Optimization of IBA Concentration for Rapid Initiation of Roots and Ultimate Growth of Kiwi Seedlings and the Association between Root System Architecture and Seedlings Growth
Optimization of IBA Concentration for Rapid Initiation of Roots and Ultimate Growth of Kiwi Seedlings and the Association between Root System Architecture and Seedlings Growth
Noorullah Khan1*, Farrukh Siyar Hamid1, Fayaz Ahmad1, Sabaz Ali Khan2, Imtiaz Ahmed1, Muhammad Abbas Khan1, Shamsul Islam1, Abdul Waheed1, Basharat Hussain Shah1 and Hussain Shah3
1PARC-National Tea and High Value Crops Research Institute (P-NTHRI), Pakistan Agricultural Research Council, Shinkiari Mansehra, Pakistan; 2Biotechnology Program, Department of Environmental Sciences, COMSATS Institute of Information Technology, 22060, Abbottabad, Pakistan; 3Plant Sciences Division Pakistan Agricultural Research Council G-5/1 Islamabad, Pakistan.
Abstract | The rooting ability in kiwifruit (Actinidia deliciosa) cuttings is very low and can be enhanced to a sufficient extent by treating cuttings with rhizogenic hormones. The present experiment was designed with the objectives to find the optimal concentration of IBA for better rooting and survival rate of seedlings from kiwi cuttings. The experiment was laid out according to randomized complete block design with three replications and 20 four budded branch cuttings of “Hayward” kiwifruit cultivar. The cuttings were treated with different concentration of IBA (0, 1000, 2000, 3000, 4000, and 5000 ppm) for ten second and then planted in polythene bags under green net shade. Application of all concentrations of IBA (1000, 2000, 3000, 4000, and 5000 ppm) significantly increased all the growth attributing traits over the control. However, the highest number of first-order adventitious roots (NFARP) plant-1 (9.5), seedling survival rate (SSR) (51.7), and plant height (85.5 cm) were recorded in plants treated with 3000 ppm IBA. Contrarily the longest roots of 50.7 cm were observed with IBA concentration of 5000 ppm. Correlation analysis revealed that SSR % and growth of kiwi seedlings are strongly associated with NFARP as we found strong correlation (P<0.001) among NFARP, SSR% and plant height. In addition, the main root length also showed moderate correlation (P<0.05) with plant height but it was not correlated with SSR%. Based on this finding, it is concluded that IBA concentration of 3000 ppm is optimum for better seedlings growth and evaluation of seedlings root systems architecture and seedling growth attributes could be used to predict the field performance of seedlings.
Received | August 20, 2019; Accepted | November 03, 2019; Published | January 20, 2020
*Correspondence | Noorullah Khan, PARC-National Tea and High value Crops Research Institute (P-NTHRI), Pakistan Agricultural Research Council, Shinkiari Mansehra, Pakistan; Email: nooragro@yahoo.com
Citation | Khan, N., F.S. Hamid, F. Ahmad, S.A. Khan, I. Ahmed, M.A. Khan, S. Islam, A. Waheed, B.H. Shah and H. Shah. 2020. Optimization of IBA concentration for rapid initiation of roots and ultimate growth of kiwi seedlings and the association between root system architecture and seedlings growth. Pakistan Journal of Agricultural Research, 33(1): 63-71.
DOI | http://dx.doi.org/10.17582/journal.pjar/2020/33.1.63.71
Keywords | Correlation, First-order adventitious roots, Kiwifruit, Rooting ability, Rhizogenic hormones, seedling survival rate
Introduction
Kiwifruit (Actinidia deliciosa and A. Chinensis) a plant originally from China, is an economically important cash crop (Zhang et al., 2015). It belongs to the family Actinidiaceae and is vigorously growing, deciduous warm-temperate perennial vine (Li et al., 2007). Kiwifruit is cultivated on more than 120,000 ha of orchard in the world and has an annual production of about 1.4 million metric tons of fresh fruit (Huang et al., 2013; Zhang et al., 2015).
