Influence of Water Activity and Time Duration on Fusarium Mycotoxins Production in Maize Grains During Post-Harvest Storage
Influence of Water Activity and Time Duration on Fusarium Mycotoxins Production in Maize Grains During Post-Harvest Storage
Fazle Akbar and Sahib Alam*
Department of Agricultural Chemistry, The University of Agriculture Peshawar, KP 25130, Pakistan
Abstract | This study investigated the influence of water activity (aw) and storage time on total fungal and Fusarium counts and mycotoxins zearalenone (ZON), trichothecenes type-A (i.e. T-2 and HT-2) and trichothecenes type-B (i.e. DON, 3-ac DON, 15-ac DON) production in maize grains. Water activity of the grains was adjusted to 0.85, 0.90 and 0.95 aw and the samples were stored at 25°C for 45 days. The control samples were kept without any aw modification. At 15 days interval, the samples were analyzed for the aforesaid parameters. The data showed that aw and storage time alone and interactively significantly (p<0.05) affected the analyzed parameters. The lowest total fungal counts (12.26×103 CFUs/g) were recorded in 0.85 aw sample at the start of storage, whereas the highest total viable counts i.e. 18.01×103 CFUs/g were documented at day 45 in the grain sample of 0.95 aw. The total Fusarium count varied from 0.42×103 CFUs/g in control sample at the start of storage time to 0.94×103 CFUs/g at day 45 in the sample modified to 0.95 aw. The lowest ZON (855.50 µg/kg), DON (1246.45 µg/kg), 3-ac DON (234.41 µg/kg), 15-ac DON (90.58 µg/kg), T-2 (221.15 µg/kg) and HT-2 (221.24 µg/kg) were recorded at the onset of storage time in the sample of 0.85 aw, whereas these mycotoxins steadily increased and attained the highest values at the uppermost marginal values of aw and storage time.. It was concluded that fungal growth and mycotoxins production in maize grains were favored by elevated aw and increasing storage time. Therefore, it is recommended that cereals grains should be kept at reduced aw to prevent fungal growth and subsequent mycotoxins production in the grains during post-harvest storage.
Received | September 16, 2019; Accepted | November 15, 2019; Published | November 26, 2019
*Correspondence | Sahib Alam, Department of Agricultural Chemistry, The University of Agriculture Peshawar, KP 25130, Pakistan; Email: dralam@aup.edu.pk
Citation | Akbar, F. and S. Alam. 2019. Influence of water activity and time duration on fusarium mycotoxins production in maize grains during post-harvest storage. Sarhad Journal of Agriculture, 35(4): 1326-1335.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.4.1326.1335
Keywords | Fusarium, Mycotoxins, Water activity, Zearalenone, Trichothecenes, Maize
Introduction
Maize (Zea mays L.) is the third major cereal crop after wheat and rice in Pakistan (Tanveer et al., 2014). Maize grains are used in the production of edible oils, animal feed and alcohol in addition to direct human consumption. In Pakistan maize was cultivated on a total area of 1.34 million hectares in 2016-2017 with a total production of 6.134 million tonnes (MNFSR, 2017). In Pakistan, the maize is mainly grown in Khyber Pakhtunkhwa and Punjab. The crop is usually grown two times in a year during July to September and February to March.
