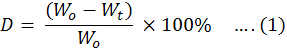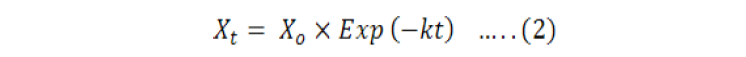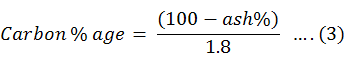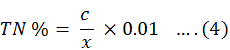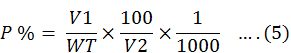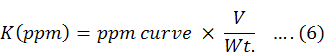Litter Fall Production and Decomposition in Deodar Forest Ecosystem
Litter Fall Production and Decomposition in Deodar Forest Ecosystem
Kamran Aziz1*, Aamir Saleem1 and Arshad Mahmood Malik2
1Department of Forestry and Range Management, Pir Mehr Ali Shah Arid Agriculture University of Rawalpindi, Pakistan; 2Department of Economics, Pir Mehr Ali Shah Arid Agriculture University of Rawalpindi, Pakistan.
Abstract | Decomposition is a natural process of nutrient cycling in which the different elements play their role to decompose the plants parts such as roots, shoots, leaves and other parts. The present study was conducted to estimate the decomposition rate, litter fall production and elemental composition of Cedrus deodara and Pinus wallichiana in the study area Dungagali, Taohidabad, Kuzagali and Khanspur situated around the Ayubia National Park. The research was carried from September, 2016 to August, 2017. Litter bag technique was used to estimate the decomposition rate, elemental composition was carried through chemical analysis in the laboratory and litter fall production was estimated by trapper technique. Under Cedrus deodara and Pinus wallichiana canopy, the leaf litter was collected and brought back to laboratory for the further chemical analysis. The Total annual litter fall production of Cedrus deodara was 4.2209t/ha/annum while the Pinus wallichiana was estimated 4.6097t/ha/annum. It showed that there is more litter fall in Pinus wallichiana as compared to Cedrus deodara. The P. wallichiana has greater decay rate as compared with C. deodara, estimated 12 % and 6% per annum respectively. The elemental composition was calculated for each of the nutrients i.e. C; N; P and K for Cedrus deodara is; 2006.67, 53.72, 21.33 and 7.91 Kg/ha/annum and for Pinus wallichiana; 2245.07, 51.69, 23.13 and 4.79 Kg/ha/annum respectively. The amount of carbon is greater in Pinus wallichiana as compared with C. deodara while the other nutrients i.e. N; P and K are greater in Cedrus deodara. It was concluded that Pinus wallichiana is better in litter fall, decay rate and in carbon and phosphorus composition.
Received | April 09, 2019; Accepted | May 22, 2019; Published | June 25, 2019
*Correspondence | Kamran Aziz, Department of Forestry and Range Management, Pir Mehr Ali Shah Arid Agriculture University of Rawalpindi, Pakistan; Email: arshadmm@uaar.edu.pk
Citation | Aziz, K., A. Saleem and A.M. Malik. 2019. Litter fall production and decomposition in deodar forest ecosystem. Pakistan Journal of Agricultural Research, 32(3): 441-448.