Currently, there are several kiwifruit species available for research and cultivation purposes (Liu et al., 2017), however, Actinidia deliciosa (green flesh) and Actinidia chinensis (yellow flesh) are the two main species of kiwifruit that are grown on commercial basis in the world.
Budding and grafting of seedlings rootstock, rooting of semi-hardwood cutting from mother and father plants and micro-propagation are four major methods used to produce kiwifruit plants (Tanimoto, 1994). However, it takes one extra year to prepare seedlings through budding and grafting as it requires the growing of seedlings rootstock, and the process of budding and grafting also requires skill. Conversely, propagation through cuttings is the quicker, convenient and feasible method of cloning important horticultural plants (Hartman et al., 2011). The problem with this method is that the rooting ability in kiwi cuttings is very low as a result the survival rate of seedlings also remains minimum (10-15%). However, this problem can be overcome to sufficient extent by treating cuttings with root growth hormones such as Indole-3-Butyric Acid (IBA) and Naphthalene acetic acid (NAA) (Testolin and Vitaglian, 1987).
Previous experiments on the propagation of kiwifruit plants through cuttings and application of different concentrations of rooting hormones have revealed variable rooting ability and survival rate of seedlings from kiwi cuttings (Testolin and Vitaglian, 1987). Abhi et al. (1991) reported that treated with IBA and NAA cuttings of Actinidia deliciosa cultivar “Monty” produced 61.5% rooted cuttings as compared to untreated cuttings (54%). Abdel-Hussain and Salman (1988) observed that wounding plus IBA treatment at the rate of 4000ppm result in the highest rooted cuttings percentage with higher number of roots, root length and root weight plant-1 of olive seedlings. Daud et al. (1989) reported increase in the number of roots of kiwi cuttings treated with 2000, 3000 and 4000 ppm IBA as compared to control.
In Pakistan, kiwifruit is a new horticultural crop. PARC-National Tea and High Value Crops Research Institute (PARC-NTHRI) in Shinkiari, Mansehra is the only research institute in Pakistan that has started organized research and development studies on kiwifruit during the last three years. PARC-NTHRI has for the first time in Pakistan introduced kiwifruit on farmer field. During the last three years, it has been observed that there is great demand of true to type plants of kiwifruit. Therefore, the present experiment was conducted with the objectives to optimize concentration of IBA for successful propagation of true to type kiwifruit plants and find the optimal concentration of IBA for better rooting and survival rate of seedlings from kiwi cuttings.
Materials and Methods
Experimental procedure
The experiment was carried out at National Tea and High Value Crops Research Institute (NTHRI) Shinkiari, Mansehra, Pakistan during 2015-16 with the objectives to test the response of kiwi cuttings to different concentrations of IBA and find the optimal level of IBA for better rooting and establishment of seedlings. The experiment was laid out according to Randomized Complete Block Design with three replications. There were twenty cuttings in each treatment. Treatments were control-1 (distilled water), control-2 (5% Methanol), 1000, 2000, 3000, 4000, and 5000 ppm Indol-3-butyric Acid (IBA). Four budded hardwood cuttings were dipped in distilled water (control-1), 5% methanol (control-2) and different concentrations of IBA for 10 seconds before planting in polythene bags on 10th March 2015. The medium utilized for planting was garden soil mixed with farm yard manure (3:1). The cuttings were planted under green net shade at NTHRI nursery.
Preparation of IBA solutions
A stock solution of 5000 ppm Indol-3-butyric Acid (IBA) was prepared by dissolving 2.5 gram IBA in small amount of Methanol (5%) and then the volume of the solution was brought to 500 milliliters by adding distilled water. Then all the other concentrations (1000, 2000, 3000, 4000, and 5000 ppm) were prepared from the stock solution. For example, 20, 40, 60, and 80 ml of the stock solution was mixed with 80, 60, 40, and 20 ml of distilled water to prepare 100 ml of each 1000, 2000, 3000, and 4000ppm concentrations of IBA.
The study lasted for eleven months, cuttings were regularly observed and the data were recorded on bud sprouting (%), seedling survival rate (SSR %) and plant height in the first seven months. The SSR % was determined by following formula.
