Monthly Variations in the Profile of Important Steroids during Annual Testicular Cycle in Catla catla: Basic Information for Future Study
Monthly Variations in the Profile of Important Steroids during Annual Testicular Cycle in Catla catla: Basic Information for Future Study
Shafaq Fatima*, Sumrin Sahar and Khalid Lone
Department of Zoology, Government College University, Lahore
ABSTRACT
Catla (Catla catla) is the most important commercial carp in South Asia due to its higher growth, flesh quality and increased market demand. Present study investigated the seasonal variations in gonadosomatic index (GSI) and profiles of sex steroids such as testosterone (T), 11-ketotestosterone (11-KT), estradiol-17β (E2), cortisol (C), progesterone (P) and 17α-hydroxyprogesterone (17α-HP) during annual reproductive cycle under semi-arid climate conditions. GSI increased during March concomitant with gradual increase in levels of T, P and 17α-HP. Highest values of these parameters were observed at different stages of gonadal development. T and 11-KT played key role in progress of testicular development and final maturation in June however, spawning in captivity was not observed. Role of P and 17α-HP was identified more as substrate of sex steroids. Higher levels of sex steroids suppressed the synthesis of cortisol presumably interfering with pituitary-inter-renal axis.
Article Information
Received 31 October 2016
Revised 17 March 2017
Accepted 15 May 2018
Available online 31 August 2018
Authors’ Contributions
SF, SS and KL participated in designing the project, field and laboratory work and statistical analysis.
Key words
Catla, Photoperiod, Sex steroids, Maturation.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.6.sc3
* Corresponding author: shafaq.fatima@y7mail.com
0030-9923/2018/0006-2375 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
The major carp, catla (Catla catla), is one of the most economically important fish species in Pakistan. Its higher growth rate and compatibility with other major carps (Labeo rohita, Cirrhinus mirigala, Cyprinus carpio) specific surface feeding habit and consumer preference have increased its popularity in carp polyculture system among the fish farmers in India, Bangladesh, Myanmar, Pakistan and Thailand (Costa-Pierce, 2005). Global production of catla has reached 2.8 million tonnes by 2014 (FAO, 2016). However traditional practices are still common in regional carp industry, particularly catla farmers thus hindering the significant improvement in annual production of this species. Impediment in growth rate has been observed during maturation in catla (Lone et al., 2009, 2012). Maturation can be controlled by multiple techniques like monosex culture (Devlin and Nagahama, 2001) and manipulation of temperature and photoperiod (Choi et al., 2010; Miguad et al., 2010; Leclercq et al., 2011). However, a descriptive study is required to understand the gonadal development and role of important sex steroids before designing a method to control occurrence of maturation in any species.
Histological development of gonads in catla during its first maturation cycle has been reported previously from semi-arid region of Pakistan (Lone et al., 2009, 2012). However, seasonal variations in important sex steroids during annual reproductive cycle of catla have not been studied in this region. Bhattacharyya and Maitra (2006) and Bhattacharyya et al. (2005) have reported annual profile of T and E2, respectively in catla but under sub-tropical climate conditions which are largely different from semi-arid climate. Therefore, present study aimed at investigating seasonal variations in profiles of T, 11-KT, E2, C, 17-α HP and P in male catla under semi-arid conditions of Pakistan. Present detailed studies are first of its kind from Pakistan and were done on fish cultured in ponds for commercial purpose. It is hoped that the results obtained will be directly beneficial and applicable to the fish farm practices.
