Oxidative Stress Biomarker in Assessing the Lead Induced Toxicity in Commercially Important Fish, Labeo rohita
Oxidative Stress Biomarker in Assessing the Lead Induced Toxicity in Commercially Important Fish, Labeo rohita
Nazish Shaukat1, Muhammad Javed1, Faiza Ambreen2* and Fariha Latif1
1Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad 38040
2Department of Zoology, GC Women University Faisalabad
ABSTRACT
Heavy metals considered as the most toxic pollutants in aquatic system. These are important inducers of oxidative stress in aquatic animals, promoting the formation of reactive oxygen species (ROS) which ultimately leads to tissue damage and oxidation of biomolecules. Aquatic organisms possess antioxidant system to cope with the oxidative stress. This research was conducted to see the effect of lead on peroxidase activity (antioxidant enzyme) in the gills and liver of Labeo rohita. Experiment was conducted in the laboratory by exposing four groups of Labeo rohita to different doses of PbCl2 viz. 96-h, 2/3rd, 1/4th and 1/5th of LC50, separately, for 30 days and compared with control group. After 30 days, fish was sacrificed and their gills and liver isolated to determine the effect of PbCl2 on peroxidase activity. Activity of peroxidase enzyme in metal stressed fish was compared with control. The physico-chemical parameters of the test media viz. pH, dissolved oxygen, carbon dioxide, total hardness, calcium, magnesium and total ammonia were monitored on daily basis. Significantly increased peroxidase activity was observed in gills and liver of Labeo rohita after exposure to PbCl2 due to all doses as compared to the control. All results were statistically significant at p<0.05. Fish liver exhibited significantly (p<0.05) higher activity of enzyme than that of gills. The physico-chemical variables viz. pH, dissolved oxygen, carbon dioxide, total hardness, calcium, magnesium and total ammonia of the test media varied significantly at p<0.05, that exerted significant effects on peroxidase activities in gills and liver of fish. This study clearly indicates the defensive nature and adaptive mechanisms of tissues (gills and liver) against free radical induced toxicity.
Article Information
Received 03 October 2016
Revised 12 May 2017
Accepted 18 October 2017
Available online 26 March 2018
Authors’ Contribution
SN performed the experiments. FA statistically analyzed the data. MJ and FL wrote the article.
Key words
Labeo rohita, Lead, Oxidative stress, Peroxidase, Toxicity.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.2.735.741
* Corresponding author: [email protected]
0030-9923/2018/0002-0735 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
The pollution of aquatic environment with heavy metals has been occurring a long time ago, although their impact becoming worst due to industrial resolution and new technologies (Shahid et al., 2013; Abbas and Javed, 2016). Metals considered as major environmental pollutants causing cytotoxic, mutagenic and carcinogenic effects in animals (More et al., 2003). Majority of heavy metals have long residence time in water sediments or in the body of aquatic organisms because, unlike the organic chemicals, they cannot be metabolized into less toxic compound (Ambreen et al., 2015). Heavy metals cause toxicity in fish through various ways out of which one is through the generation of reactive oxygen species (ROS) causing oxidative stress (Valko et al., 2005). Metals are able to disrupt the integrity of the physiological and biochemical mechanisms in fish.
Exposure of fish to metals may result in increase in ROS such as hydrogen peroxide (H2O2), superoxide radicals and hydroxyl radicals (OH) leading to impairment of normal oxidative metabolism and finally to oxidative stress (Lushchak, 2011). Many xenobiotics use general toxicity mechanism by causing oxidative stress in fish which is a pathological process related to increase production of ROS. Different parameters can be used to evaluate the oxidative stress generated by metal toxicity. These include lipid peroxidation (LPO), formation of protein carbonyls, as well as antioxidant enzymes in fish tissues (Campana et al., 2003; Farombi et al., 2007). This antioxidant system includes various enzymes such as superoxide dismutase (SOD) which catalyze the conversion of superoxide radicals to H2O2, as well as catalase (CAT) and glutathione peroxidase (GPx) which acts to convert H2O2 into water and oxygen (Meyer et al., 2003; Dauremepuits et al., 2004; Farombi et al., 2007).
