Pharmacognostic Evaluation of Oxalis pes-caprae L. (Family Oxalidaceae)
Pharmacognostic Evaluation of Oxalis pes-caprae L. (Family Oxalidaceae)
Syeda Naila, Muhammad Ibrar, Fazal Hadi* and Muhammad Nauman Khan
Department of Botany, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan
Abstract | The present study was conducted on Oxalis pes-caprae L. (Family Oxalidaceae) to analyze its morphological and pharmacognostic features. The results revealed that it leaves are compound, perennial and summer deciduous with upper epidermis followed by mesophyll region with vascular bundle and the lower epidermis. The leaf features showed that palisade ratio was (7.35), vein islets number (9), and vein termination number (9.1), stomata number (47) and stomatal index were (42.72). Transverse section of the root showed outer epidermal cells, cortex and pericycle, xylem and phloem cells. Powder drug microscopy, ash analysis, extractive values and fluorescence analysis were also studied. Preliminary phytochemical screenings of different extracts of Oxalis pes-caprae L. showed the presence of carbohydrates, phenols, flavonoids, tannins, saponin, fats and oils.
Received | January 29, 2018; Accepted | December 06, 2019; Published | January 12, 2020
*Correspondence | Fazal Hadi, Department of Botany, University of Peshawar, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: [email protected]
Citation | Naila, S., M. Ibrar, F. Hadi and M.N. Khan. 2020. Pharmacognostic evaluation of Oxalis pes-caprae L. (Family oxalidaceae). Sarhad Journal of Agriculture, 36(1): 70-80.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.1.70.80
Keywords | Oxalis pes-caprae, Oxalidaceae, Pharmacognosy, Physicochemical analysis
Introduction
The word pharmacognosy was initially used between 1811 and 1815 and was formerly concerned to information of medicine constituents (Evans, 1996). Pharmacognosy can also be defined as practical discipline which conducts biochemical, biologic and economical characteristics of natural drug and constituent (Tyler et al., 1988). In current years, investigation in crude drugs has experienced a vast attention (Verpoorte, 2000). Biochemical, chemical, physical and biological characteristics of drugs and exploration of novel drugs from natural sources are studied in field of pharmacognosy (Balunas and Kinghorn, 2005). People have consumed plants as source of food, shelter, clothing, medicine, cosmetics, and for searching release from adversity of life; in a continuous strive to get better their quality of life (Betoni et al., 2006). The pharmacognostic parameters are important for authentication, identity and quality of crude drugs. Pharmacognostic standards are set up to ensure quality and to lessen chances of adulteration and contamination during collection and preparation of drugs (Bhattacharya and Kamaruz, 2009). By stepwise pharmacognostic studies, the procedure of standardization can be accomplished (Ozarkar, 2005). It facilitates in recognition and confirmation of the plant material and ensures quality. Morphological, anatomical and biochemical evaluations are simple pharmacognostic methods employed in standardization of plant material (Anon, 1998). Organoleptic assessment can be made by sense organs. It gives the easiest and fastest way to determine identity, quality and purity of drugs. Morphological observation of plants through naked eye or magnifying lens is called the macroscopic study (Shah et al., 2012). Different ash values are used to detect the pureness and quality of sandy, earthy or exhausted materials in crude drug. Total ash value shows occurrence of several earthy impurities like carbonates, oxalates and silicates. Water soluble ash is conducted to evaluate presence of exhausted drugs in genuine one, while the acid insoluble ash (comprises of mainly silica) point out adulteration with earthy material (Aron and Mehalingam, 2012).
Oxalis pes-caprae L. is a 12-24 cm tall, perennial, herbaceous plant with bulbiferous and underground stem belonging to family Oxalidaceae (Nasir, 1971). Because of pleasant sour taste, it is also called as “sourgrass” or soursob. High content of oxalic acid is cause of its sourness. The rhizomes and tubers of plants have the ability to increase aeration, water infiltration and decrease the growth of other weeds but is responsible for lessening yield of crops. It is also a weed of pastures, orchards, vineyards, vegetables, fallows, gardens, roadsides and disturbed areas (Peirce, 1997). The present study was carried out to analyze the pharmacognostic profile of Oxalis pes-caprae L. Similar studies were made by Simpson and Morris (2014), Maria et al. (2015), Tripoda (2015), Girma and Wurko (2016) and Vasco-correa and Zapata (2017) on various aspects of medicinal plants and pharmacognosy.