Materials and methods
This study was conducted according to procedures approved by the Government College University Animal Ethics Committee. Fish were reared at a commercial fish farm (Himalaya Fish Hatchery, Sheikhupura) under ambient water temperature and photoperiod throughout the study period (November, 2007 – October, 2008; Age: 18 months – 29 months). At each monthly sample point, a total of ten fish were randomly collected and transferred to University Aquaculture Facility. Fish were kept in tanks for 24 h to be released from stress of capture. Fish could not be distinguished into male and female due to lack of sexual dimorphism, therefore, number of male fish varied at each sample point from the required number of five. At sampling, fish were killed by killed by anesthetic overdose (30 µl L-1 AQUI-S). Total body weight (near to 0.1 g) and total body length (near to 0.1 cm) and gonadal weight (near to 0.1 g) were measured. GSI was measured by using following formula:
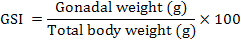
Blood samples were stored on ice until centrifugation (3000 rpm for 15 min). The plasma was stored at -80°C until assayed to determine the levels of sex steroids by ELISA (BIORAD). Detection limits of assay (ELISA) for T, 11-KT, E2, C, 17α-HP and P were 0 – 20 ng/ml, 0.00078 – 0.1 ng/ml, 0.25 – 1 ng/ml, 0.049 – 200 ng/ml, 0 – 1 ng/ml and 0.3 – 40 ng/ml, respectively.
Monthly variations were analyzed by one way ANOVA after analyzing the data by Levene’s test of homogeneity. Tukey’s post-hoc test was applied for comparison of means where P<0.05 indicated significant differences.
Results
Highest value of GSI (F11, 56 = 8.40, P < 0.5) was observed in June (0.48 ± 0.07 %) (Fig. 1) which dropped in July and remained low during rest of the study period. Levels of T showed significant (F11, 56 = 5.05, P < 0.5) rise in July (0.67 ± 0.10 ng/ml which dropped in August and remained low till end of the study (Fig. 2A). 11-KT exhibited two peaks during the months of May and September (F11, 56 = 3.96, P < 0.5) (Fig. 2B). l’[Levels of E2 remained within the range of 0.5 ± 0.01 – 0.13 ± 0.02 ng/ml over the study period (F11, 56 = 13.10, P < 0.5) (Fig. 2C). A significant rise in levels of C was observed during July (620.00 ± 30.50 ng/ml) (Fig. 2D). Over the study period, concentration of C remained within the range of 329.01 ± 40.43 – 639.15 ± 59.35 ng/ml (F11, 56 = 4.30, P < 0.5). Observed range of P (F11, 56 = 5.30, P < 0.5) and 17α-HP (F11, 56 = 7.30, P < 0.5) over the study period were 0.47 ± 0.02 – 0.47 ± 0.03 ng/ml and 0.20 ± 0.01 – 0.66 ± 0.03 ng/ml, respectively (Fig. 2E, F).
Discussion
The present study investigated the gonadal development and its endocrine and environmental control during annual testicular cycle of catla under semi-arid climate conditions. Higher levels of P and 17α-HP at this stage can be correlated with progression in spermatogonial proliferation exhibiting their role as substrate of T and 11-KT thus initiating spermatogenesis (Miura et al., 2007) in this species. Relatively higher concentration of E2 at this stage could be associated with its role in spermatogonial proliferation as reported previously (Song and Gutzeit, 2003). Further testicular development i.e. initiation of meiosis and formation of spermatids might be regulated by high concentration of T during May and June as observed in other teleosts including catla (Bhattacharyya and Maitra, 2006). Peak in GSI was observed in June preceded by the peak of 11-KT while concomitant with peak in T. This finding is in contrast with that Bhattacharyya and Maitra (2006), who reported the highest value of GSI in July in same species. At this stage, milt could be extracted on manual stripping showing the final stages of development (milt hydration). These developmental changes were presumably found as a result of action of T and 11-KT and release of other growth factors mediated by these both androgens from sertoli cells (Kobayashi et al., 1991).
In catla, cortisol profile showed a negative correlation with those of T and 11-KT. Cortisol secretion started decreasing from April and reached at its minimum value in June which is the period of the active spermeiogenesis. During this period concentrations of sex steroids were very high opposite to that of C consistent with previous studies (Pottinger et al., 1996; Semenkova et al., 2002). According to Mommsen et al. (1999) elevated levels of gonadal steroids modify the pituitary-inter-renal axis thus suppressing the levels of cortisol as observed in present study. Spawning was not observed in catla under captivity however, milt could not be stripped manually after July. This indicates regression of testes associated with decline in levels of androgens at this stage. This regressed phase in catla lasted from August till October exhibiting. Very low concentrations of testosterone and 11-KT. This decrease might reflect a shift in steroidogenesis pathways from synthesis of C19 androgens to production of C21 progestins production (Kokokiris et al., 2000) as levels of P and 17α-HP showed slight increase during this period of regressed testicular activity. Endocrine changes observed during testicular cycle of catla in present studies showed seasonal differences from previous study on catla (Bhattacharya and Maitra, 2006). They observed the peak of testosterone between July and August. This difference might be due to sub-tropical climate and geographical conditions in previous study (Sivakumaran et al., 2003). Temporal differences in occurrence of developmental events under different climates further highlight the importance and need of present study.