Lead is non-essential metal which enter in aquatic organisms from point sources related to lead mining and industrial process. Lead is redox inactive metal and produce oxidative stress in the aquatic organisms by inducing alterations in the activities of antioxidant enzymes leading towards the increase in production of ROS like O2-, H2O2 and OH by the Haber-Weiss and Fenton reactions (Ercal et al., 2001). Increased production of ROS can cause the oxidation of biomolecules and also produces changes in the redox status of cell and gene expression (Livingstone, 2003). Oxidative stress occurs due to imbalance between production and degradation of ROS by the antioxidant defense system (Nishida, 2011).
Among antioxidant enzymes, one of the enzymes is peroxidase found in the mitochondrial matrix and cell, it has selenium molecules on the active sites and requires glutathione as a cofactor for its functioning and act as a donor and consumer of H2O2 and also plays a significant role in the metabolism and phagocytosis (Rodriguez et al., 2003; Aruljothi and Samipllai, 2014).
Gills are the most important organ of the fish, play multifunctional role in performing dynamic functions such as osmoregulation, acid-base balance, respiration and excretion of nitrogenous wastes (Evans et al., 2005). Metals can enter into the gills by attaching with mucus layer of the gills and cause alterations in the ultrastructure and general morphology of fish gills (Athikesavan et al., 2006). Liver is the major place for the detoxification of toxic chemicals and also plays a significant role in the metabolism and excretion of toxic substances (Ferreira et al., 2005). Fish liver is the main source of an antioxidant enzyme GPx and shows higher activity of this enzyme as compared to the other organs to overcome the oxidative stress caused by heavy metals (Murugan et al., 2008). Labeo rohita (rohu) is the most important fish among three Indian major carps belongs to the Cyprinidae family, found in freshwaters of South and South-East Asia. Labeo rohita is the most important and widely used in poly-culture systems of carps (Vutukuru et al., 2007). Due to its high meat quality, it is widely consumed fish in Asia and it is also suitable to examine the level of water pollution (Ramani et al., 2002). Therefore, present research work was planned for assessing the PbCl2 induces toxicity on peroxidase activity in L. rohita.
Materials and Methods
The present research work was conducted under controlled laboratory conditions at Fisheries Research Farms, Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad. The fingerlings of L. rohita were brought to the laboratory for acclimation. After the acclimation period, healthy fish fingerlings of similar weights and lengths were selected for enzymatic studies. Fingerlings of L. rohita were exposed to PbCl2 (LC50 34.20±1.80 mgL-1 96 h) at different sub-lethal concentrations as determined by Abdullah et al. (2007) for 30 days.
After 30 days of lead exposure, fish organs viz. gills and liver were isolated to check the peroxidase activity. Each test was conducted with three replications for each concentration/treatment and activity of peroxidase in the selected organs (gills and liver) was compared with the control group. The gills and liver of fish were rinsed with phosphate buffer of pH 6.5 (0.2M) and homogenized in cold buffer (1:4W/V) by using a blender. The homogenate were centrifuged at 10,000 rpm, at 4ºC, for 15 min. The clear supernatants were preserved at -4ºC for the enzyme assay.
The fish samples were subjected to enzyme assay by following the method as described by Civello et al. (1995) for the determination of peroxidase activity. The enzyme peroxidase activity was determined Spectrophotometrically at a wavelength of 470 nm by measuring the conversion of guaiacol to tetraguaiacol. Reagents viz. phosphate buffer of pH 6.5 (0.2M), guaiacol and hydrogen peroxide were used in peroxidase enzyme assay. Guaiacol (750 µL) was added to phosphate buffer (47 ml) and mixed well on vortex agitator. After agitation, H2O2 (300 µL) was added to buffer solution. Reaction mixture contain buffered substrate solution (300µL), enzymes extract (60µL). A cuvette containing the 3ml of blank solution was placed into the spectrophotometer and set it to zero at wave length of 470 nm. Then a cuvette containing buffered substrate was placed into the spectrophotometer and reaction was started after adding 0.06 ml enzyme extract. The reaction time was 3 min and after that absorbance was recorded and activity of enzyme was calculated by using the following formula:
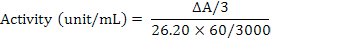
Water pH and dissolved oxygen were measured and recorded by electronic meter HANNA HI-9146 while pH was recorded by digital meter HANNA HI-8424. However, CO2, total ammonia, total hardness, calcium and magnesium were measured by following the methods of APHA (1998).
Factorial experiment with the three replications for each test concentration was performed to find-out statistical differences among various parameters. The treatment means were compared by using Tukey’s/Student Newman-Keul test while the relationships among different parameters were determined by using regression and correlation methods.
