Larvicidal Effect of Ipomoea stolonifera on Laboratory-Reared and Field-Collected Aedes aegypti Mosquitoes in Enugu, Nigeria
Larvicidal Effect of Ipomoea stolonifera on Laboratory-Reared and Field-Collected Aedes aegypti Mosquitoes in Enugu, Nigeria
Godwin Ikechukwu Ngwu1*, Maria Ifeyinwa Ngwu2, Fabian Chukwuemenam Okafor1, Aruh Ottah Anaga3 and Christopher Didigwu Nwani1
1Department of Zoology and Environmental Biology, University of Nigeria, Nsukka-410001
2Department of Pharmaceutical Microbiology and Biotechnology, University of Nigeria, Nsukka-410001
3Department of Veterinary Physiology and Pharmacology, University of Nigeria, Nsukka-410001
ABSTRACT
Comparative study of the effects of methanolic crude extracts of Ipomoea stolonifera on 4th instars larvae of both laboratory-reared and field-collected Aedes aegypti were carried out. The 4th instars larvae of laboratory-reared and the field-collected A. aegypti mosquitoes were exposed to 5000mg/L, 2000 mg/L, 1000 mg/L, 500 mg/L, 100 mg/L, 50 mg/L of the extract. The solvent for the extract, dimethyl sulphoxide (DMSO) was used as control. The results indicate concentration dependent mortalities and significant differences (P<0.05) in the mean percentage mortality between the laboratory-reared and field-collected Aedes mosquitoes. The mortality rate due to the extract was found to be less in the field collected A. aegypti (LC50 = 2.81 ± 0.14; LCI = 2.55; UCI = 3.11) than the laboratory-reared ones (LC50=1.24±0.09; LCI = 1.07; UCI = 1.43) after 24 h exposure. The median larvicidal concentration LC50 values of the extract were 1.24 ± 0.09g/L and 2.81 ± 0.14g/L for 4th instars larvae of both laboratory-reared and field-collected mosquito larvae respectively. Further research is recommended on the isolation of the active ingredients of I. stolonifera for use in effective control of mosquito.
Article Information
Received 31 January 2019
Revised 14 May 2019
Accepted 07 September 2019
Available online 02 April 2020
Authors’ Contribution
GIN and FCO conceived and designed the study. GIN, MIN and AOA conducted the study. GIN, AOA and CDN collated the data and did the statistical analysis. GIN, FCO, AOA and CDN wrote the manuscript. FCO supervised the study.
Key words
Aedes aegypti, Larvae, Ipomoea stolonifera, Larvicidal effect
DOI: https://dx.doi.org/10.17582/journal.pjz/20190131150142
* Corresponding author: [email protected]
0030-9923/2020/0004-1371 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Vector-borne diseases affect over a billion people and lead to the death of more than one million of their victims annually (WHO, 2014). Mosquito-borne diseases are the most common constituent of these vector borne diseases (Burkett-Cadena and Vittor, 2018). Aedes mosquitoes are, in the meantime, the culprits for the transmission of various arboviral diseases. Aedes aegypti, found in Africa, Asia and Central and South America particularly transmit yellow fever, dengue and, of recent interest Chikungunya and Zika viruses, in different parts of the world (Ali et al., 2012; Paixão et al., 2017). These diseases have heightened over the years, principally due to insecticide and drug resistance developed by the mosquito vectors and the pathogens (Liu, 2015). Diverse vector control measures deployed to checkmate threats from these disease vector mosquitoes have continued to be thwarted by their growing resistance to synthetic insecticides due to misuse and overuse (Bass and Jones, 2016; Farooqi et al., 2019). The current challenge demands the development of new and environmentally friendly insecticides that can be used as effective larvicides (Borovsky, 2003) hence the need for continued search for novel ones that will be efficacious in checkmating their activities.