DOI | http://dx.doi.org/10.17582/journal.pjar/2019/32.3.441.448
Keywords | Decomposition, Litterfall, Himalaya, Nutrients, NPK
Introduction
In forest, decomposition plays a basic role in primary production (Barg and Laskowski, 2006; Barg and McClaugherty, 2014; Swift et al., 1979). Decomposition is carried out through mineralization of dead natural organisms through digestion by microorganism (Presscott and Grayston, 2013). Litter decomposition is vital as it added nutrients in forest ecosystem and regulate carbon by its accumulation into the soil and it is also a source of inorganic material to plants (Heal et al.,1997; Barg and McClaugherty, 2008). In addition, litter decomposition also helps in elemental regulations and as well as plant chemical elements (Couteaux et al., 1995; Guckland et al., 2009). Plant humus is the composition of the litter and nutrients available in the soil and as well as on the ground surface. The litter present on the soil surface usually contains leaf litter, branches, twigs and fruits. Underground litter mostly contains fine roots having distance about 2 mm. In eco arrangement of backwoods, the accompanying sorts of litter are, by and large, equivalent in mass per annum supply of litter fall. (McClaugherty et al., 1982; Vogt et al., 1986). Decay of the root and of leaf litter mainly occurs on the surface of soil and the decomposition of roots occur within the soil surface. So, in the forest floor leaf decomposition occur in different stages. In many studies, researcher and environmentalist put leaf litters bags on soil surface. (Cusack et al., 2009; Gholz et al., 2000; Ostertag and Hobbie, 1999). Soil surface contains higher quality litter and litter decomposition (Cortez et al., 1996). The decomposition of litter is also effected by many of the driving factors such as climatic factors that consist of air, moisture and temperature etc. Chemical components of litter fall and biotic factors such as microbial activities also effect the litter (Heal et al., 1997). Many of the other factors which include moisture, temperature and solar radiation reaching the forest floor also have some effects on forest canopy. The herbivores which are grazing on forest floor may also affect decomposition (Bardgett et al., 1998; Pineiro et al., 2010).
Cedrus deodara is one of the most common moist temperate species of Himalayan and neighbouring country named Afghanistan at eastern aspect. It is locally known as deodar or diyar particularly in Khyber Pakhtun khwa, some parts of India, Himachal Pradesh, Sikkim, Darjeeling, Jammu and Kashmir, Uttar akhand territories, south-western most and some parts of Nepal at southern aspect, occurring at 1,500–3, 200m (4,921–10, 499 ft) elevation. It’s a massive needle leaved evergreen plants attaining 40– 50 m (131– 164 ft) tall, especially 60 m (197 ft) with a trunk up to 3 m (10 ft) in breadth. It has a conic crown with level branches and hanging branchlets. The leaves are needle-like, generally 2.5– 5 cm (0.98– 1.97 in) long, infrequently up to 7 cm (2.8 in) long, slim (1 mm (0.039 in) thick), borne independently on long shoots and in thick bunches of 20– 30 on short shoots. They differ from splendid green to glaucous blue-green in shading. The female cones are barrel-formed, 7– 13 cm (2.8– 5.1 in) long and 5– 9 cm (2.0– 3.5 in) wide and deteriorate when develop in a year to discharge the winged seeds. The male cones are 4– 6 cm (1.6– 2.4 in) long and shed their dust in pre-winter. It is broadly developed as a fancy tree, regularly planted in parks and extensive greenhouses for its hanging foliage. General development is constrained to territories with mellow winters, with trees much of the time killed by temperatures beneath about −25 °C (−13 °F), restricting it to USDA zone 7 and hotter for dependable development, (Odum, 1985).
In forest ecosystem, soils are the major carbon pool. Estimation of net soil balance in litter decay is needed because small changes in carbon pool could significantly increase in atmospheric carbon dioxide (Davidson and Janssens, 2006; Harmon et al., 2011). Decomposition of litter would be affected by management practices, silvicultural operations and grazing, etc.
Objectives of the study were;
- • To determine the amount and elemental composition of leaf litter fall from cedrus deodara and Pinus wallichiana
- • To estimate the decay rate of cedrus deodara and Pinus wallichiana
Materials and Methods
Study area
The research area Dungagali, Taohidabad, Kuzagali and Khanspur are situated around the Ayubia National Park (Figure 2 map). These sites are the most and the best indicatives of moist temperate forest of Pakistan. There are around 200 types of herbs and bushes and around 10 types of Gymnosperm trees (Deodar, Blue pine, Chir pine, Rubinia, Poplar, Horse chestnut and Reen) found in park area. Ayubia National Park is a part of Galliyat Reserved forest that is situated on the slopes running north-south near Abbottabad and at the north-western end of Murree. It is situated along 34o: 01’ to 34o: 3.8’ N latitude and 73o: 22.8’ to 73o: 27.1’ E longitude (WWF- Pakistan, 2010).