Conclusion
Overall, present study provided the basic information on endocrine control of testicular development in catla during second year of life, when fish matures first time in pond culture. This study is the first of its kind in Pakistan and provided the basal line reference values for reproductive parameters and will become the base for many advanced studies on the control of reproduction of this fish in semi-arid regions.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Bhattacharyya, S. and Maitra, S.K., 2006. Biol. Rhythm Res., 37: 87-110. https://doi.org/10.1080/09291010500124605
Bhattacharyya, S., Dey, R. and Maitra, S.K., 2005. Acta Zool. (Stockholm), 86: 71-79. https://doi.org/10.1111/j.1463-6395.2005.00188.x
Choi, S., Lee, C.H., Park, W., Kim, D.J. and Sohn, Y.C., 2010. Zool. Sci., 27: 24-32. https://doi.org/10.2108/zsj.27.24
Costa-Pierce, B., 2005. Urban aquaculture. CABI Publishers, Wallingford, pp. 234. https://doi.org/10.1079/9780851998299.0000
Devlin, R.H. and Nagahama, Y., 2002. Aquaculture, 208: 191-364. https://doi.org/10.1016/S0044-8486(02)00057-1
FAO, 2016. The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome, pp. 5. http://www.fao.org/3/a-i5555e.pdf
Kobayashi, M., Aida, K. and Stacey, N.E., 1991. Zool. Sci., 8: 389-393.
Kokokiris, L., Mourot, B., Le Menn, F., Kentouri, M. and Fostier, A., 2000. Fish Physiol. Biochem., 23: 1-11. https://doi.org/10.1023/A:1007882807782
Leclercq, E., Taylor, J.F., Sprague, M. and Migaud, H., 2011. Aquacult. Eng., 44: 35-47. https://doi.org/10.1016/j.aquaeng.2010.12.001
Lone, K.P., Fatima, S. and Sahar, S., 2009. Pakistan J. Zool., 41: 483-494.
Lone, K.P., Sahar, S. and Fatima, S., 2012. Pakistan J. Zool., 44: 159-172.
Migaud, H., Davie, A. and Taylor, J.F., 2010. J. Fish Biol., 76: 27-68. https://doi.org/10.1111/j.1095-8649.2009.02500.x
Miura, C., Higashino, T. and Miura, T., 2007. Biol. Reprod., 77: 822-828. https://doi.org/10.1095/biolreprod.107.061408
Mommsen, T.P., Vijayan, M.M. and Moon, T.W., 1999. Rev. Fish Biol. Fish., 9: 211-268. https://doi.org/10.1023/A:1008924418720
Pottinger, T.G., Carrick, T.R., Hughes, S.E. and Balm, P.H.M., 1996. Gen. Comp. Endocrinol., 104: 284-295. https://doi.org/10.1006/gcen.1996.0173
Semenkova, T., Barannikova, .I, Kime, D.E., Mc Allister, B.G., Bayunova, L., Dyubin, V. and Kolmakov, N., 2002. J. appl. Ichthyol., 18: 375-381. https://doi.org/10.1046/j.1439-0426.2002.00368.x
Sivakumaran, K.P., Brown, P., Stoessel, D. and Giles, A., 2003. Environ. Biol. Fish., 68: 321-332. https://doi.org/10.1023/A:1027381304091
Song, M. and Gutzeit, H.O., 2003. Develop. Growth Differen., 45: 327-337. https://doi.org/10.1046/j.1440-169X.2003.00701.x
To share on other social networks, click on any share button. What are these?