Materials and Methods
Fresh plant material of Oxalis pes-caprae L. at flowering stage were collected, cleaned, washed and garbled. For macroscopic and microscopic characteristics, fresh samples were used and remaining collection was dried in shade at about 25±3 0C and powdered with electric grinder. For protecting powder from molds, insects attack and moisture, these were stored in air tight bottles. Morphological observations of the leaf, stem, root and flower of O. pes-caprae were examined by organoleptic methods. Leaf observation included nature, size, color, odor, taste, phyllotaxis, insertion, leaf base, stipule, petiole, lamina (venation, texture, composition, apex, base, margin, general outline, surface and incision). Stem was studied for habit, shape, odour, taste, colour, surface, duration, branches and position. Roots were studied for branches, color, odor, taste, size, shape, surface, duration and rootlets. Flowers were studied for type, petals, sepals, filament and flowering period. Bulb was studied for color, shape, taste and size (Wallis, 1985; Evans, 2002). With the help of sharp razor thin transverse sections of selected plants parts were made with large numbers. Thin sections were selected for study to mount on glass slide and studied under Nikon microscope fitted with camera (Chaffey, 2001).
Many pieces of leaves were cut into 1 sq mm pieces and boiled in chloral hydrate solution. The cleared pieces were mounted on slides and observed under Labomed microscope provided with overhead digital IVU 3100 camera. Beginning at margin all vein islets were counted in the square and on boundary of square. Vein-terminations were counted in square. 10 readings were taken in continuous squares and vein-islets were counted forgetting exact and standard values (Trease and Evans, 2002).
Palisade ratio
Palisade cell ratio is the average number of palisade parenchyma cells present beneath each upper epidermal cell (Trease and Evans, 2002).
Procedure: Small pieces of leaf (2mm square) were cut between midrib and margin, boiled in 100% concentrated chloral hydrate solution. Cleared pieces were mounted and observed under Labomed microscope provided with overhead digital IVU 3100 camera. Many sets of each 4 upper epidermal cells were examined. Then palisade cells lying beneath each set of four epidermal cells were focused with fine adjustment and counted. Palisade ratio was then obtained by dividing the number by four. In order to obtain accurate values, ten readings were taken from various fragments (Trease and Evans, 2002).
Stomatal number and stomatal index
Number of stomata per square mm of epidermis from both surfaces of leaf is known as stomatal number. Percentage measure of stomatal density per unit area taken by epidermal cells is stomatal index (Trease and Evans, 2002).
Procedure: To prevent leaves from dessication dipped them in water. Epidermis from both surfaces of leaf was removed with help of sharp razor blade. Separated epidermis was placed on slide and examined under microscope for following parameter (Choudhary and Imran, 1997).
- • Absence and presence of stomata.
- • Number of stomata per square mm
- • Number of epidermal cells
- • Stomatal index was calculated by using formula
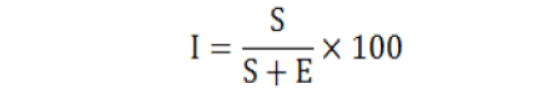
Where;
I= Stomatal index; S= No of stomata per unit area; E= No of epidermal cells per unit area.
Powder drug microscopy
The whole plant powdered material was treated with warm 200% concentrated chloral hydrate solution. Drops from this was taken on slide, covered by cover slip and examined for different structures under microscope fitted with camera (Wallis, 2005).
Physicochemical study
Powder drug fluorescence study: The fluorescence analysis of the whole dry plants powders treated with various reagents (50% HCl, ethanol, methanol, NH3 solution, 10% FeCL3 solution, acetic acid, 50% HNO3 and iodine solution) was conducted by examining samples under visible and Ultra Violet light of short and long wave lengths (Evans, 2002).
Ash analysis: Total ash examination
Methodology: Silica crucible having flat bottom was completely washed and for drying kept in oven at 70 0C for 30 minutes. Crucible was then heated, tarred, cooled in desiccators and weighted (W1). 4gm of plant powder of O. pes-caprae was shifted to crucible and then heated on Benson burner. After this shifted to muffle furnace in which temperature was slowly rised to 550 0C. The process continued up to several hours to burnt carbon in drug becoming white in color. Then crucible having ash was shifted to desiccators cooled and weighted (W2). Calculation of total and percent ash was done by following method (Wallis, 1985).
Weight of empty crucible = W1
Weight of empty crucible + ash = W2
Total ash (mg/g) = W2 – W1 of sample= mg/gram
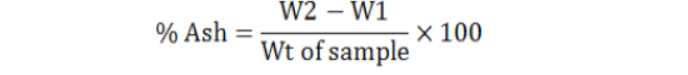
Acid insoluble ash analysis
The drugs containing different amount of Calcium oxalate crystal and adulterated with lime, sand, clay and lime are examined by this analysis (Wallis, 1985; Rangari, 2002)
Methodology: To the crucible having known quantity of ash, added 25ml HCl, covered by watch glass and boiled it for five minutes. Watch glass was washed with 5ml of hot distilled water and added the liquid to crucible. On an ash less filter paper, insoluble matter was collected, washed the filter paper with hot water to neutralize the filtrate. Then shifted this filter paper to its crucible, dried on hotplate and heated at 500 0C in furnace. Then shifted the crucible to desiccator for cooling (30 minutes), weighted without delay. Calculated the acid-insoluble ash by following method (Wallis, 1985).