The Ayubia National Park and its periphery meets the requirement of rivers i.e. the River Jhelum on eastern side, the Rivers Dawar and Haru on the western and Kunhar on the northern end. As part of the ecological group of Himalayan moist temperate forest, this area mainly consists of Western Mixed Coniferous Forest. The Park is spaced over 3,312 ha and major vegetation type is sub tropical pine forest, Himalayan moist temperate forest and sub alpine meadows. The area is very rich in biodiversity and provides an excellent home to important plants and wildlife species (Jalaluddin, 1993).
The Galliyat forests of Nathia Gali are situated in Abbotabad District, Hazara Division of KP, approximately between 33:55 north latitude and 34:29 east longitudes. The Gallies forests are situated among the mountainous region of district Abbottabad, north to south on geological pattern, near 40km to Abbottabad city. The city of Abbotabad lies on the western periphery of the Ayubia National Park. On the other side (southern aspect) of the park, Murree city is situated and some other cities are Havailian and Mansehra about 25-30km from its outer periphery and Nathia Gali, DungaGali and Thandiani hill stations in the central part of the forests. The reserved forests of the Gallies were originally surveyed and demarcated in 1971. These forests are in many pieces and are surrounded by the Guzara Forests (Jalaludin, 1993).
Experimental design
Litter fall collection: Litter fall for spp. Cedrus deodara and Pinus walichiana collected for the time period of one year started from September 2016 to August 2017. 18 trappers were used. Material used to make this instrument is nylon net. Mesh size is of 1 mm, all the trappers were installed in the study sites. Litter trappers were installed above the forest ground to collect the litter fall under the forest canopy of target species. The species branches having diameter more than 2cm, cones, dead barks and fruits were exempted from the site to collect litter fall, but only foliage litter fall was collected as per our study concern. Litter fall was collected at the interval of 29 days. After the data collection from the field that was in raw or litter form. The litter was stored into plastic bags, transferred into oven to dry, for 24 hours at 70 degrees Celsius.
Leaf litter decomposition analysis: For the analysis of leaf litter decomposition, a sample 0f 24 bags (dimensions 9x5 inches) of mesh net was collected. Litter fall was placed in different bags comprising of 10 grams in each bag and placed in field area. After every 45 days, three bags were collected from the field and weighed on electronic balance to check the mass loss. Percentage of leaf decay was calculated with the help of Equation 1.
Litter mass loss or decay rate was estimated by the given formula.
where;
D= Litter mass after weighing (%age); wo = Litter mass before weighing; wt = Litter mass after weighing.
The decay rate and the constant K was calculated by the Olsen formula 1963 as given in Equation 2.
Such as;
xt = Litter mass after t time interval; xo = Litter fall initial mass; K = decay rate co-efficient; st= time.
Litter fall analysis: The collected foliage litter was then dried in the oven at 70-degree C for 24 hours to get a constant weight and weighed on electric weigh balance, to find out the monthly weight in (grams). To determine the amount of litter elements (carbon and N, P, K) after the grinding the samples was labelled and stored in the lab. The samples were analysed at university laboratory to determine the amount of elements. The collection of foliage litter fall was tested in university laboratory to check their elemental composition. The whole process was repeated twice during the spring and winter session.
Preparation of sample: The litter of both the species were separated. The litter samples were cleaned with deionised water. The samples of litter were dried in the oven to get a constant weight. The welly mill was used to grind the samples, the mesh was used of 20 sieves, the respective grinded material were brought to laboratory in air tight bags for analysis.
Total carbon (C)
To check the amount of carbon in every sample the percentage of ash was calculated. The digested sample of five grams were taken into china dish for this purpose. After this the dishes were kept into muffle furnace at 500-degree C for 2-4 hours. Through this procedure the whole sample were converted to the ash. To determine the percentage content, residues of all china dishes were weighed and this content was calculated by percentage formula. Carbon percentage was calculated using Equation 3.