So far, plant extracts seem to serve as appropriate alternatives to synthetic insecticides because they are safe, biodegradable, cost effective and readily available worldwide (Susheela, 2016). Though several plants from different families have been reported to be bioactive against different mosquito stages, only very few of them have moved from the laboratory to field use (Murthy and Rani, 2009) which necessitates continued search for appropriate bioactive plant products that can be used in the field. Aquatic macrophytes, some of which pose problems to mosquito control (Kant and Srivastava, 2004), may have allelochemicals detrimental to these organisms (Ghobrial et al., 2015). These preliminary results demand in-depth study of their phytochemical constitutions, bioactivities and efficacy as possible mosquito control agents.
MATERIALS AND METHODS
Test mosquitoes
Mosquito eggs of A. aegypti reared in the laboratory to the fifth generation down the line were collected from Arbo-Viral Research Laboratory, Federal Ministry of Health Enugu, Nigeria, while the wild mosquito eggs of the A. aegypti were trapped in the Zoological Garden of University of Nigeria, Nsukka. The eggs were hatched and allowed to develop into the 4th instars larvae. The 4th instars larvae appeared within 3 to 4 days after egg-hatching.
Plant collection and extraction
Whole plant (stems, roots and leaves) of Ipomoea stolonifera were collected from the ‘Ohe’ pond in Nsukka Urban (Nsukka Central Local Government Area) of Enugu state, Nigeria. Bio-Resources Development and Conservation Programme, Nsukka centre (BDCP) identified the plants. Absolute methanol of analytical grade (Sigma – Aldrich, Germany) diluted to 70% with distilled water was used for the crude extraction from the plant. A 304 g quantity of I. stolonifera dried at room temperature in the laboratory was pulverised in Thomas Wiley Mill, Model. 4 and then macerated in 70% methanol for 24 h. It was first filtered with muslin cloth and then with Whatman no.1 (24.0 cm) filtre papers. The filtrate was then subjected to rotary evaporation to recover the methanol and then all traces of methanol in the resulting jelly extract removed by keeping it at room temperature for complete evaporation to dryness. The percentage yield of the extract was then determined gravimetrically as follows:
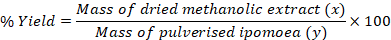
Brine shrimp cytotoxicity assay
The brine shrimp, Artemia salina (Leach) was used in bioassay procedure to estimate the cytotoxicity as well as the biological activity of the I. stolonifera methanolic extracts using standard methods (Mclaughlin and Chnag, 1991; Meyer et al., 2007). The result was analysed using probit analysis (minitab for windows release 12.21) to determine the LC50 at 95% confidence interval.
Phytochemical tests
Small dry quantities of the plant extract were subjected to phytochemical tests following standard methods (Iwu and Chiori, 1984).
Egg hatching and larval rearing
Tap water, in a plastic bowl, used for the egg hatching and larval rearing was to stand undisturbed for 24 h to acclimatize. A piece of white linen cloth containing the eggs was soaked in the treated water. The linen was totally submerged in such a way that the surface with the trapped eggs faced up in the bowl. Eggs started to hatch within 30 minutes but were left overnight, to ensure eggs were completely hatched, before removing the white linen. The young larvae appeared, in a plastic bowl, as tiny motile organisms when viewed with hand lens. The emerging wrigglers were fed with fine powder of brewer’s yeast (Iyaloo and Facknath, 2017) spread over the water and subsequently with more animal, growers’ marsh, chicken feed, product of Vita Feed Nigeria Limited, for quicker generation of detritus which the larvae fed on.
Surface film, scum, which occasionally formed on the surface of the water, was daily skimmed off with a piece of paper. The 4th instars larvae which were reached after the 3rd instars had molted were pooled into a beaker of clean tap water.