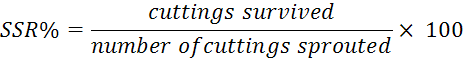
To avoid wasting of and damage to seedlings the root architecture data (number of adventitious roots and average root length) were recorded in February, 2016 when the seedlings were dormant.
Statistical analysis
The data were subjected to analysis of variance (ANOVA), correlation analysis and least significant difference (LSD) test with the help of computer based statistical software Statistix 8.1 (McGraw-Hill, 2008).
Results and Discussion
Bud sprouting rate (%)
Analysis of the data (Table 1) indicated that there were no significant differences among various IBA concentrations for bud sprouting rate (BSR %) of the kiwi cuttings. However, the BSR % was higher than 95% in all treatments. This higher BSR % in all treatment may be due the reserved food present in the cutting and available for the plant to grow for some weeks without formation of the root Similar non-significant findings have also been documented by other study where different concentration of IBA had no significant effect on the bud sprouting rate in kiwi cuttings (Riaz et al., 2007).
Number of first-order adventitious roots plant-1 (NFARP)
Data relating to number of first-order adventitious roots plant-1 (NFARP) have been presented in Figure 1. Analysis of variance (Table 1) revealed that application of different concentrations of IBA highly significantly (P<0.0001)increased the NFARP of seedling from kiwi cutting in this experiment. The average NFARP of different treatments ranged from minimum of 2.13 to maximum of 9.5 adventitious roots plant-1. The highest NFARP (9.5) was recorded in those plants where cuttings were treated with 3000 ppm IBA followed by 4000 ppm IBA with NFARP value of 8.1 which was at par with 3000 ppm IBA as against the minimum NFARP of 2.1 and 2.1 recorded in control-1 (distilled water) and control-2 (5% methanol) respectively (Figure 1).
The root system architecture of a species which is determined by the length of the primary/adventitious and density of the lateral root plays a vital part in establishment of a crop by supplying water, nutrients and anchoring the plant into the soil (Khan et al., 2012). The root architecture of plant could be used in estimating whether a plant will establish and become successful in a particular environmental condition (Malamy and Benfey, 1997). The lateral roots develop from main or adventitious roots while the adventitious roots develop from vegetative parts such as stem or leaf derived cell (Bellini et al., 2014). Induction and development of adventitious root is a crucial stage in vegetative propagation of true to type important horticultural and woody plants and enable clonal propagation and quick evolution of superior genotypes (Han et al., 2009). Development of adventitious roots is a complicated procedure and is believed to be regulated by numerous factors including phytohormones, soil moisture, light intensity, temperature, nutritional status and genetic characteristic of the plant (Han et al., 2009). Due to these complexities the rooting ability in kiwi cuttings is very low as a result the survival rate of seedling usually remains minimum (10-15%). However, phytohormones, particularly auxin such as IAA, IBA NAA play an important role in regulating root initiation, development and thus improving the seedling survival rate (Testolin and Vitaglian, 1987; Connor, 1982). This important role of IBA was also obvious from the present study where application of all concentration of IBA significantly increased the number of adventitious roots plant-1 over the control treatments. In the present study the increase in number of roots plant-1 with 3000-4000 ppm IBA is in partial agreement (Is it partial agreement or complete agreement as the best level in your study is 3000 and 4000 ppm?) with Riaz et al. (2007) and Yasar et al. (2010) who reported that application of 4000 and 5000 ppm IBA resulted in the highest number of roots per plant in kiwi cuttings. However, in our case increase in IBA concentration beyond 4000 ppm had detrimental effect on NFARP formation as there was significant decrease in NFARP when the concentration of IBA increased to 5000 ppm. This may be due to the toxic effect of higher dose of IBA on the formation and development of lateral roots. De Klerk et al. (1999) reviewed that dedifferentiation of cells occurs through the first 24 h after taking the cuttings. During this lag period cells begin to make a response to the rooting stimulant, auxin and accumulate starch during these initial 24 h. The next stage is the induction stage which extends up to 72 to 96 hours. During this induction phase,
Table 1: Analysis of Variance (ANOVA) of response of measured traits to different concentrations of IBA.