Weight of empty crucible = W1
Weight of empty crucible + sample = W2
Acid insoluble ash = W2 –W1 = X mg/gm
Water-soluble ash analysis
For detection of water exhausted material, this test is conducted (Jarald and Jarald, 2007).
Methodology: To pre-weighted crucible (W1) having known quantity of ash (W2), added 25ml water, covered by watch glass and boiled it for five minutes. Watch glass was washed with 5ml of hot distilled water and added the liquid to crucible. On an ash less filter paper, insoluble matter was collected, and then shifted this filter paper to its crucible, dried on hot plate and heated at 500 0C for 15minutes in furnace. Then shifted the crucible to desiccator for cooling (30 minutes), weighted without delay (W3), from which insoluble matter was calculated (X mg/gm). The amount of water-soluble ash (Y mg/gm) was then calculated by subtracting this amount from total ash (Wallis, 1985).
Weight of empty crucible = W1
Weight of empty crucible + sample = W3
Weight of sample = W3 - W1 = X mg/gm
Water soluble ash = W2 – X = Y mg/gm
Extractive value
Extractive value is the amount of the extractable or soluble material of a drug in a specific solvent.
Methodology: By following methodology of Ansari et al. (2006) extractive values were investigated. In airtight bottles, 500ml of different solvents and 100gm powder of plants were mixed and kept for 7 days with occasional shaking. After this extracts were filtered and in rotary evaporators filtrates were dried. Calculation for percent extractive values were done by following formula:
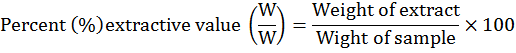
Phytochemical screening
For detection of alkaloids, carbohydrates, phytosterols, proteins, flavinoids, triterpenoids, saponins, phenols, tannins, fat and oil in various extracts of O. pes-caprae, different qualitative phytochemical tests were carried out.
Tests for carbohydrates
Molisch test: To extract solutions, some drops of alcoholic α-napthol were added. To sides of test tube, 0.2 ml of concentrated H2SO4 was added. Appearance of purple to violet color ring at junction, indicate the existence of carbohydrates (Evans, 2002).
Benedict test: Some drops of Benedict reagent added to extract solutions and boiled on water bath. Reddish brown precipitate indicated existence of reducing sugar (Evans, 2002).
Fehling test: Some drops of sample solutions was treated with equal volumes of Fehling’s A and Fehling’s B and boiled. Brick red precipitate of cuprous oxide formation showed the existence of reducing sugar (Evans, 2002).
Detection of protein
Millon test: To extracts, 2ml of Millon’s reagent was added. The appearance of white precipitate in solution which turned red by heating confirms the presence of protein (Evans, 2002).
Ninhydrine tests: 0.2% solution of Ninhydrine was boiled with extract solutions in test tube. Proteins were indicated by violet color appearance (Kumar and Kiladi, 2009).
Tests for alkaloid
Mayer test: Some drops of Mayer reagent were added to extract solution. The appearance of creamy white precipitates confirms the presence of alkaloids (Khandelwal, 2004).
Wagner test: Some drops of Wagner reagent were added to extract. Formation of reddish brown precipitate indicates existence of alkaloids (Khandelwal, 2004).
Hager test: To extract solution some drops of Hager reagent were added. Appearance of yellow precipitates confirmed existence of alkaloids (Khandelwal, 2004).
Tests for phytosterol and triterpenoid
Libermann-Burchard test: Some drops of acetic anhydride were added to extracts, boiled, cooled and then from sides of test tube, concentrated H2SO4 was added. At junction of 2 layers if brown ring made, green color of upper layer confirmed existence of sterol and deep red color of lower layer confirmed existence of triterpenoid (Harborne, 1998).
Detection of phenol
Ferric chlorides test: In test tube, 2ml extract solution and 2ml FeCl3 were mixed. Phenols indicated by production of deep bluish solution (Dahiru et al., 2006).
Detection of flavonoid
Alkali reagents test: NaOH solution was mixed with extract solutions. Yellow to red precipitates indicates existence of flavonoid (Kokate, 1994).
Tannins
Ferric chlorides test: Extract solutions and FeCl3 were mixed. Blue green colouration confirmed existence of tannins (Kokate, 1994).
Alkali reagents test: Extract solutions and NaOH solution were mixed. Yellow to red precipitates production in short time confirmed existence of tannins (Kokate, 1994)
Dtection of saponins
Frothing test: In test tube, 5ml aqeous solution of extracts was shaken. Saponin indicated by production of froth for some time (Chaouche et al., 2011).
Detection of fats and oil
Spot test: Extracts were individually pressed between 2 filter papers. Existence of fixed oil indicated by formation of oil stains on papers (Gomathi, 2010).