Counting-in of larvae and larvicidal screening tests
Twenty 4th instars larvae were counted into each of all labelled 250ml beakers containing only tap water and the set up left to stand on the bench for 24 h in order to acclimatize. Mortality effects of the methanolic extract of I. stolonifera to the larvae were evaluated using graded concentrations method. Stock solution of the methanolic extract was prepared by dissolving 1 g dry-weight of the extracts in 4 ml of dimethyl sulphoxide (DMSO) for every 100 ml stock solution in tap water. Six graded concentrations of 5000mg/L, 2000mg/L, 1000mg/L, 500 mg/L, 100mg/L and 50mg/L were each prepared in triplicates in 100 ml amounts from the stock solution. The control was made up of 40 ml/L of the DMSO solvent.
The larvicidal effects of the different concentrations of the extract were then checked 6 hourly for a period of 24 h. Thus, the larvicidal effects of graded concentrations of the extract were determined within 24 h for the 4th instars larvae of both the laboratory-reared and field-collected mosquitoes.
Statistical analysis
One-way analysis of variance (ANOVA) for comparing the effect of the various concentrations of the extract on the 4th instars larvae was utilised and expressed as mean ± SD. A comparison of lab-reared and field-collected Aedes mosquito was carried out using independent samples t-test. The median lethal concentration bioassay value, LC50 was obtained using probit analysis (Minitab for windows release 12.21). All analyses were carried out using Stata 20 statistical package.
RESULTS
Phytochemical tests
Phytochemical tests performed on the I. stolonifera extracts showed that some compounds were absent (-), present in small concentration (+), present in moderately high concentration (++) or present in very high concentration (+++). The result is as follows: saponins (++), flavonoids (+), glycosides (+++), proteins (+++), tannins (+++), steroids (+), terpenoids (+), reducing sugar (-), carbohydrate (-), resins (-), alkaloids (-), acid (-), fats and oil (-).
Table I. Comparison of mortality effect of substance administered on 4th instars larvae of laboratory-reared and field-collected mosquitoes (Mean + SD).
|
Extract concentration |
Laboratoryreared |
Field- collected |
t–Staistic♣ |
|
5000mg/L |
96.67%±1.67 |
83.33%±7.26 |
1.7889 |
|
2000mg/L |
81.67%±4.41 |
50.00%±2.87 |
.0083*** |
|
1000mg/L |
58.33%±3.33 |
23.33%±4.41 |
.3317*** |
|
500mg/L |
16.67%±4.41 |
0.00%±0.00 |
.7796** |
|
100mg/L |
11.67%±4.41 |
0.00%±0.00 |
2.6458* |
|
50mg/L |
0.00%±0.00 |
0.00%±0.00 |
-- |
|
40ml/L(DMSO) |
0.00%±0.00 |
0.00%±0.00 |
-- |
Test statistic testing for the difference in means of number dead within 24 h between laboratory reared and field-collected mosquito larvae at different concentrations; *, **, *** Significant at 10%, 5% and 1% levels respectively.
Percentage yield of the extract used
The percentage yield (w/w) of the crude methanolic extract of I. stolonifera was 23.06%.
Brine shrimp mortality bioassay
Result of mortality bioassay of methanolic extract of the plant on brine shrimp was LC50 1625.3 ppm at 95% confidence interval.
Larvicidal evaluation
Mean mortality effects of graded concentrations of the extract on 4th instar larvae of both laboratory-reared and field-collected Aedes mosquitoes after 24 h exposure are shown in Figure 1. The result showed that the mean mortality effect of the extract on laboratory-reared mosquitoes was highest (96.67 ± 1.67a %) at 5000mg/L concentration and least (11.67 ± 4.41d %) at 100 mg/L. There were no mortality effects at 50mg/L of the extract. Also, all the larvae in 40ml/L DMSO control were alive and vibrant.
The field-collected mosquitoes also showed highest mortality effect of 83.33±7.26a % at 5000mg/L concentration but least effect of 23.33% ±4.41c at 1000mg/L. None of the larvae at concentrations of 500mg/L, 100mg/L and 50mg/L died. The larvae in the 40ml/L DMSO control of this group were also alive.