| MS | ||||||
| S. O.V. | df | BSR (%) | NFARP | MRL (cm) | SSR (%) | PH(cm) |
| Replication | 2 | 2.05 | 2.4914 | 58.926 | 35.76 | 315.74 |
| Treatments | 6 | 2.00NS | 149.21** | 333.91* | 682.52** | 1832.18** |
| Error | 12 | 1.05 | 0.878 | 39.83 | 38.19 | 17.82 |
| F-value | - | 1.91 | 28.32 | 8.38 | 17.90 | 102.79 |
| P-value | - | 0.16 | P<0.0001 | P<0.001 | P<0.0001 | P<0.0001 |
| LSD @alpha =0.05) | - | NS | 1.67 | 11.23 | 11.05 | 7.51 |
**= means significant difference at P <0.0001 and *= means significant difference at P<0.01 level of probability and NS means non-significant difference. (MS= Mean Square, S.O.V. =Source of variation, df= Degree of Freedom), BSR%=Bud Sprouting Rate%, NFARP= Number of First-order Adventitious Roots per Plant, MRL (cm) = Main Roots Length in cm, SSR%=Seedling Survival Rate. PH (cm)= Plant Height in cm.
the already stimulated cells become progressively dedicated for the development of root primordia by the rhizogenic action of IBA. Within this phase of 72 to 96 hours, the rhizogenic hormone pulses promote the maximum number of adventitious roots. At later stage after 96 hours, the rooting hormone is no further required, and their higher concentrations of rooting hormones IBA that are promising for the development of root meristemoids have detrimental effect on root formation during this phase. These results are supported by Edson et al. (1991) who observed decline in rooting ratio with increase in hormone (IBA) concentration. They concluded that higher concentrations of auxin (IBA) hormones Which Hormones?) have adverse influence on adventitious root formation and development.
Main root length (cm)
Data relating to main root length (MRL) have been presented in Figure 2. Analysis of variance (Table 1) revealed that application of different concentrations of IBA significantly increased MRL. Comparison of means of the data indicated that higher IBA concentration of 5000 ppm induced the longest MRL (50.67 cm) followed by 4000 and 2000 ppm (43.11 and 40.22 cm) while the shortest MRL of 21.89 and 24.22 cm recorded in control treatments (Conrol-1 and Conrol-2 respectively).
Table 2: Correlation among different growth attributing traits.
| BSR (%) | NFARP | MRL | SSR (% ) | PH(cm) | |
| BSR (%) | 1 | ||||
| NFARP | 0.35 NS | 1 | |||
| MRL | -0.12 NS | 0.65 NS | 1 | ||
| SSR (% ) | 0.42 NS | 0.96** | 0.61Ns | 1 | |
| PH(cm) | 0.33 NS | 0.95** | 0.77* | 0.97** | 1 |
**= the correlation is significant at P value <0.001 and *= the correlation is significant at P value <0.05, BSR%=Bud Sprouting Rate%, NFARP= Number of First-order Adventitious Roots per Plant, MRL (cm) = Main Roots Length in cm, SSR%=Seedling Survival Rate. PH (cm)= Plant Height in cm.