Nitrogen (N)
The amount of total nitrogen was calculated by Anderson and Ingram (1993). The sample was digested to perform the procedure. Digestion is mandatory for this procedure. The mixture of 0.42 g selenium powder and 14 g of lithium sulphate were added to 30% hydrogen per oxide (H2O2) and prepared for digestion. The sample of one gram was taken into digestion flask and digestion mixture of 4.4 ml was mixed. To make the sample colourless we put it into digestion process at 360 degree c for 2 hours. After cooling the sample, we added 50 ml of distilled water. To obtain the clear solution we put it for a time.
The N1 reagent had been made by dissolving sodium salicylate (34 grams), sodium cirate (25 grams) and sodium titrate (25 grams) into 750 millilitres of water. And 30 g of sodium hydro oxide into 750 ml of water was dissolved to get the N2 reagent. To prepare NH4+ -N standard we added 4.714 dry ammonium sulphate into the water and 1000 ml volume was obtained by water addition. The 100ug/ml of NH4+ solution was used to prepare the other standard of 1, 10, 15, 20, 25, and 30 ppm.
To determine the amount of nitrogen method of colorimetric is used. 0.1 gram of the sample standard was brought to the laboratory for the further tests. The sample was left for 15 minutes after addition of 5ml of N1 reagent. Now by addition of 5 ml of N2 reagent the sample is left to stand for some time to make the colourful solution. After all of this the absorbance of sample was noted at 655 nm by using spectrometer. The concentration of solution was calculated by constructing a graph between absorbance and standard solution. The percentage of nitrogen was estimated with the help of Equation 4.
Whereas;
c is the concentration of the nitrogen after error, v is the final substance volume and W is the matter weight amount.
Phosphorus (P)
The amount of phosphorous was measured by wet digestion method. The litter fall samples were analysed to check phosphorous by spectrophotometer at wavelength of 410 nm. 22.5 g of ammonium hepta moly bate was dissolved into deionised Water to prepare the reagent. After this 1.25 g of ammonium metawandate was dissolved into 300 ml of Water. Mixed two reagents on the letter flask. Concentrated 250ml of HNO3 was dissolved into water and raised to volume of 1 litre. We took 250 g of dried potassium dihydrogen phosphate and was dissolved into a volume of 1 litre. Solution of 0.5, 1-0, 1.5, 2.0and 2.5 ppm were obtained. The concentration of phosphorous was calculated by the Equation 5.
Whereas;
V1 is the overall digested capacity of plant material into millilitre, V2 is the volume of plants digestion used for estimation into ml, WT is the total dry mass of sample into gms.
Potassium (K)
The overall concentration of K was calculated by dry ash method. 165.6ml of concentrated HCL was dissolved into deionised water and produced a volume of 1litre. The litter sample was dried well, 1 g of sample was placed into 50 ml porcelain flask. Now the flask is put into muffle furnace at 550 degree c for 5 hours. The ash was dissolved in 5 ml 2NHCI and mix properly for 30 minutes, then analysed by flame photometer.
The total potassium concentration (ppm) was calculated by given formula.
Whereas;
V is overall total substance nutrient (ml), Wt. is the mass of moisture less plants in grams.
Statistical analysis
One-way ANOVA was utilized to test the distinctions in mass misfortune, decay rate, soil fauna densities and the circumstances at which half and 95% litter deterioration will accomplished among the diverse work sizes for the shisham specie. Pearson’s connection examination will be utilized to test the relationship between’s dirt dampness substance and decay rate.
Results and Discussion
Litterfall production
The annual or overall production of litterfall was recorded as maximum of Pinus wallichiana at study site. The most of the litterfall was consist of leaves in the both of the species and minimum number of other parts, such as cones and fruits. The collection of Litterfall was affected by heavy snowfall during the month of January to March. The annual Litterfall varies from month to month which made a complex interaction of different edaphic and climatic factors. The monthly Litterfall was recorded as low in the months of January to March.