Results
The experiment was designed to determine the effect of lead on peroxidase activity in the gills and liver of L. rohita. The fish were exposed to PbCl2 for 30 days and physico-chemical parameters viz. water temperature, pH, dissolved oxygen, carbon dioxide, total ammonia, total hardness, calcium and magnesium were also monitored throughout the trial.
Peroxidase biomarker
Peroxidase belongs to the family of antioxidant enzymes which provides protection to the aquatic organisms against toxic effects of ROS which inhibits a situation of oxidative stress. To check the variations in the peroxidase activity at different concentrations of PbCl2 in the gills and liver of L. rohita, the data were subjected to the statistical analysis by following the factorial designs. A significant increase was observed in the peroxidase activity in the lead treated groups as compared to control group. Peroxidase activity was found to be higher in the gills and liver at LC50 exposure as compared to other treatments (96- hr, 2/3rd, 1/4th, 1/5th of LC50) indicating high level of dose dependent peroxidase activity to overcome the toxic effects of ROS produced due to exposure of metal. Table I represents the activity of peroxidase in the gills and liver of L. rohita with different treatments of lead. In the gills of L. rohita, the significantly highest peroxidase activity was observed at LC50 concentrations that was 0.485±0.004UmL-1 and second highest was found due to 2/3rd concentration exposure (0.343±0.003 UmL-1) while this was least in control fish with the value of 0.112±0.007 UmL-1. Comparison of means indicated that the peroxidase activity tends to be elevated in all treatments as compared to control. In liver of L. rohita, the highest activity of peroxidase was measured as 0.615±0.005UmL-1 at LC50 concentration while it was significantly lower (0.143±0.005 005 UmL-1) in control fish groups. In L. rohita the peroxidase activity was more pronounced in the liver than in gills. Therefore, after exposure to metal the peroxidase activity was significantly increased to show quick response in preventing the cells against oxidative stress caused by heavy metal therefore, the activity of peroxidase was found to be higher in the liver as compared to gills (Table II).
Table I.- Effect of chronic exposure to lead on peroxidase activity (UmL-1) in the gills and liver of Labeo rohita.
| Organs |
Treatments |
Overall means ± SD |
||||
|
96-hr LC50 |
2/3rd LC50 |
1/4th LC50 |
1/5th LC50 |
Control |
||
| Gills |
0.485± 0.003a |
0.343± 0.002b |
0.275± 0.004c |
0.123± 0.003d |
0.112± 0.004e |
0.268± 0.004 |
| Liver |
0.615± 0.005a |
0.425± 0.007b |
0.358± 0.002c |
0.286± 0.004d |
0.143± 0.005e |
0.366± 0.005 |
| Overall Means ± SD |
0.550± 0.004 |
0.384± 0.005 |
0.317± 0.005 |
0.205± 0.004 |
0.126± 0.006 |
|
| Source of variation |
Degree of freedom |
Sum of squares |
Mean squares |
F-value |
| Replications |
2 |
0.00011 |
0.00005 |
NS |
| Treatments |
4 |
0.64504 |
0.16126 |
9179.91p˂0.05 |
| Organs |
1 |
0.07193 |
0.07193 |
4094.80p˂0.05 |
| Treatments × organs |
4 |
0.01530 |
0.00382 |
217.67p˂0.05 |
| Error |
18 |
0.00032 |
0.00002 |
|
| Total |
29 |
0.73269 |
|
|
The mean with similar letter in single row and column are statistically non-significant at p<0.05. NS, non-significant; Significant at p<0.05; SE (treatments) = 1.711; SE (organs) = 1.082; SE (Treatments×Organs) = 2.420
Table II.- Effect of different concentrations of lead on physico-chemical characteristics of the test media.