Initiation of fast growing and deep root system is essential during the initial growth stage of seedlings for competition with weeds, acquiring of nutrients from deeper layers of soil and absorbance of water during drought season (Robinson, 1994). A deep root system plays a vital part in flourishing a plant in a specific environment (Malamy and Benfey, 1997). In the present experiment there is gradual increase in root length (RL) and other growth attributing traits with increase in IBA concentration from 0 to 2000 ppm while there is sudden decrease at 3000 ppm IBA concentration which is optimal for NFARP and other growth parameters. This decrease in RL may be due to the competition among increased number of roots plant-1 at this concentration of IBA. While at higher concentration of IBA (5000 ppm) there is significant decrease in NFARP and other growth attributing traits. This shows that longer root system may be beneficial for the growth of plants but not on the cost of reduction in NFARP as NFARP in this study is more related with all the growth attributing traits as compared MRL. It is not obvious whether the increase in MRL at higher concentration is due to the timely induction of roots at higher dose of the hormone and acquisition of nutrients by the plants as suggested by Ingle (2008) or due to minimum number of roots and lack of competition among the roots. The increase in MRL in this research is in partial agreement with those obtained by Bradley (2011) who recorded higher rooting percentage, fresh and dry root weight and root length in kiwi cuttings at the concentration of 5000 ppm IBA as compared to control (distilled water), 1000 ppm and 2500 ppm IBA. Ercisli et al. (2002) also reported highest root length when they treated kiwifruit cuttings with 6000 ppm IBA.
Seedling survival rate (SSR)
Data regarding Seedling survival rate (SSR) have been presented in Figure 3. Analysis of variance of the data (Table 1) exhibited statistically highly significant differences for SSR % among different IBA concentrations. The highest SSR (%) of 51.67 was recorded in those treatments where cuttings were treated with 3000 ppm IBA followed by 4000 ppm (43.67%) which was at par with 3000 ppm IBA treatment (Figure 3). The lowest SSR (%) of 11.66 or 12.33 % were recorded in control-1(distilled water) and Contol-2 (5% methanol). Seedling survival rate (SSR %) is an important growth parameter which can be used as a good indicator to predict seedling growth and establishment. The increase in SSR% with application of all concentrations of IBA as compared to the control could be due to the formation of higher number of roots and their faster growth mediated by IBA. While the highest SSR% of seedlings at 3000 and 4000 ppm IBA could be due to the balance dose of IBA at this concentration for kiwi cuttings. These results are in partial agreement with Ercisli et al. (2003), Riaz et al. (2007), who also reported gradual increase in SSR% of seedlings from kiwi cuttings at all concentrations of IBA applied to the kiwi cuttings. However, they recorded the highest SSR% at 4000 to 5000 ppm IBA with which our results are not in agreement. The data also revealed that there was gradual decrease in SSR% when the concentration of IBA increased beyond 3000 ppm to 4000 and 5000 ppm (Figure 3).
This decrease in SSR% at higher concentration of IBA (5000 ppm) may be due to some toxic effect on the initiation of roots as one year old kiwi shoots are semi hardwood (Preet and Vishal, 2011) contrary to hardwood cuttings as in case of apple or peach (Halil et al., 2009). The rooting and survival ability in kiwi cuttings is generally very low (10-15%) without the application of growth hormones as reported by Riaz et al. (2007) and Muhammad et al. (2014). Nevertheless, several studies have reported variable improvement in seedling survival through the application of growth hormones (Preet and Vishal, 2011). The data regarding SSR% in this study resulted in a sigmoid model or S-curve shape where there is gradual increase in SSR% with increase in IBA concentration from 0 to 3000 ppm and then gradual decrease at higher concentrations of IBA (4000 and 5000 ppm). These results are in partial agreement with Ercisli et al. (2003), Riaz et al. (2007), who reported gradual increase in SSR% of seedlings from kiwi cuttings with increase in IBA concentration up to 5000 ppm and they also reported decrease in SSR% at higher concentration of IBA (6000 ppm). This difference in the results may be due to the method of application of IBA as well as different environmental conditions.
Plant height (cm)
Data regarding plant height (PH) is presented in Figure 4. Analysis of variance (Table 1) indicated that application of IBA highly significantly increased plant height of the seedlings. The tallest seedlings (85.52 cm) was recorded in those plots where kiwi cuttings were treated with 3000 ppm IBA followed by 4000 ppm IBA with plant height of 79.38 cm which was statistically at par with plant height of 85.52 cm. While the lowest seedlings height of 23.67 and 25.62 cm recorded in control-1 and 2. In addition, the data also followed nearly the same pattern as the data of NFARP and SSR (%) which is obvious from Figures 1 and 3.