Litterfall production of Cedrus deodara
The annual litterfall was collected for the species of Cedrus deodara and Pinus wallichiana for a time period of one year starting from September 2016 to August 2017. The monthly litterfall varied from month to month. Total annual litterfall of Cedrus deodara was 4.2209 tons per ha per annum documented in the study results. The maximum litterfall was recorded in the month of December 0.8144 t/ha while the lowest amount was 0.255 in the month of February Figure 1. The gradual increase in litterfall of this species can be seen from the months of September towards December. The litterfall for the months of March, April, May, June, July and August was 0.3055, 0.2605, 0.2794, 0.2572, 0.2827 and 0.2838 tons per hectares respectively. The litter fall was affected by the following factors i.e. snowfall, rainfall, hills storms and wind. Many of the other factors which includes; temperature, elevation and humidity and slopes also affect the pattern of litterfall.
Litterfall production of Pinus wallichiana
The Pinus wallichiana is the companion species of the Cedrus deodara and heavily spread over in the most of the study area. The annual litterfall was recorded as 4.6097 t/ha/annum. Monthly litterfall varied from month to month, increasing from August to December. The maximum litterfall results 0.8877 t/ha were seen in the month of December and minimum was 0.2588 t/ha. The results of other months are 0.4317, 0.2861, 0.295, 0.3483 and 0.3411 t/ha given by respective months i.e. November, January, March, April and May. The results recorded maximum for the Pinus wallichiana has with the comparison of Cedrus deodara. Needles of Pinus wallichiana are bigger in length than Cedrus deodara, the litterfall of pinus is more than deodara species and vice versa. It was recorded that the both the species have greater litterfall in the same seasons like November to December and lower in January to February.
Decay rate of Cedrus deodara
On the basis of 45 days interval, mass loss was checked out of 10 grams and the remaining 9.923 grams was calculated. After 90 days the remaining leaf litter calculated was 9.725 grams out of 10 grams. After the next interval of 136 days, three bags of weight 10 grams each was collected and weighed it on the electronic balance and was found 9.323 grams. Moving to the next interval, three bags were taken (after 182 days) off, from the field to calculate the net remaining weight of the leaf litter decay. This time we noted 9.186 grams weight. The next interval was of 226 days, the remaining mass was 9.043 grams out of 10 grams of the three bags. On the other interval of 272 days the remaining mass was 8.881 grams. This is the second last interval of 316 days; the remaining mass was recorded 8.792 grams. The last value was calculated after 363 days which was 8.682 grams out of the 10 grams (Figure 2). The overall annual decompositions rate was 6 percent of the leaf litter mass. As we know that these are evergreen species, that’s why the decomposition rate is too slow. The percentage and the decay coefficient ‘K’, the mass loss and remaining leaf litter are illustrated in the graphical form.
Leaf litter decay rate of Pinus wallichiana
Pinus wallichiana mostly covered the study site. We applied the same time interval and weight of the leaf litter for the Pinus wallichiana as we applied for the Cedrus deodara above. The different and variant results found. At the different time interval, we recorded variant results in mass loss and as well as leaf litter decay rates with respect to time elongation. More the time consumed, the more decay rate was recorded. The minimum mass loss in the first 45 days interval was 0.9663% recorded, and the mean maximum mass loss was 12.533%, the whole results of decay coefficient ‘’K’’, percentage decay rate and mass loss are documented (Figure 3).
Elemental composition of litterfall
The other aim of study is to estimate the nutritional value of litter fall such as carbon, nitrogen, phosphorus and potassium, as these elements play a vital role in growth of plant, also determine the structure, composition and annual yield of plant and primary factor of nutrient cycling in an ecosystem and carbon storage.
Elemental composition of litterfall in Cedrus deodara and Pinus wallichiana
The sample of litter fall was collected throughout the year after the interval of one month. The samples were tested in the laboratory to determine the percentage rate of the Carbon, Nitrogen, Phosphorus and Potassium. The % age was calculated in milligrams per gram.