The results of dosage responses for the 4th instars larvae of both the laboratory-reared and field-collected mosquitoes at LC50 were 1.24±0.09 and 2.81±0.14 respectively at 95% confidence intervals. Test statistic testing for the difference in means of number dead within 24 h between laboratory-reared and field-collected mosquito larvae at different concentrations are shown in Table I.
DISCUSSION
Tannin formed the highest component (+++) of our crude extract while saponin (++) was next to it. Flavonoids (+), steroids (+) and terpenoids (+) were present in minute amounts. Pure samples of all of these extracts have been reported to be bioactive in various degrees and dimensions. Tannin has been reported to be toxic to Culex. pipiens, Aedes taxa, A. aegypti, A. albopictus and A. rustics (Rey et al., 1999; Tharkur et al., 2004) while saponin has been reported as having larvicidal activity (Jawale, 2014) and especially very efficacious against A. aegypti and C. pipiens (Wiesman and Chapagain, 2003; Chapagain and Wiesman, 2005). Flavonoids, steroids and terpenoids have also been reported to be capable of inducing larval mortality or retarding larval development (Arivoli and Tennyson, 2011; Ghosh et al., 2012). The various constituents of our extract combine effectively and most probably synergistically to boost the efficacy of I. stolonifera as a bioactive agent against the Aedes mosquito sampled. Plant saponins are particularly widely distributed amongst plants and have a wide range of biological properties (Sparg et al., 2004). Since bioactivity of crude plant extracts depend on one or more of its phytochemical constituents, I. stolonifera as good reservoir of these constituents might have contributed significantly to the mortality effect of the crude extract on A. aegypti larvae recorded. The plant, I. stolonifera can therefore be used as bioactive agent in A. aegypti control either as purified sample or as crude extract.
We recorded various degrees of mortality on the 4th instars larvae which were concentration and breeding condition dependent. Susceptibility is known to be concentration and breeding conditions dependent (Gutierrez et al., 2014). The 4th instar larvae of the laboratory-reared mosquitoes were more susceptible to the extract than those of the field-collected. This could be attributed to several factors including insecticide use for controlling crop pests in agriculture as selective pressures favouring resistance; the presence of anthropogenic pollutants common in urban, agricultural or industrial areas, and the impact of plant chemicals (Nkya et al., 2013). The last ones might be more responsible since mosquito larvae are thought to ingest particulate matters like debris or detritus which abound in much of the water bodies where they grow. Interactions between these xenobiotics influence mosquito detoxification pathways (Moon, 1985; Chahine and O’Donnell, 2011) as opposed to the laboratory-reared ones in friendlier and pre-conditioned environment.
The result of log probit analysis showed that LC50 values of the extract were 1.24 ± 0.09g/L and 2.81 ± 0.14g/L for 4th instars larvae of both laboratory-reared and field-collected mosquito larvae. This extract has comparably low bioactivity (Promsiri et al., 2006) which could be attributed to variation in toxicity potentials of the active components of different plants. Phytochemical constituents and concentrations also vary from plant to plant and screening for larvicidal activity of plant extracts could lead to the discovery of new agents for pest and vector control (Kamaraj et al., 2008).
The confinement of the lab-reared Aedes mosquito could also have influenced their higher level of susceptibility as opposed to the field-collected ones which enjoyed free-range life and were thus prone to environmental influences. The methods of application of extracts affect their biological activities as well (Boschitz and Grunwald, 1994). Sensitivity is also influenced by the presence and level of some enzymes like cytochrome P-450, esterase, and glutathione-S-transferase. It has been reported that cytochrome P-450 and esterases are involved in the detoxification of tannins (Rey et al., 1999), a toxic constituent of many plant species. Ipomoea stolonifera is quite available in many aquatic bodies and can be grossly exploited in mosquito control if further research is carried out on how to harness the active ingredients for this purpose.