The increase in plant height with increase in IBA concentration may be due to the formation of increased number of roots through which the plants absorbed sufficient nutrients from the soil and thus achieved maximum height. These results are supported by the results obtained by Chandramouli (2001) during his study on Bursera penicillata cuttings, who reported that increase in IBA concentration also increases seedling height and number of leaves plant-1. He attributed the increase in seedling height to the better acquisition of nutrients from the soil through better growth of roots induced by the rooting hormones. Riaz et al. (2007) and Gjeloshi et al. (2013) also recorded the highest plant height at 3000 and 4000 ppm IBA applied to kiwi cuttings. The data also revealed that there was gradual decrease in PH with increase in IBA concentration beyond 3000 ppm. The decrease in plant height at higher concentration (4000 or 5000 ppm) might be due to inhibitory effect of IBA at supra-optimal concentration (De-Klerk et al., 1999; Han et al., 2009). These results are consisted with those obtained by Riaz et al. (2007) and Gjeloshi et al. (2013) who also reported adverse effect of higher concentration (5000 and 6000 ppm) of IBA on plant height and root formation.
Correlation between phenotypic traits
Statistically highly significant and positive correlations were observed among different phenotypic traits (Table 2). The R2 value for the significant Pearson correlation among different growth parameter varied from 0.77 to maximum 0.97. The highest correlation of R2 0.97, 0.96 and 0.95 was observed between PH and SSR %; NFARP and SSR% and NFARP and PH respectively (Table 2). The lowest significant correlation of R2 value 0.77 and P value <0.04 was recorded between MRL and PH. While the trait, BSR % was statistically not correlated with any other growth parameter. The strong positive correlation among PH, SSR and NFARP may be due to the increasing effect of IBA on enhancement of root traits. On the other hand, the lack of correlation between MRL and SSR% and NFARP may be due to the fact that SSR% and NFARP were adversely affected by higher concentrations of IBA compared to MRL. While the trait, BSR % was statistically not correlated with any other growth parameter. This may be due to the fact that BSR% was not affected by the application of IBA as compared to the other growth attributing traits. These findings are consistent with those obtained by Mobli and Baninasab (2009) during their experiment on three Pistacia vera species who reported that survival rate of the seedlings was highly significant and positive correlated with number, length, fresh and dry weight of roots. They concluded that increasing effect of IBA on survival rate of seedlings is through enhancements of root traits. Khan et al. (2012) who reported highly significant and positive correlation among lateral root number, root fresh weight, root dry weight and root diameter with seedling shoot fresh weight, shoot dry weight, shoot length and shoot diameter during their experiment on a recombinant inbred lines (RIL) population of tomato. They also observed weak correlation between main root length and other growth attributing traits. Kormanik and Ruehle (1989) during their study on sweet gum seedlings also reported significantly positive correlations between numbers of roots and seedling survival rate, seedling height, and root collar diameter after first growing season in the field. Based on this finding, they concluded that evaluation of numbers of seedlings roots are one of the best method for predicting seedling survival, establishment, field performance, and overall quality of out-planted seedlings. Although seedling physiology is perhaps the ultimate factor assessing seedling quality, but, performing time-consuming and labor-intensive physiological tests are more difficult for nursery growers than simple morphological measurements which can be carried out as seedlings are prepared for shipping. The strong linear correlations among NFARP, SSR% and other plant growth parameters reveal that evaluation of seedling root systems could be used to predict the potential, development and successful establishment of seedlings in the field.
Conclusions and Recommendations
Based on the findings of the present study, it is concluded that application of IBA in all concentrations improved the rooting ability of kiwi cuttings as a result improves seedlings growth, survival rate and establishment of good quality out planted kiwi seedlings. However, the best results are obtained when kiwi cuttings are treated with 3000 to 4000 ppm IBA. It is also concluded that higher concentration of IBA (5000 ppm) has detrimental effect on seedlings survival and growth due to their inhibitory effect on adventitious root formation. It is further concluded that seedling survival and growth in kiwi cuttings is more associated with NFARP compared to MRL. From the strong correlations between NFARP and seedling performance, it is concluded that numbers of seedlings roots are one of the best predictors of survival, field performance, competitive ability and overall quality of out-planted seedlings compared to root length. To the best of our knowledge this is the first time to establish such association between NFARP and seedling performance in kiwifruit plant. Based on these results, it is recommended that application of IBA concentration in the range of 3000 to 4000 ppm for achieving best results from kiwi cuttings. However, further research trials are needed to test the intermediary dose between 2500 to 3500 ppm if one can get better results than the ones obtained during this study. It is also recommended to take in consideration NFARP of kiwi seedlings while checking the quality of kiwi seedlings for their overall field performance.