Results and Discussion
In pharmacognostic studies Oxalis pes-caprae was evaluated for identification and standardization.
Macroscopic (Morphological) features
Macroscopic parameters such as shape, surface, size and organoleptic parameters such as taste, odor, color etc of leaf, stem, root, flower and bulb of O. pes-caprae was conducted for identification and standardization.
Leaf: Macroscopic study of leaf depicted that the leaf of O. pes-caprae is palmate compound, perennial summer deciduous. Upper surface is medium green in color with purple spots and lower surface is paler. Leaflets are cuneate-obcordate in shape with deeply notched apex, obcordate base entire margin and 5-15 mm in length, 10-30 mm in breath with reticulate venations (Figure 1a, b, c). More characteristics of leaf are listed in (Table 1).
Stem: Macroscopical characteristics of stem revealed that it is hollow; pale brown in color. Subterranean, bulbous in nature with slight sour taste. Some other features of the stem are listed in Table 1.
Root: Morphological feature of O. pes-caprae root showed that it is cylindrical in shape, white to brown in color and 2-5 cm long with no odor and sour in taste (Figure 2).
Flower: Inflorescence is of subumbellate type, petals 15-20 mm long yellow in color, sepals 5-7 mm long lanceolate. Flowering period is from January to April (Table 1).
Table 1: Various morphological features of leaf, stem, root, flower and bulb of Oxalis pes-caprae.
| Part | Feature | Oxalis pes-caprae |
|
Leaf |
Nature | Perennial summer deciduous, bulbiferous plant |
| Size | 5-15mm long, 10-30mm broad | |
| Color | Medium green and often purple zoned or spotted on upper surface, paler beneath | |
| Odor | Odorless | |
| Taste | Sour | |
| Phyllotaxis | No definite phyllotaxy | |
| Insertion | Radical | |
| Stipule | Exstipulate | |
| Petiole | 4-25cm long | |
| Venation | Reticulate | |
| Apex | Deeply notched | |
| Base | Obcordate | |
| Surface | Upper glabrous, lower sparsely apressed- hairy | |
| Texture | Soft | |
| Margin | Entire | |
| Shape | Leaflet broadly cuneate-obcordate and sub-bilobed or rarely triangular in outline and deeply lobed | |
| Type | Compound | |
| Fracture of dry leaf | Brittle | |
|
Stem |
Habit | Subterranean, bulbous, aerial stem absent |
| Shape | Hollow | |
| Odor | Odorless | |
| Taste | Sour | |
| Color | Pale brown | |
| Surface | Rough | |
| Duration | Perennial | |
| Branches | Present | |
| Position | Underground | |
|
Root |
Branches | Filiform branches present |
| Color | White to brown | |
| Odor | Odorless | |
| Taste | Sour | |
| Size | 2-5 cm long | |
| Shape | Cylindrical | |
| Surface | Rough | |
| Duration | Perennial | |
| Rootlets | Present | |
|
Inflorescence |
Type | Subumbellate |
| Petals | 15-20 mm long, yellow | |
| Sepals | 5-7 mm long, lanceolate | |
| Filament | Glabrous | |
| Flowering period | January- April | |
|
Bulb |
Color | White to brown |
| Shape | Tear shape | |
| Taste | Sour | |
| Size | Small |
Bulb: Bulbs are small, white to brown in color, tear shape and sour in taste (Table 1).
Similar studies were also conducted by many of the researchers e.g. Reddy and Lakshmi (2012) carried out macroscopic studies of Oxalis corniculata. Wahab (2013) analyzed physico-chemical and pharmacognostical properties for Averrhoa carambola. Shailajan et al. (2014) worked on the leaf bud of Ficus benghalensis L. Sonkar et al. (2014) carried out morphological investigation on leaves of Momordica dioica Roxb. These studies confirm that pharmacognostic investigation of crude drug is chief requisite for identification and authentification, determining quality and purity of drugs.
Microscopic study
Anatomical study: In the present study anatomical study of O. pes-caprae was carried out as given below.
Leaf anatomy: Transverse section of O. pes-caprae leaf depicted that it is comprised of upper epidermis followed by mesophyll region having vascular bundle in midrib region and lower epidermis. Upper epidermis is single layered consist of wavy shaped cells. Collenchymatous cells are existing below upper epidermis. Leaf has unicellular covering trichomes. Shape of palisade parenchyma cells is cylindrical which arranged in single layer. Spongy parenchyma cells are round shaped cells arranged with intercellular spaces. Vascular bundle is consisting of xylem and phloem present in midrib region (Figure 3 and 5).
Root anatomy: Tranverse section of the root showed that it is composed of single layered of outer epidermal cells. The epidermal cells are slightly wavy. Inner the epidermis is a two layered endodermal cells which are round in shape and closely packed. After endodermis is a cortex which is many layered, round and compact cells. Inner to cortex pericycle exists. Pericycle consists of xylem and phloem cells and pith. Xylem cells are arranged in star shape and in the arms of xylem, phloem cells are present which small rounded shape are. In the centre of pericycle, pith cells are present (Figure 4).