| Treatments |
pH |
Dissolved oxygen (mgL-1) |
Carbon dioxide (mgL-1) |
Total hardness (mgL-1) |
Calcium (mgL-1) |
Magnesium (mgL-1) |
Total ammonia (mgL-1) |
|
96-hr LC50 |
8.58± 0.06 a |
4.83± 0.04 e |
0.96± 0.03 a |
298.18± 0.06 a |
28.44± 0.03 a |
58.27± 0.05 a |
1.70± 0.04 a |
|
2/3rd LC50 |
8.51± 0.04 a |
5.15± 0.06 d |
0.86± 0.03 b |
284.32± 0.04 b |
25.84± 0.05 b |
56.28± 0.04 b |
1.63± 0.03 b |
|
1/4th LC50 |
8.38± 0.07 b |
5.28± 0.04 c |
0.75± 0.03 c |
278.52± 0.07 c |
23.54± 0.04 c |
54.70± 0.05 c |
1.37± 0.02 c |
|
1/5th LC50 |
8.23± 0.05 c |
5.46± 0.03 b |
0.65± 0.02 d |
267.28± 0.07 d |
21.70± 0.07 d |
52.37± 0.03 d |
1.34± 0.04 d |
| Control |
8.18± 0.03 d |
5.75± 0.02 a |
0.53± 0.032 e |
250.40± 0.06 e |
20.86± 0.07 e |
51.69± 0.04 e |
1.06± 0.05 e |
Physico-chemistry of lead test media
Water pH, dissolved oxygen, carbon dioxide, total ammonia, total hardness, calcium and magnesium of the test media were monitored on 12 hourly basis. The pH is an important parameter of water which determines the acidic or basic nature of water. The significantly acidic or basic water may interrupt the biochemical reaction of the aquatic organisms. The pH value of the water found to be increased with increasing concentrations of lead. Aquatic organisms require dissolved oxygen (DO) for metabolism and respiration. They also have to pay the costs of dependence on DO when reactive oxygen species are produced by various pollutants results in oxidative stress. During lead exposure the consumption of DO by the fish decreased significantly with the increased concentration of the lead. DO of water decreased (4.83±0.04 mgL-1) significantly at LC50 as compared to the control (5.75±0.02 mgL-1). The maximum and minimum mean values of carbon dioxide contents of the test media ranged from 0.96±0.03 to 0.53±0.02mgL-1 at LC50 and control group, respectively. A gradual increase in total ammonia concentration was observed with the increase in concentrations of lead. Total ammonia of water increased (1.75±0.04 mgL-1) significantly at LC50 treatment as compared to the control (1.06±0.05 mgL-1). The regression of peroxidase activity in the gills and liver on pH of the test media was significantly positive with R2 values of 0.958 and 0.901, respectively. The peroxidase activity in both liver and gills was found to be increased with the increasing pH of the lead exposed test media. The dependence of peroxidase activity in both gills and liver on DO contents of the test media was significantly inverse with R2 values of 0.924 and 0.994, respectively. Due to lead exposure, the DO decreased in the test media and the peroxidase activity of both gills and liver increased significantly. Linear regression between peroxidase activity in the gills and liver on carbon dioxide showed that the enzyme (peroxidase) activity was positively significant at p˂0.05 with R2 values of 0.927 and 0.962, respectively. Carbon dioxide concentration increased resulting into enhanced activity of peroxidase in gills and liver of the fish. Statistically significant and direct dependence of peroxidase activity in liver and gills and total ammonia was observed with the computed R2 values of 0.834 and 0.927, respectively. Fitted line plots of peroxidase activity in both the organs against total ammonia revealed that with an increase in total ammonia of the metal exposed test media, the enzyme activity also increased. A strong and direct dependence of peroxidase activity in both the organs viz. gills and liver was found, on total hardness of lead exposed media. The regression analysis between peroxidase activity in gills and liver on calcium contents exhibited a positive and significant relationship with R2 values of 0.979 and 0.946, respectively. Peroxidase activity was increased with the increasing magnesium contents of the test media (Supplementary Table 1).
Discussion
Contamination of aquatic environment due to the industrial, domestic and human activities has severe effects on the aquatic fauna (Canli and Atli, 2003). Metals can cause oxidative damage to the cell due to higher production of ROS by Fenton- and Haber-Weiss type mechanisms, that can cause an induction in lipid peroxidation, protein modifications and DNA damage (Baysoy et al., 2012). Heavy metals have ability to generate oxidative stress in the fish because of their strong oxidative nature. Peroxidase is one of the important antioxidant enzymes which plays an important role in the cellular defenses by the decomposition of H2O2 into water and oxygen and prevents the tissues from oxidative damage (Dursun et al., 2001).