Acknowledgments
The authors would like to thank Pakistan Agriculture Research Council (PARC) for the funding and financial support under the RADP sub project title “Promotion of kiwifruit in Mansehra Area” with Grant No. F. No. –CS-89/RADP/2014-15/ (PARC) to carry out this research.
Author’s Contribution
Noorullah Khan, Farrukh Siyar Hamid, Fayaz Ahmad and Sabaz Ali Khan conceived and designed the experiment (15%). Noorullah Khan, Farrukh Siyar Hamid, Fayaz Ahmad and Shamsul Islam collected and analyzed the data and wrote the paper (35 %). Hussain Shah provided the chemicals/materials (15%). Imtiaz Ahmed, Muhammad Abbas Khan, Abdul Waheed and Basharat Hussain Shah provided technical assistance at every stage of the experiment (15%). Noorullah Khan, Farrukh Siyar Hamid, Fayaz Ahmad, Sabaz Ali Khan, Shamsul Islam, Imtiaz Ahmed, Muhammad Abbas Khan, Abdul Waheed, Bashatat Hussain Shah and Hussain Shah critically reviewed and revised the article (20%).
References
Abhi, N., M.Z. Ahmadi and H. Sadeghi. 1991. Effects of different factors on rooting percentage of hardwood and semi-hardwood kiwifruit “Monty” cuttings in Mazandaran, Iran. N.Z. J. Crop Hortic. Sci. 19: 365-367. https://doi.org/10.1080/01140671.1991.10422877
Bellini, C., D.I. Pacurar and I. Perrone. 2014. Adventitious roots and lateral roots: similarities and differences Ann. Rev. Plant Bio. 65: 639-666. https://doi.org/10.1146/annurev-arplant-050213-035645
Connor, D.M. 1982. Cutting propagation of Actinidia chinensis (kiwifruit). Comb. Proc. Int. Plant Prop. Soc. 32: 329-333.
Daud, D.A., J.T. Agha, K.H. Abu-Lebda and M.S. Al-Khaiat. 1989. Influence of IBA on rooting leafy olive cuttings. Olive. 6: 28-30.
De-Klerk, G., W.V. Derkieken and J.C. De-Jong. 1999. The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant. 35: 189–199. https://doi.org/10.1007/s11627-999-0076-z
Edson, L.J., D.L. Wenny and L. Fins. 1991. Propagation of western larch by stem cuttings. Western J. Appl. For. 6: 47-49. https://doi.org/10.1093/wjaf/6.2.47
Ercisli, S., A. Esitken, R. Cangi and F. Sahin. 2003. Adventitious root formation of kiwifruit in relation to sampling date, IBA and Agrobacterium rubi inoculation. Plant Growth Regul. 41: 133–137. https://doi.org/10.1023/A:1027307720934
Halil, K., R. Aslantas, G. Ozkan and M. Guleryuz. 2009. Effects of indol–3-butyric acid (IBA), plant growth promoting rhizobacteria (PGPR) and carbohydrates on rooting of hardwood cutting of MM106 Apple rootstock. Afr. J. Agric. Res. 4: 60-64.
Han, H., H. Zhang and X. Sun. 2009. A review on the molecular mechanism of plants rooting modulated by auxin. Afr. J. Bio. 8: 348-353.