Zalke et al. (2014) examined microscopy of roots of Combretum albidum G. Don. Gatade et al. (2015) investigated the microscopical characters of leaves of Blumea eriantha DC. Shende et al. (2015) worked on leaves of Strobilanthes sessilis for microscopic characters. Pulate et al. (2015) conducted pharmacognostic studies on Canthium parviflorum. Observations of the present microscopical study of O. pes-caprae are in line with the result of these workers.
Quantitative microscopy of leaf surface
The vein islets number for O. pes-caprae was in the range of 5-14 with an average of 9. The vein termination was in the range of 7-15 with an average of 9.1 (Table 2, Figure 6). The upper epidermal cells were slightly wavy shape and closely packed having no space between them. Stomata were present only on lower epidermis (hypostomatic). Epidermal cells of lower epidermis were rounded and loosely arranged having space between them. Both unicellular and multicellular trichomes were present (Figure 6c). Stomatal number is in range of 32-60 with the average of 47 (Table 2). Stomatal index ranged from 40-45 with average number 42.5 (Table 2). The palisade ratio was in the range of 5-10 with the average of 7.35 (Table 2, Figure 6).
Key: a, b, c, unicellular and multicellular trichomes; d, calcium oxalate crystals; e, columnar palisade cells; f, epidermis with palisade cells; g, fragment of epidermal cells; h, fragment of ground tissue; I, fragment of leave showing vein islet; j, needle shape crystals; k, parenchyma cells; l, phloem fibers; m, starch grains; n, vessels with annular thickening; o, xylary trachieds; p, xylem vessels.
Same work has done by researchers such as Nagar et al. (2013), Ukwubilec (2013) and Deepthy et al. (2015) examined Tephrosia collina var lanuginocarpa, Ficus abutilifolia. and Tamilnadia uliginosa. These studies strengthen our present study. These parameters are very important for establishing pharmacognostic parameters of medicinal plants, because these help in the identification and evaluation of medicinal drugs.
Table 2: Leaf surface study of Oxalis pes-caprae.
| Parameters | Oxalis pes-caprae | |
| Range | Mean | |
| Stomatal frequency | 32-60 | 47 |
| Stomatal index | 40-45 | 42.5 |
| Vein islet No. | 5-14 | 9 |
| Vein termination No. | 7-15 | 9.1 |
| Palisade ratio | 5-10 | 7.35 |
Powder drug microscopy
Powder microscopy of the whole plant powder of O. pes-caprae showed unicellular and multicellular trichomes, calcium oxalate crystals, columnar palisade cells, epidermis with palisade cells, fragment of epidermal cells, fragment of ground tissue, fragment of leave showing vein islet, needle shape crystals, parenchyma cells, phloem fibers, starch grains, vessels with annular thickening, xylary trachieds and xylem vessels (Figure 6). Shailajan et al. (2014), Zalke et al. (2014), Gatade et al. (2015) and Pulate et al. (2015) carried out powder drug microscopy of Ficus benghalensis L., Combretum albidum G. Don., Blumea eriantha DC. and Canthium parviflorum Roxb. All these studies supported our present findings.
Physicochemical study
In the present study powder drug of O. pes-caprae was examined for the following physicochemical studies.
Powder drug fluorescence study: Through UV light, crude drugs are evaluated qualitatively. It is an essential parameter for pharmacognostic assessment of crude drug (Zhao et al., 2011). In the present study fluorescence study of O. pes-caprae was conducted by treating with various chemical reagents and observing under visible and both UV-254 and UV-310 for characteristic fluorescence. Untreated powder was light green in visible light, light brown in both UV-256 and UV-310. The results for other reagents are shown in Table 3.
Fluorescence study performed by many researchers. Gatade et al. (2015), Sonkar et al. (2014) and Zalke et al. (2014) examined Blumea eriantha, Momorica dioica and Combretum albidum for fluorescence study under visible and both UV-254 and UV-310. Our current results are in line with these findings.
Table 3: UV and visible florescence study of powder of Oxalis pes-caprae with different chemical reagents.
|
S. No |
Treatment | Oxalis pes-caprae | ||
| Visible light | UV 256 | UV 310 | ||
| 1. | Untreated | Light green | Light brown | Light brown |
| 2. | 50% HCl | Blackish green | Grayish brown | Grayish black |
| 3. |
50%HNO3 |
Whitish orange | Light orange | Orange |
| 4. |
NH3 Sol. |
Yellowish green | Yellowish brown | Dark brown |
| 5. |
FeCl3 Sol. |
Light green | Yellow | Light brown |
| 6. | Iodine Sol. | Light brown | Brown | Grayish brown |
| 7. | Ethanol | Dark brown | Dark brown | Blackish brown |
| 8. | Methanol | Dark brown | Brown | Blackish brown |
| 9. | Acetic acid | Reddish black | Dark brown | Purplish brown |
Ash analysis: In the current work ash analysis (total ash, acid insoluble and water soluble ash) were conducted for O. pes-caprae. Values of this study are listed in Table 4. Total ash, acid insoluble ash and water-soluble ash was 28%, 0.17w/w and 19.27w/w respectively.