During the present study, peroxidase activity at all sub-lethal concentrations was significantly increased in both the gills and liver of L. rohita as compared to the control fish. Liver is the main detoxifying organ as it contains antioxidant enzymes to overcome the lethal effects of ROS produced by the toxicity of heavy metals (Fernandes et al., 2007). Gills are more susceptible to oxidative damage because of direct contact with water and thin epithelial cells; therefore, metals can easily penetrate in the gills (Evans et al., 2004). Bangeppagari et al. (2014) also observed that the peroxidase activity significantly increased in the liver and gills of lead exposed L. rohita. Activity of lipid peroxidase was increased significantly in the liver of cadmium exposed L. rohita (Dabas et al., 2014). The results are also in line with the findings of Mohanty et al. (2013) who found that the cadmium exposure can cause a significant increase in the activity of lipid peroxidase in the gills and liver of L. rohita. Cadmium induced significant alterations in the activities of antioxidant enzymes in the gills and liver of the freshwater fish, L. rohita, leading towards oxidative stress (Kumari et al., 2014).
Lead caused a significant alteration in the activity of GPx in the liver of freshwater fish, Oreochromis niloticus (Eroglu et al., 2014). Similar results were obtained by Maiti et al. (2010) who found that lead exposure caused an increase production of reactive oxygen species at cellular level and leads towards increased activity of lipid peroxidase in the brain of fish (Clarias batrachus). Similarly, Awoyemi et al. (2014) observed that lead chloride caused a significant induction in the glutathione peroxidase activity in the liver and gills of Oreochromis niloticus and Clarias gariepinus. GPx was significantly increased in the liver of lead exposed Clarias gariepinus (Saliu and Bawa-Allah, 2012). Baysoy et al. (2012) also reported a significant increase in the activity of lipid peroxidase in the liver of the lead stressed Oreochromis niloticus. Similarly Brucka-Jastrzebska (2010) observed enhanced activity of lipid peroxidase activity in the liver and kidney of lead stressed fish as compared to control group. In contrast to our findings, Sujatha et al. (2013) observed significantly decreased GPx activity in L. rohita after cadmium exposure.
The physico-chemical parameters of the test media also altered by the lead exposure leading towards more toxic effects on the metal stressed fish. During the whole experiment, the concentration of dissolved oxygen was observed to be decreased and the level of carbon dioxide and total ammonia was decreased with the increasing lead concentrations. These results are confirmed by the findings of Abdullah et al. (2007) who observed that DO concentration was decreased when the concentrations of total ammonia, carbon dioxide, and total hardness were increased after exposure of lead. Leung and Furness (2001) observed that the pH, carbon dioxide, total ammonia and total hardness significantly induced increase in peroxidase activity in the metal exposed fish. Sampaio et al. (2012) observed decrease oxygen consumption rate of fish while level of carbon dioxide and total ammonia increased significantly leading towards oxidative stress in the fish, Piaractus mesopotamicus.
Aknowledgement
Authors gratefully acknowledge the support offered by the University of Agriculture, Faisalabad, Pakistan.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2018.50.2.735.741
Statement of conflict of interest
There is no conflict of interest.
References
Abbas, S. and Javed, M., 2016. Growth performance of Labeo rohita under chronic dual exposure of water-borne and dietary cobalt. Pakistan J. Zool., 84: 257-264.
Abdullah, S., Javed, M. and Javid, A., 2007. Studies on acute toxicity of metals to the fish (Labeo rohita). Int. J. Agric. Biol., 9: 333-337.
Ambreen, F., Javed, M. and Batool, U., 2015. Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of mixtures. Pakistan J. Zool., 2: 339-407.
APHA, 1998. Standard methods for the examination of water and wastewater, 20th Ed. American Public Health Association, New York, pp. 1193.
Aruljothi, B. and Samipillai, S.S., 2014. Effect of arsenic on lipid peroxidation and antioxidants system in freshwater fish, Labeo rohita. Int. J. Mod. Res. Rev., 2: 15-19.
Athikesavan, S., Vincent, S., Ambrose, T. and Velmurugan, B., 2006. Nickel induced histopathological changes in the different tissues of freshwater fish, Hypophthalmichthys molitrix (Valenciennes). J. environ. Biol., 27: 391-395.
Awoyemi, O., Kafilat, Bawa-allah, M.A., Adebay, A. and Otitoloju, A., 2014. Accumulation and antioxidant enzymes as biomarkers of heavy metal exposure in Clarias gariepinus and Oreochromis niloticus. Appl. Ecol. environ. Sci., 5: 114-122.