Total carbon
The total carbon was estimated 2006.67kg/ha/annum of the litter fall of Cedrus deodara (Figure 4). The maximum accumulation of carbon was recorded in December 432.3 kg/ha, the minimum was 99.35 kg/ha estimated table the amount of carbon varies from month to month in Cedrus deodara. The total amount of carbon 2245.07 kg/ha/y was estimated. Maximum amount of carbon was recorded 467.3 kg/ha in December and minimum in January 120.3 kg/ha was calculated. We found the higher carbon content in Pinus wallichiana than Cedrus deodara (Figure 5).
Total nitrogen
Nitrogen is the basic nutritional element of plants, plays a vital role in manufacturing of protein and chlorophyll. Its deficiency leads to chlorosis in the plants the total nitrogen was recorded during the year 53.72 kg/ha/y. The maximum nitrogen content was estimated in December 11.08 kg/ha and the minimum was 2.35 kg/ha recorded. While in Pinus wallichiana the total amount of nitrogen content 51.69 kg/hectare/annum and the mean maximum 10.74 and the mean minimum 2.73 kg/ha were estimated in the month of December and January respectively.
Total potassium
Potassium regulates flow of gaseous through the opening and closing of stomata of the plants. Its deficiency leads to scorching and curling of leaves and also retards the growth of plant, root development and seed formation. The total potassium concentration in Cedrus deodara was 21.33kg/ha/annum, the mean maximum was 4.4 kg/ha in the month of December and the minimum amount 1.15 kg/ha in February estimated. In Pinus wallichiana, total potassium was estimated as 23.13 kg/ha/annum. Its maximum and minimum values 4.92 kg/ha in December and 1.21 kg/ha in February were recorded.
Total phosphorus
Phosphorus is the major element for the growth of shoots and roots development. It regulates metabolism and plays a central role in cell division. Its deficiency retards the genetic process and plant growth. The total amount of phosphorus was 7.91 kg/hac/annum and is maximum i.e. 1.35 in December and minimum 0.42 kg/ha calculated, while the total amount of phosphorus in Pinus wallichiana is 4.79 kg/ha/annum. Its maximum and minimum amounts in the months of December and February were estimated 0.98 and 0.23 kg/ha respectively. Results indicated that Cedrus deodara has greater amount of phosphorus than Pinus wallichiana.
Economic implications
Removal of litterfall support in improving wood quality indirectly by saving wood from buring. It provides alternative to fire wood burning and save precious wood. Pinus wallichiana being better in litter fall and decay is more economic tree than Cedrus deodara.in market price analysis the Cedrus deodara is better than Pinus wallichiana having high market price.
Conclusion and Recommendations
The study was of comparative nature and was designed to analyze the litter fall and decay rate in two species of Cedrus deodara and Pinus wallichiana. The results indicated that Pinus wallichiana is better than Cedrus deodara in both parameters. On the basis of chemical composition, it was found that carbon and phosphorus is relatively more in Pinus wallichiana and nitrogen and potash in Cedrus deodara.
Authors Contribution
Kamran Aziz: Concieved the idea, wrote abstract, Methodology, Conclusion, Data collection, Data entry in SPSS and analysis, Result and discussion, Introduction, References.
Aamir Saleem: Did SPSS analysis, Conclusion, Technical Input at every step, Overall Management of the article, Data analysis, Result and discussion, Introduction, References.
Arshad Mahmood Malik: Did SPSS analysis, Conclusion, Technical input at every step, Overall management of the article, References.
References
Anderson, J. M. and J. S. I. Ingram. 1993. Tropical soil biology and fertility: A handbook of methods (2nd ed.). Wallingford: CAB International.
Bardgett, R.D., D.A. Wardle and G.W. Yeates. 1998. Linking above-ground and below- ground interaction: How plant responses to foliar herbivory influence soil organisms. Soil Biol. Biochem. 30(14): 1867-1878. https://doi.org/10.1016/S0038-0717(98)00069-8
Barg, B. and Laskowski, R. 2006. Advances in ecological research. Litter decomposition: A guide to carbon and nutrient turnover. 38:421.
Barg, B. and McClaugherty. 2014. Plant litter: Decomposition, humus formation, Carbon sequestration, 3rd ed. Springer, Verlag, Berlin, Heidelberg, Germany.