ACKNOWLEDGEMENT
We are highly indebted to the laboratory staff of Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, for their cooperation during the laboratory study.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Ali, M.S., Ravikumar, S. and Beula, J.M., 2012. Bioactivity of seagrass against the dengue fever mosquito Aedes aegypti larvae. Asian Pac. J. trop. Biomed., 2: 570–573. https://doi.org/10.1016/S2221-1691(12)60099-9
Arivoli, S. and Tennyson, S., 2011. Larvicidal and adult emergence inhibition activity of Abutilon indicum (Linn.) (Malvaceae) leaf extracts against vector mosquitoes (Diptera: Culicidae). J. Biopestic., 4: 27–35.
Bass, C. and Jones, C.M., 2016. Mosquitoes boost body armor to resist insecticide attack. Proc. natl. Acad. Sci., 113: 9145 – 9147. https://doi.org/10.1073/pnas.1610992113
Borovsky, D., 2003. Trypsin-modulating oostatic factor: a potential new larvicide for mosquito control. J. exp. Biol, 206: 3869–3875. https://doi.org/10.1242/jeb.00602
Boschitz, C. and Grunwald, J., 1994. The effect of NeemAzal on Aedes aegypti (Diptera: Culicidae). J. appl. Parasitol., 35: 251 – 256.
Burkett-Cadena, N.D. and Vittor, A.Y., 2018. Deforestation and vector-borne disease:Forest conversion favors important mosquito vectors of human pathogens. Basic appl. Ecol., 26: 101–110. https://doi.org/10.1016/j.baae.2017.09.012
Chahine, S. and O’Donnell, M.J., 2011. Interactions between detoxification mechanisms and excretion in Malpighian tubules of Drosophila melanogaster. J. exp. Biol., 214: 462–468. https://doi.org/10.1242/jeb.048884
Chapagain, B. and Wiesman, Z., 2005. Larvicidal activity of the fruit mesocarp extract of Balanites aegyptiaca and its saponin fractions against Aedes aegypti. Dengue Bull., 29: 203 – 207.
Farooqi, M.A., Irsa, B., Ali, S., Sajjad, A., Hassan, M.W., and Akhtar, S., 2019. Impact of selected insecticides on Apis mellifera L. (Hymenoptera: Apidae) under controlled conditions. Pakistan J. Zool., 52: 193-198. https://dx.doi.org/10.17582/journal.pjz/2020.52.1.193.198
Ghobrial, M.G., Nassr, H.S. and Kamil., A.W., 2015. Bioactivity effect of two macrophyte extracts on growth performance of two bloom-forming cyanophytes. Egypt. J. aquat. Res., 41: 69–81. https://doi.org/10.1016/j.ejar.2015.01.001
Ghosh, A., Chowdhury, N. and Chandra, G., 2012. Plant extracts as potential mosquito larvicides. Indian J. med. Res., 135: 581–598.
Gutierrez, P.M., Antepuesto, A.N., Eugenio, B.A.L. and Santos, M.F.L., 2014. Larvicidal activity of selected plant extracts against the Dengue vector Aedes aegypti mosquito. Int. Res. J. biol. Sci., 3: 23–32.
Iwu, M.M. and Chiori, C.O., 1984. Antimicrobial activity of Eupatorium odoratum extracts. Fitoterapia, 6: 354–356.
Iyaloo, D.P. and Facknath, S., 2017. Optimization of Aedes albopictus rearing procedures: Preliminary steps towards large-scale rearing of the species within the laboratory in Mauritius. J. Ent. Zool. Stud., 5: 46–53.
Jawale, C.S., 2014. Larvicidal activity of some saponin containing plants against the dengue vector Aedes aegypti. Trends Biotechnol. Res., 3: 1–11.