Huang, S., J. Ding, D. Deng, W. Tang, H. Sun, D. Liu, L. Zhang, X. Niu , X. Zhang, M. Meng, J. Yu, J. Liu , Y. Han, W. Shi, D. Zhang , S. Cao , Z. Wei, Y. Cui, Y. Xia, H. Zeng, K. Bao, L. Lin, Y. Min, H. Zhang , M. Miao, Xiaofeng Tang1,2, Yunye Zhu1 , Yuan Sui1 , Guangwei Li1 , Hanju Sun1 , Junyang Yue1 , Jiaqi Sun2, F. Liu, L. Zhou, L. Lei, X. Zheng, M. Liu, L. Huang, J. Song, C. Xu, J. Li, K. Ye, S. Zhong, B. Lu, G. He, F. Xiao, H. L. Wang , H. Zheng, Z. Fei, and Y. Liu. 2013. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 4: 2640. https://doi.org/10.1038/ncomms3640
Ingle, M.R. 2008. Effect of growth regulators and environments on rooting of Stevia cuttings (Stevia rebaudiana Bertoni). Univ. Agric. Sci. Dharwad, MSc thesis.
Khan, N., R.H. Kazmi, L.A.J. Willems, A.W. Van-Heusden, W. Ligterink and H.W.M. Hilhorst. 2012. Exploring the natural variation for seedling traits and their link with seed dimensions in tomato. PLoS One. 7: e43991. https://doi.org/10.1371/journal.pone.0043991
Li, J.Q., X. W. Li and D.D. Soejarto. 2007. Actinidiaceae. In: Flora of China. (Wu Z.Y., Raven P.H. and Hong D.Y., eds.). Science Press, Beijing and Missouri Botanical Garden Press, St. Louis. Vol. 12: 334-362.
Liu, Y., B. Zhou, Y. Qi, X. Chen, C. Liu, Z. Liu and X. Ren. 2017. Expression differences of pigment structural genes and transcription factors explain flesh coloration in three contrasting kiwifruit cultivars. Front. Plant Sci. 8: 1-15. https://doi.org/10.3389/fpls.2017.01507
Malamy, J.E. and P.N. Benfey. 1997. Down and out in Arabidopsis: The formation of lateral roots. Trends Plant Sci. 2: 390–396. https://doi.org/10.1016/S1360-1385(97)90054-6
Mobli, M. and B. Baninasab. 2009. Effect of indole-butyric acid on root regeneration and seedling survival after transplanting of three pistaciaspecies. J. Fruit Orn. P. Res. 17: 5-13.
Muhammad I., A. Rab, J. Rahman, M. Sajid, I. Khan, S. Ali, M.Razaq and Sallahuddin. 2014. Influence of different planting dates and media on growth of kiwifruit (Cv.Hayward) cuttings. Sarhad J. Agric. 30: 419-424.
Preet P. and S.R. Vishal. 2011. Effect of pre-conditioning treatments, IBA and collection time on the rooting of semi- hardwood cuttings of kiwifruit, Actinidia deliciosa Chev. Int. J. Farm Sci. 1: 30-36.
Riaz, A., K.U. Rahman, M. Ilyas, M. Ibrahim and M.A. Rauf. 2007. Effect of indole butyric acid concentrations on the rooting of kiwi cuttings. Sarhad J. Agric. 23: 293-296.
Robinson, D. 1994. The responses of plants to non-uniform supplies of nutrients. New Phytol. 127: 635–674. https://doi.org/10.1111/j.1469-8137.1994.tb02969.x
Testolin, R. and C. Vitagliano. 1987. Influence of temperature and applied auxins during winter propagation. Hort. Sci. 22: 573-574.
Yasar, E., S. Ercisli, A. Haznedar and R. Cakmakcl. 2010. Effects of plantgrowth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 43: 91-98. https://doi.org/10.4067/S0716-97602010000100011
Zhang, H.Y., H.M. Liu and X.Z. Liu. 2015. Production of transgenic kiwifruit plants harboring the Sbt Cry1Ac gene. Genet. Mole. Res. 14 (3): 8483-8489. https://doi.org/10.4238/2015.July.28.16
To share on other social networks, click on any share button. What are these?