Table 4: Ash analysis of Oxalis pe-caprae powder.
| Powder | Total Ash % | Acid insoluble ash (mg/g) | Water soluble ash (mg/g) |
| Oxalis pes-caprae | 28 | 0.17 | 19.27 |
Similar studies were conducted by various researchers like Nagar et al. (2013), Shailajan et al. (2014) and Sonkar et al. (2014) examined Tephrosia collina, Ficus benghalensis and Momordica dioica. The findings of these workers confirming our current studies.
Extractive value: For determination of crude drugs, extractive values are helpful. These values also provide information about nature of chemical constituents existing in drug. Solvents also have the ability to dissociate the quantities of desired substances. Percent extractive values of O. pes-caprae were analyzed using different solvents including ethanol, methanol, acetone, n-hexane and chloroform. Highest extractive value was obtained in methanol (20.6 %), followed by chloroform (8.43 %), ethanol (5.31 %), acetone (2.76 %) and n- Hexane (1.75 %) (Table 5). Many studies have been done on this subject like Zalke et al. (2014), Gatade et al. (2015), Pulate et al. (2015) and Shende et al. (2015) determined Combretum albidum G. Don., Blumea eriantha DC., Canthium parviflorum and Strobilanthes sessilis for extractive value. This proposes that extractive values estimation is an essential mean for assessment of drugs and for finding a range of intentional and unintentional adulterations in drug. It also gives us clue for the selection of best solvent for extraction.
Phytochemical screening: Qualitative preliminary phytochemical screenings of O. pes-caprae in ethanolic, methanolic, acetone, n-hexane and chloroform extracts were tested with various chemical reagents to notice phytoconstituents existing in each extract. All extracts exhibited occurrence of carbohydrates, phenols, flavonoids and tannins. Saponin were present in ethanolic, methanolic, acetone and chloroform extract while absent in n-hexane extract. Fat and oil were present in ethanolic and n-hexane extract. Proteins, alkaloids, phytosterols and triterpenoids were absent in all the extracts (Table 6).
Table 5: Percent extractive value of Oxalis pes-caprae with various solvents.
| Plant | Solvent | % Extracts |
| Oxalis pes-caprae | Ethanol | 5.31 |
| Methanol | 20.6 | |
| Acetone | 2.76 | |
| n-Hexane | 1.75 | |
| Chloroform | 8.43 |
Similar studies were done by various workers. Nagar et al. (2013) analyzed stem, leaf and root of Tephrosia collina for phytochemical screening. Sonkar et al. (2014) tested the extracts of leaves of Momordica dioica Roxb for preliminary phytochemical analysis. Our study is in line with these workers.
Table 6: Qualitative chemical analysis of extracts of Oxalis pes-caprae.
| S. No | Constituents | Test name | Ethanolic extract | Methanolic extract | Acetone extract | n-Hexane extract | Chlorofrom extract |
| 1. | Carbohydrate | Molish test | + | + | + | + | + |
| Benedict test | + | + | + | + | + | ||
| Fehling test | + | + | + | + | + | ||
| 2. | Protein | Millon test | - | - | - | - | - |
| Ninhydrin test | - | - | - | - | - | ||
| 3. | Alkaloids | Mayer test | - | - | - | - | - |
| Wagner test | - | - | - | - | - | ||
| Hager test | - | - | - | - | - | ||
| 4. | Phytosterols and triterpenoids | Libermann-Burchard test | - | - | - | - | - |
| 5. | Phenol | Ferric chloride test | + | + | + | + | + |
| 6. | Flavonoids | Alkali test | + | + | + | + | + |
| 7. | Tannins | Ferric chloride test | + | + | + | + | + |
| Alkali test | + | + | + | + | + | ||
| 8. | Saponins | Frothing test | + | + | + | - | + |
| 9. | Fat and oil | Spot test | + | - | - | + | - |
Novelty Statement
The present research work will contribute a lot in understanding the Macro- and Microscopic feature of Oxalis pes-caprae and different organic molecules, which indicates the importance of this plant in the fields of pharmacognosy and medicines.
Author’s Contribution
Syeda Naila: Carried out the reserach work.
Muhammad Ibrar: Supervised the overall research and helped in experiments.
Fazal Hadi: Designed the researcha nd wrote the manuscript.
