptospirosis in Sub-Saharan Africa: a systematic review. International Journal of Infectious Diseases, 2014; 28: 47–64. https://doi.org/10.1016/j.ijid.2014.06.013
- • Feikin, D.R., Olack, B., Bigogo, G.M., Audi, A., Cosmas, L., Aura, B., Burke, H., Njenga, M.K., Williamson, J. and Breiman, R.F. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One, 2011; 6: e16085. https://doi.org/10.1371/journal.pone.0016085
- • Firebaugh, G. 1997. Analyzing repeated surveys. Thousand Oaks, Sage, CA. https://doi.org/10.4135/9781412983396
- • Gachohi, J.M., Njenga, M.K., Kitala, P., Bett, B. Modelling vaccination strategies against Rift Valley fever in livestock in Kenya. PLoS Negl Trop Dis 10(12): e0005049. https://doi:10.1371/journal. pntd.000504
- • Gaynor, K., Katz, A.R., Park, S.Y., Nakata, M., Clark, T.A. and Effler, P.V. Leptospirosis on Oahu: an outbreak associated with flooding of a university campus. American Journal of Tropical Medicine and Hygiene, 2007; 76: 882–885.
- • Githeko, A.K. and Ndegwa, W. Predicting malaria epidemics in the Kenyan highlands using climate data: a tool for decision makers. Global Change and Human Health, 2001; 2: 54-63. https://doi.org/10.1023/A:1011943131643
- • Hanski, I.A. and Gilpin, M.E. 1997. Metapopulation biology: ecology, genetics, and evolution. Academic Press, San Diego, CA.
- • Hartskeerl, R.A., Collares-Pereira, M. and Ellis, W.A. Emergence, control and re-emerging leptospirosis: dynamics of infection in the changing world. Clinical Microbiology and Infection, 2011; 17: 494–501. https://doi.org/10.1111/j.1469-0691.2011.03474.x
- • Halliday, J.E.B., Knobel, D.L., Allan, K.J., de Bronsvoort C.B.M., Handel, I., Agwanda, B., Cutler, S.J., Olack, B., Ahmed, A., Hartskeerl, R.A., Njenga, M.K., Cleaveland, S. and Breiman, R.F. Urban leptospirosis in africa: A cross-sectional survey of Leptospira infection in rodents in the Kibera urban settlement, Nairobi, Kenya. American Journal of Tropical Medicine and Hygiene, 2013; 89: 1095–1102. https://doi.org/10.4269/ajtmh.13-0415
- • Holt, J., Davis, S. and Leirs, H. A model of leptospirosis infection in an African rodent to determine risk to humans: seasonal fluctuations and the impact of rodent control. Acta Tropica, 2006, 99: 218–225. https://doi.org/10.1016/j.actatropica.2006.08.003
- • Hotez, P.J., Fenwick, A., Savioli, L. and Molyneux, D.H. Rescuing the bottom billion through control of neglected tropical diseases. Lancet, 2009; 373: 1570–1575. https://doi.org/10.1016/S0140-6736(09)60233-6
- • Ismail, T.F., Wasfy, M.O., Abdul-Rahman, B., Murray, C.K., Hospenthal, D.R., Abdel-Fadeel, M., Abdel-Maksoud, M., Samir, A., Hatem, M.E., Klena, J., Pimentel, G., El-Sayed, N. and Hajjeh, R. Retrospective sero-survey of leptospirosis among patients with acute febrile illness and hepatitis in Egypt. American Journal of Tropical Medicine and Hygiene, 2006; 75: 1085–1089.
- • Kawaguchi, L., Sengkeopraseuth, B., Tsuyuoka, R., Koizumi, N., Akashi, H., Vongphrachanh, P., Watanabe, H. and Aoyama, A. Sero-prevalence of leptospirosis and risk factor analysis in flood-prone rural areas in Lao PDR. American Journal of Tropical Medicine and Hygiene, 2008; 78: 957-961.
- • Lau, C.L., Smythe, L.D., Craig, S.B. and Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: fuelling the fire? Transactions of the Royal Society of Tropical Medicine and Hygiene, 2010; 104: 631–638. https://doi.org/10.1016/j.trstmh.2010.07.002
- • Lee, B.Y., Brown, S.T., Korch, G.W., Cooley, P.C., Zimmerman, R.K., Wheaton, W.D., Zimmer, S.M., Grefenstette, J.J., Bailey, R.R., Assi, T.M. and Burke, D.S. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine, 2010; 28: 4875–4879. https://doi.org/10.1016/j.vaccine.2010.05.002
- • Lloyd-Smith, J.O., George, D., Pepin, K.M., Pitzer, V.E., Pulliam, J.R.C., Dobson, A.P., Hudson, P.J. and Grenfell, B.T. Epidemic dynamics at the human-animal interface. Science, 2009; 326: 1362–1367. https://doi.org/10.1126/science.1177345
- • Maciel, E.A.P., de Carvalho, A.L.F., Nascimento, S.F., de Matos, R.B., Gouveia, E.L., Reis, M.G. and Ko, A.I. Household transmission of Leptospira infection in urban slum communities. PLoS Neglected Tropical Diseases, 2008; 2(1): e154. https://doi.org/10.1371/journal.pntd.0000154
- • Reis, R.B., Ribeiro, G.S., Felzemburgh, R.D.M., Santana, F.S., Mohr, S., Melendez, A.X.T.O., Queiroz, A, Santos, A.C., Ravines, R.R., Tassinari, W.S., Carvalho, M.S., Reis, M.G. and Ko, A.I. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Neglected Tropical Diseases, 2008; 2(4): e228. https://doi.org/10.1371/journal.pntd.0000228
- • Rudge, J.W., Webster, J.P., Lu, D.B., Wang, T.P., Fang, G.R. and Basáñez, M.G. Identifying host species driving transmission of schistosomiasis japonica, a multihost parasite system, in China. Proceedings of the National Academy of Sciences of the USA, 2013; 110: 11457–11462. https://doi.org/10.1073/pnas.1221509110
- • Sarkar, U., Nascimento, S.F., Barbosa, R., Martins, R., Nuevo, H., Kalafanos, I., Grunsteinm, I., Flannery, B., Dias, J., Riley, L.W., Reis, M.G. and Ko, I. Population-based case-control investigation of risk factors for Leptospirosis during an urban epidemic. American Journal of Tropical Medicine and Hygiene, 2002, 66:605–610.
- • Triampo, W., Baowan, D., Tang, I.M., Nuttavut, N., Wong-Ekkabut, J. and Doungchawee, G. A simple deterministic model for the spread of Leptospirosis in Thailand. International Journal of Biological Sciences, 2007; 2:22–26.
- • UN-Habitat (United Nations-Habitat) 2003. Slums of the World: The face of urban poverty in the new millennium? Monitoring the Millennium Development Goal, Target 11-World-wide Slum Dweller Estimation Working Paper. pp. 94.
- • Wanyangu, S.W., Angolio, A. and Wamwayi, H.M. Further serological evidence of caprine leptospirosis in Kenya. East African Agricultural and Forestry Journal, 1993; 59:137–143.
To share on other social networks, click on any share button. What are these?