Banggeppagari, M., Gooty, J.M., Tirado, J.O., Mariadoss, S., Thangaswamy, S., Maddela, N. R. and Ortiz, D.R., 2014. Therapeutic efficiency of Spirulina against lead acetate toxicity on the fresh water fish Labeo rohita. Am. J. Life Sci., 2: 389-394. https://doi.org/10.11648/j.ajls.20140206.19
Baysoy, E., Atli, G., Gurler, C.O., Dogan, Z., Eroglu, A.A., Kocalar, K. and Canli, M., 2012. The effects of increased freshwater salinity in the biodisponibility of metals (Cr, Pb) and effects on antioxidant systems of Oreochromis niloticus. Ecotoxicol. environ. Safe., 84: 249-253. https://doi.org/10.1016/j.ecoenv.2012.07.017
Brocka-Jastrzebska, E., 2010. The effect of aquatic cadmium and lead pollution on lipid peroxidasion and superoxide dismutase activity in freshwater fish. Polish J. environ. Stud., 19: 1139-1150.
Campana, O., Sarasquete, C. and Blasco, J., 2003. Effect of lead on ALA-D activity, metallothionein levels and lipid peroxidation in blood, kidney and liver of the toadfish Halobatrachus didactylus. Ecotoxicol. environ. Safe., 55: 116-125. https://doi.org/10.1016/S0147-6513(02)00093-3
Canli, M. and Atli, G., 2003. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut., 121: 129-136. https://doi.org/10.1016/S0269-7491(02)00194-X
Civello, P.M., Arting, G.A., Chaves, A.R. and Ann, M.C., 1995. Peroxidase from strawberry fruit by partial purification and determination of some properties. J. Agric. Fd. Chem., 43: 2596-2601. https://doi.org/10.1021/jf00058a008
Dabas, A., Nagpure, N.S., Mishra, R.M., Kushwaha, B., Kumar, R. and Kumar, P., 2014. Investigation of cadmium-induced genotoxicity and oxidative stress response in Indian major carp, Labeo rohita. Human Ecol. Risk Assess., 20: 510-526. https://doi.org/10.1080/10807039.2012.702591
Dautremepuits, C., Paris-Palacios, S., Betoulle, S. and Vernet, G., 2004. Modulation in hepatic and head kidney parameters of carp (Cyprinus carpio) induced by copper and chitosan. Comp. Biochem. Physiol., 137: 325-333. https://doi.org/10.1016/j.cca.2004.03.005
Dursun, N., Dogan, P. and Donmez, H., 2001. Plasma and erythrocyte lipid peroxide levels in workers with occupational exposure to lead. Biol. Trace Elem. Res., 82: 29-40. https://doi.org/10.1385/BTER:82:1-3:029
Ercal, N., Gurer-Orhan, H. and Aykin-Burns, N., 2001. Toxic metals and oxidative stress part I: Mechanism involved in metal-induced oxidative damage. Curr. Top. med. Chem., 1: 529-539. https://doi.org/10.2174/1568026013394831
Eroglu, A., Dogan, Z., Atli, G. and Canli, M., 2014. Effect of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ. Sci. Pollut. Res., 22: 1-11.
Evans, D.H., Piermarini, P.M. and Choe, K.P., 2005. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol. Rev., 85: 97-177. https://doi.org/10.1152/physrev.00050.2003
Evans, M.D., Dizdaroglu, M. and Cooke, M.S., 2004. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res., 567: 1-61. https://doi.org/10.1016/j.mrrev.2003.11.001
Farombi, E.O., Adelowo, O.A. and Aimuko, Y.R., 2007. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. environ. Res. Publ. Hlth., 4: 158-165. https://doi.org/10.3390/ijerph2007040011
Fernandes A.F., Jorge, V., Ferreira, C., Santos, S.G., Monteiro, S.M. and Matos, J.C.P., 2007. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesq. Vet. Bras., 27: 29-45.
Ferreira, M., Ferreira, M.P. and Henriques, M.A., 2005. Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat. Toxicol., 71: 39-48. https://doi.org/10.1016/j.aquatox.2004.10.009
Kumari, K., Khare, A. and Dange, S., 2014. The applicability of oxidative stress biomarkers in assessing chromium induced toxicity in the fish Labeo rohita. BioMed. Res. Int., 2014: 1-11. https://doi.org/10.1155/2014/782493
Leung, K.M.Y. and Furness, R.W., 2001. Metallothionein induction and condition index of dogwhelks Nucella lapillus (L.) exposed to cadmium and hydrogen peroxide. Chemosphere, 44: 321-325. https://doi.org/10.1016/S0045-6535(00)00297-6
Livingstone, D.R., 2003. Oxidative stress in aquatic organism in relation to pollution and agriculture. Rev. Med. Vet., 154: 427-430.