Muhammad Nauman Khan: Collected and compiled the data and helped in writing the manuscript.
References
Anonymous. 1998. Macroscopic and microscopic examination: Quality Control Methods for Medicinal Plant Materials, WHO, Geneva.
Aron, A. and P. Mehalingam. 2012. Pharmacognostic and preliminary phytochemical investigation on leaf of Aeschynomene indica L. J. Biosci. Res., 3(1): 100-105.
Ansari, M.M., J. Ahmad and S.H. Ansari. 2006. Pharmacognostic evaluation of the stem bark of Balanites aegyptica Delile-Hingot. Hamdard Medicus., 50(1): 82-94.
Balunas, M.J. and A.D. Kinghorn. 2005. Drug discovery from medicinal plants. Life Sci., 78 (5): 431–441. https://doi.org/10.1016/j.lfs.2005.09.012
Betoni, J.E.C., R.P. Mantovani, L.N. Barbosa, L.C.D. Stasi and A.F.J. Mem. 2006. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Inst. Oswaldo Cruz., 101 (4): 387-390. https://doi.org/10.1590/S0074-02762006000400007
Bhattacharya, S. and M.Z. Kamaruz. 2009. Pharmacognostical evaluation of Zanthoxylum nitidum Bark. Int. J. Pharm. Tech. Res., 1 (2): 292-298.
Chaffey, N.J. 2001. Putting plant anatomy in its place. Trends Plant Sci., 6: 439-440. https://doi.org/10.1016/S1360-1385(01)02050-7
Chaouche, T., F. Haddouchi and F.A. Bekkara. 2011. Phytochemical study of roots and leaves of the plant Echium pycnanthum Pomel. Der. Pharmacia Lett., 3 (2): 1-4.
Chaudhary, N. and M. Imran. 1997. Comparative study of stomata in some members of Malvaceae and Euphorbiaceae. Pak. J. Plant Sci., 3 (1): 33-45.
Dahiru, D., J.A. Onubiyi and H.A. Umaru. 2006. Phytochemical screening and antiulcerogenic effect of Moringa oleifera aqueous leaf extract. Afr. J. Trad., 3 (3): 70-75. https://doi.org/10.4314/ajtcam.v3i3.31167
Deepthy, M.M.J., P.M. Radhamany and A.V. Jalaj. 2015. Pharmacognostic Studies on Leaf of Tamilnadia uliginosa (Retz.) Tirveng. and Sastre (Rubiaceae). Int. J. Adv. Res., 3 (12): 118 – 123.
Evans, W.C. 1996. Pharmacognosy, 14th edn. W.B. Saunders, London.
Evans, W.C. 2002. Pharmacognosy. 15th ed. English language book, society baillere tindall, Oxford University Press. Harcourt Publ. Limitted. 312-545.
Gatade, A.T., A.K.M. Azmina, G.A. Rupali and D.J. Gandhi. 2015. Pharmacognostic studies and HPTLC fingerprinting of Blumea eriantha (Asteraceae) leaves. Int. J. Pharm. Sci., 7 (8): 97-100.
Girma, E. and T. Worku. 2016. Extraction and characterization of pectin from selected fruit peel waste. Int. J. Sci. Res., 6: 447-454.
Gomathi, V., B. Jayakar, R. Kothai and G. Ramakrishnan. 2010. Antidiabetic activity of leaves of Spinacia oleracea Linn. in Alloxan induced diabetic rats. J. Chem. Pharm. Res., 2 (4): 266-274.
Harnborne, J.B. 1998. Phytochemical methods (3rd Edn). Chapman and Hall, New York.
Jarald, E.E. and S.E. Jarald. 2007. A text book of pharmacognosy and phytochemistry (1st Edn). CBS publishers and distributors, New Delhi, India. pp. 6.
Khandelwal, K.R. 2004. Practical pharmacognosy. Techniques and experiments (12th Edn). Nirali Prakashan, Punne India. pp. 157.
Kokate, C.K. 1994. Practical pharmacognosy. 4th Edn. Vallabh Prakashan, New Delhi. pp. 120- 156.
Kumar, B.J.R. and C.P. Kiladi. 2009. Preliminary phytochemical and pharmacognostic studies of Holoptelea integrifolia Roxb. Ethnobot. Leaflets., 13: 1222-1231.
Maria, B., V. Gama, C. Silvab, L.M. Silvab and A. Abudc. 2015. Extraction and characterization of pectin from citric waste. Chem. Eng. Trans. 44: 259-264.
Nagar, S.P. and K. Bhambra. 2013. Preliminary phytochemical and pharmacognostic studies on Tephrosia collina var Lanuginocarpa Vs Sharma- An endangered species of Western India Gangadeep. Int. J. Pharm. Pharm. Sci., 5 (4): 99-103.
Nasir, Y. 1971. Flora of Pakistan. Department of botany, Univ. Karachi: Karachi. 4: 1-3.