Berg, B. and C. McClaugherty. 2008. Plant litter: Decomposition, Humus formation, Carbon sequestration, second ed. Springer-Verlage Heidelberg Berlin. 3: 338-352.
BjoÈrn, B. 2000. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manage. 133: 13-22. https://doi.org/10.1016/S0378-1127(99)00294-7
Bocock, K.L. and O. Gilbert. 1957. The disappearance of leaf litter under different woodland conditions. Plant soil. 9(2): 179-185. https://doi.org/10.1007/BF01398924
Bothwell, L., P. Selmants, C. Giradina and C. Litten. 2014. Leaf litter decomposition rates increase with rising mean annual temperature in Hawaiian tropical montane wet forests. Peer. J. 2: 685-697. https://doi.org/10.7717/peerj.685
Ciusack, D.F., W.W. Chou, W.H. Yang, M.E. Harmone and W.L. Silver. 2009. Controls on long term root and leaf litter decomposition in neo tropical forests. Glob. Change. Boil. 15: 1339-1355. https://doi.org/10.1111/j.1365-2486.2008.01781.x
Cornelissen, J. 1996. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J. Ecol. 48(4): 573-582. https://doi.org/10.2307/2261479
Cortez, J., J.M. Demard, P. Bottner and L. JocteurMonrozier. 1996. Decomposition of Mediterranean leaf litters: A microcosm experiment investigating relationships between decomposition rates and litter quality. Soil Biol. Biochem. 28(4-5): 443-453. https://doi.org/10.1016/0038-0717(96)00005-3
Gholz, H. L., D. A. Wedin, S. M. Smitherman, M. E. Harmon and W. J. Parton. 2000. Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol. 6:751-765.
Guckland, A., M. Jacob, H. Flessa, F. M. Thomas and C. Leuschner. 2009. Acidity, nutrient stocks and organic matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagussylvatica L.). J. Plant Nutr. Soil Sci. 172: 500-511.
Harmon, M. E., B. Lamberty, J. Tang and R. Vargas. 2011. Heterotrophic respiration in disturbed forests: A review with examples from North America. J. Geophys. Res. Biogeosic. 116 (G4).
Heal, O. W., J. M. Anderson and M. J. Swift. 1997. Plant litter quality and decomposition: an historical overview driven by nature Plant litter quality and decomposition. Pp. 3-30. Wallingford: CAB International.
Jalaluddin, M. 1993. Revised working plan for the Gali reserved forest NWFP. Forest pre investment center Peshawer, Pakistan.
McClaugherty, C. A., J. D. Aber and J. M. Melillo. 1982. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems (Massachusetts). Ecology: A Publication of the ecological society of America.
Odum, S. 1985. Report on frost damage to trees in Denmark after the severe 1981/82 and 1984/85 winters. Horsholm Arboretum, Denmark.
Ostertag, R., & S. E. Hobbie. 1999. Early stages of root and leaf decomposition in Hawaiian forests: Effects of nutrient availability. Oecologia. 121(4): 564-573.
Pineiro, G., J. M. Paruelo, M. Oesterheld and E. G. Jobbagy. 2010. Pathways of grazing effects on soil organic carbon and nitrogen. Rangeland Ecol. Manag. 63: 109-119.
Presscott, C. E., and S. J. Grayston. 2013. Tree Species influence on microbial communities in litter and soil: current knowledge and research needs. Forest Ecol. Manag. 309:19-27.
Swift, M. J., Heal, O. W. and Anderson, J. M. 1979. Decomposition in terrestrial ecosystems (vol.5). Uni. Of California Press.
Vogt, K. A., C. C. Grier, and D. J. Vogt. 1986. Production, turnover and nutrient dynamics of above and below ground detritus of world forests. In Advances in ecological research, Vol. 15, pp. 303-377.Academic Press
WWF, Pakistan. 2010. Pakistan annual report. WWF Pakistan head office.PO box 5180, Ferozpur Road Lahore, Pakistan.