Lushchak, V., 2011. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol., 101: 13-30. https://doi.org/10.1016/j.aquatox.2010.10.006
Maiti, A.K., Nimai, C.S. and Paul, G., 2010. Effect of lead on oxidative stress, Na+ K+ ATPase activity and mitochondrial electron transport chain activity of the brain of Clarias batrachus L. Bull. environ. Contam. Toxicol., 84: 672-676. https://doi.org/10.1007/s00128-010-9997-9
Meyer, J.N., Smith, J.D., Winston, G.W. and Giulio, R.T., 2003. Antioxoxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model pro-oxidants: short term and heritable responses. Aquat. Toxicol., 65: 377-395. https://doi.org/10.1016/j.aquatox.2003.06.001
Mohanty, B.P., Mahananda, M.R. and Pradhan, S., 2013. Cadmium induced toxicity and antioxidant activities in Labeo rohita (Hamilton). Environ. Ecol. Res., 1: 41-47.
More, T.G., Rajput, R.A. and Bandela, N.N., 2003. Impact of heavy metals on DNA content in the whole body of bivalve, Lamelleiden, Marginalis. Environ. Sci. Pollut. Res., 22: 605-616.
Murugan, S.S., Karuppasamy, R., Poongodi, K. and Puvaneswari, S., 2008. Bioaccumulation pattern of zinc in freshwater fish, Channa punctatus (Bloch) after chronic exposure. Turk. J. Fish. aquat. Sci., 8: 55-59.
Nishida, Y., 2011. The chemical process of oxidative stress by copper (II) and iron (III) ions in several neurodegenerative disorders. Monat. Fur. Chem., 142: 375-384. https://doi.org/10.1007/s00706-010-0444-8
Ramani, M.B., Mercy, T.V.A., Nair, J.R. and Sherief, P.M., 2002. Changes in the proximate composition of Labeo rohita (Halmiton) exposed to sub lethal concentrations of monocrotophos. Indian J. Fish., 49: 427-432.
Rodriguez, A., Esteban, M.A. and Meseguer, J., 2003. Phago-cytosis and peroxidase release by Seabream (Sparus aurata L.) leukocytes in response to yeast cells. Anat. Rec., 272: 415-423. https://doi.org/10.1002/ar.a.10048
Saliu, J.K. and Bawa-Allah, K.A., 2012. Toxicological effects of lead and zinc on the antioxidant enzyme activities of post juvenile Clarias gariepinus. Resour. Environ., 2: 21-26. https://doi.org/10.5923/j.re.20120201.03
Sampaio, F.G., Boijink, C.D., Santos, L.R.B., Oba, E.T., Kalinin, A.L., Luiz, A.J.B. and Rantin, F.T., 2012. Antioxidant defenses and biochemical changes in the neotropical fish pacu, Piaractus mesopotamicus: Responses to single and combined copper and hypercarbia exposure. Comp. Biochem. Pysiol. Toxicol. Pharmacol., 156: 178-186. https://doi.org/10.1016/j.cbpc.2012.07.002
Shahid, M., Ferrand, E., Schreek, E. and Dumat, E.C., 2013. Behavior and impact of zirconium in the soil-plant system: Plant update and phytotoxicity. Rev. Contam. Toxicol., 221: 107-127. https://doi.org/10.1007/978-1-4614-4448-0_2
Sujatha, S.P., Pugazhendy, K., Jayachandran, K., Prabakaran, S. and Jayanthi, C., 2013. Protective role of Denolex elata against the cadmium toxicity in the glutathione peroxidase (GPx) in the fresh water fish Labeo rohita (Hamilton). Int. J. Develop. Res., 3: 037-039.
Valko, M., Morris, H. and Cronin, M.T.D., 2005. Metals toxicity and oxidative stress. Curr. Med. Chem., 12: 1161-1208. https://doi.org/10.2174/0929867053764635
Vutukuru, S.S., Prabhath, N.A., Raghavender, M. and Yerramilli, A., 2007. Effect of arsenic and Cr on the serum amino-transferase activity in Indian major carps, Labeo rohita. Int. J. environ. Res. Publ. Hlth., 4: 224-227.
To share on other social networks, click on any share button. What are these?