Ozarkar, K.R. 2005. Studies on anti-inflammatory effects of two herbs Cissus quadrangularis Linn. and Valeriana wallichi DC. Using mouse model. Ph. D. thesis, Univ. Mumbai, Mumbai.
Pulate, P.V., W.N. Aziz and V.R. Deshmukh. 2015. Phytochemical, ethnomedicinal and anatomical study of Canthium parviflorum. World J. Pharm. Pharm. Sci., 4 (11): 1464-1482.
Peirce, J.R. 1997. The Biology of Australian Weeds. Oxalis pes-caprae L. Plant Prot. Q., 12: 110-119.
Rangari, V.D. 2002. Pharmacognosy and phytochemistry. Volume-1. Career publications, Maharashtra, India. pp.1.
Reddy, K.Y. and S.M. Lakshmi. 2012. Pharmacognostical and phytochemical investigation of whole plant of Oxalis corniculata L. Int. J. Phytother., 2 (1): 34-53.
Shah, G., M. Kaur, P.S. Singh, S. Rahar, F. Dhabliya, Y. Arya and R. Shri. 2012. Pharmacognostic parameters of Eucalyptus globulus leaves. Phcog J., 4 (34): 38-43. https://doi.org/10.5530/pj.2012.34.7
Shailajan, S., S. Menon, P. Punyarthi, S. Jana, N. Sayed and B. Tiwari. 2014. Pharmacognostic evaluation of Vatankur - An Ayurvedic Drug. Int. J. Pharm. Sci. Rev. Res., 27 (1): 89-93.
Shende, V.S., S.D. Jadhav, N.H. Aloorkar, A.S. Kulkarni and S.V. Suryavanshi. 2015. Pharmacognostic and phytochemical evaluation of Strobilanthes sessilis Nees leaves. Int. J. pharm., 2(6): 310-314.
Simpson, G.A. and Morris. 2014. The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioactive Carbohydrates. Diet. Fibre., 3: 106-114. https://doi.org/10.1016/j.bcdf.2014.03.003
Sonkar, S., V.K. Arya, A. Baranwal, N. Sutar and R. Sutar. 2014. Phytopharmacognostical and anatomical studies of Momordica dioica Roxb. (Cucurbitaceae) leaf. World. J. Pharma. Pharma. Sci., 3 (5): 813-821.
Trease, G.E. and W.C. Evans. 2002. Pharmacognosy. 15th edition. English language book, society baillere Tindall. Oxf. Univ. Press. 17, 417, 546- 547.
Tyler, V.E., L.R. Brady and J.E. Robbers. 1988. Pharmacognosy. 9th edn. Leaf. and Febiger, Philadelphia.
Vasco-correa, J. and A.D.Z. Zapata. 2017. Enzymatic extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) at laboratory and bench scale. LWT-Food Sci. Technol. https://doi.org/10.1016/j.lwt.2017.02.024
Tripodo, M.M., 2015. Enzymatic extraction of pectin from Opuntia ficus-indica Cladodes. Proc. VIII IC Cactus Pear and Cochineal 93: 393-398. https://doi.org/10.17660/ActaHortic.2015.1067.54
Simpson., G.A. and Morris. 2014. The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioactive Carbohydrates. Dietary Fibre. 3: 106-114. https://doi.org/10.1016/j.bcdf.2014.03.003
Ukwubilec, A. 2013. Comparative pharmacognostic study of Ficus abutilifolia Miq. (Moraceae) Plant Leaf, Stem bark, and Root. Int. J. Adv. Pharm. Bio. Chem., 2 (1): 90-98.
Verpoorte, R. 2000. Pharmacognosy in the new millennium: leadfinding and biotechnology. J. Pharm. Pharmacol., 52: 253-262. https://doi.org/10.1211/0022357001773931
Wahab, S. 2013. Authentication and quality evaluation of an important ayurvedic drug Averrhoa carambola Linn leaves. Asian J. Pharm. Clin. Res., 6 (4):52-56.
Wallis, T.E. 2005. Text Book of Pharmacognosy. CBS publishers, Delhi. 572- 575.
Wallis, T.E. 1985. Text book of pharmacognosy. 5th ed. CBS Publisher and Distributors, New Delhi, India. 572-575.
Zalke, A.S., B. Duraiswamy and U.B. Gandagule. 2014. Pharmacognostic study of root of Combretum albidum G. Don. Phcog. J., 6 (1): 28-33. https://doi.org/10.5530/pj.2014.1.5
Zhao, Z., Z. Liang and P. Guo. 2011. Macroscopic identification of Chinese medicinal materials: Traditional experiences and modern understanding. J. Ethnopharmacol., 131: 556-561. https://doi.org/10.1016/j.jep.2011.01.018
To share on other social networks, click on any share button. What are these?