Kamaraj, C., Rahuman, A. and Bagavan, A., 2008. Antifeedant and larvicidal effects of plant extracts against Spodoptera litura (F.), Aedes aegypti L. and Culex quinquefasciatus Say. Parasitol. Res., 103: 325–331. https://doi.org/10.1007/s00436-008-0974-8
Kant, R. and Srivastava, H. C., 2004. Observations on anopheline breeding in relation to aquatic plants in different breeding habitats of Kheda (Gujarat). J. Commun. Dis., 36: 187–194.
Kauffman, E.B. and Kramer, L.D., 2017. Zika virus mosquito vectors: Competence, biology, and vector control. J. Infect. Dis., 216: S976–990. https://doi.org/10.1093/infdis/jix405
Liu, N., 2015. Insecticide resistance in mosquitoes: impact, mechanisms and research directions. Annu. Rev. Ent., 60: 537–559. https://doi.org/10.1146/annurev-ento-010814-020828
Mclaughlin, J.L. and Chnag, C., 1991. Bench-top bioassays for the discovery of bioactive natural products: an update. In: Studies in natural products chemistry (eds. B.V. Elsevier and S. Ur-Rahman). Amsterdam: Elsevier, pp. 383–409.
Meyer, B.N., Ferrigni, N.R., Putman, J.E., Jacobson, L.B., Nichols, D.E. and Mclaughlin, J.L., 2007. Brine shrimps: a convenient general bioassay for active plant constituents. Pl. Med., 45: 31 – 34. https://doi.org/10.1055/s-2007-971236
Moon, R.D.,1985. A brief overview of the life history, physiology and ecology of Minnesota mosquitoes. J. Minnesota Acad. Sci., 50: 6–8.
Murthy, J.M. and Rani, P.U., 2009. Biological activity of certain botanical extracts as larvicides against the yellow fever mosquito, Aedes aegypti L. J. Biopestic., 2: 72–76.
Nkya, T.E., Akhouayri, I., Kisinza, W., and David, J.P., 2013. Impact of environment on mosquito response to pyrethroid insecticides: Facts, evidences and prospects. Insect Biochem. mol. Biol., 43: 407–416. https://doi.org/10.1016/j.ibmb.2012.10.006
Paixão, E.S., Teixeira, M.G. and Rodrigues, L.C., 2017. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Hlth., 3: e000530. https://doi.org/10.1136/bmjgh-2017-000530
Promsiri, S., Naksathit, A., Kruatrachue, M. and Thavara, U., 2006. Evaluation of larvicidal activity of medicinal plant extracts to Aedes aegypti (Diptera: Culicidae) and other effects on a non-target fish. Insect Sci., 13: 179–188. https://doi.org/10.1111/j.1744-7917.2006.00080.x
Rey, D., Cuany, A., Pautou, M. and Meyran, J., 1999. Earth and environmental science. J. chem. Ecol., 25: 537–548. https://doi.org/10.1023/A:1020953804114
Sparg, S.G., Light, M.E. and Van, S.J., 2004. Biological activities and distribution of plant saponins. J. Ethnopharmacol., 94: 219–243. https://doi.org/10.1016/j.jep.2004.05.016
Susheela, C.P., 2016. Evaluation of larvicidal action of natural extracts on mosquito larvae of Aedes aegypti (Diptera: Culicidae). Int. J. Mosq. Res., 3: 26–30.
Tharkur, N., Mainkar, S.P., Pandit, R.A. and Indap, M.M., 2004. Mosquito larvicidal potential of some extracts obtained from the marine organisms-prawn and sea cucumber. Indian J. Mar. Sci.., 33: 303–306.
WHO., 2014. A global brief on vector-borne diseases. Retrieved from http://apps.who.int/%09iris/bitstream/10665/111008/1/WHO_DCO_WHD_2014.1_eng.pdf
Wiesman, Z. and Chapagain, B.P., 2003. Laboratory evaluation of natural saponins as a bioactive agent against Aedes aegypti and Culex pipiens. Dengue Bull., 27: 168–173.
To share on other social networks, click on any share button. What are these?