Maize crop is infected by several genera of fungi including Penicillium, Aspergillus, Fusarium, Rhizopus and Mucor during the pre- and post-harvest stages (Hell et al., 2000; Nesci et al., 2003; Abbas et al., 2006). Maize infestation by Fusarium species starts from standing crop and prevails during post-harvest storage of grains (Krnjaja et al., 2013). Deleterious species of Fusarium such as Fusarium langsethiae, F. graminearum, F. poae, F. culmorum, F. sporotrichioides, F. crookwellense and F. equiseti secrete toxic secondary metabolites known as mycotoxins (Trung et al., 2001). Toxicologically important Fusarium mycotoxins are categorized into zearalenone (ZON), trichothecenes family [i.e. trichothecenes type-A comprised of T-2 toxin (T-2) and HT-2 toxin (HT-2) and trichothecenes type-B include deoxynivalenol (DON), 3-acetyldeoxynivalenol (3-ac DON), 15-acetyldeoxynivalenol (15-ac DON)] and fumonisins (Antonissen et al., 2014). Recent survey conducted by BIOMIN across 72 countries of the world showed the infection of maize by mycotoxins especially trichothecenes, zearalenone and fumonisin (Taschl and Jenkins, 2018). Temperate climatic conditions favor Fusarium infestation of cereal crops, since they need comparatively lower temperature for their growth and mycotoxin production than other toxigenic fungi, for instance, Aspergillus species.
Fusarium species attack maize crop at any stage of production, harvesting and storage. Their infestation result in substantial quantity and quality deterioration of maize grains and cause sufficient economic losses to the farming community. The major factors that influence Fusarium infection and their subsequent production of mycotoxins include environmental relative humidity, temperature, moisture availability or water activity (aw) of the grains, pre- and postharvest handling, infestation and physical damage caused by insects and storage structure (Sanchis and Magan, 2004).
The most relevant parameter when considering moisture in relation to fungal growth is not the water content of a substrate but the water activity. It is a prime tool practiced for care and increasing shelf life of food and feed with respect to physicochemical properties, speed of degradative reactions and microbial growth (Vega-Mercado and Barbosa-Canovas, 1994). Water activity represents the free water content that is not physically or chemically bound to the substrate and is therefore instantly available for microbial growth. Fungal growth is therefore directly related to water activity rather than water content per se (Magan and Olsen, 2004). The aw of a hygroscopic substance is equivalent to its per cent equilibrium humidity and is measured on a scale from 0.0 (completely dry) to 1.0 (pure water). Most of the food spoilage fungi cannot grow at aw level less than 0.70, for maize this corresponds to 15% moisture content (Multon, 1988). In an in vitro study Hope and Magan (2003) found that the range of aw for mycotoxins production was narrower than the range for the growth of fungi. They also demonstrated that mycotoxin production was enhanced by increasing aw level. Likewise, there is a positive relation between mycotoxin accumulation in grains and storage length (Kaaya and Kyamuhangire, 2006; Liu et al., 2006).
In the forth-going situation of Fusarium mycotoxin contamination in Pakistani maize (Khatoon et al., 2012), this research was carried out to determine the impact of aw and storage time on total fungal and Fusarium population and production of the mycotoxins ZON and trichothecenes type-A (T-2 and HT-2) and trichothecenes type-B (DON, 3-ac DON, 15-ac DON) in maize grains during storage.
Materials and Methods
Water activity modification and storage of maize grains
This study was conducted at the Department of Agricultural Chemistry, the University of Agriculture Peshawar during 2017-18. Maize grain composite sample (5 kg) was obtained from Agricultural Research Farm, the University of Agriculture Peshawar. The experiment was designed in completely randomized design with two factors. The factors included aw at levels of 0.95, 0.90 and 0.85 and incubation period at 0, 15, 30 and 45 days of storage. Sterilized distilled water was used for the rehydration (i.e. aw modification) of maize grain samples. The samples (400 g each) were taken in sterilized food jars (Ampula Ltd, UK) having porous lids and water was added to them. The required quantity of water to attain the desired aw levels of the sample was calculated from the moisture sorption isotherm prepared for maize grains (Figure 1). Initially the jars kept at 4°C for 48 hrs with intermittent shaking for uniform absorption of water by the grains. The control treatment was kept without addition of water. A total of twelve replicate jars were prepared for each treatment aw level and incubated at 25°C in closed containers. A beaker filled with glycerol-water solution at the same aw level was kept alongside the sample jars in order to sustain uniform equilibrium relative humidity (ERH) inside the containers. The containers were tightly closed to prevent external contamination to maintain uniform ERH throughout the experimental period. Three replicate jars were sampled at the start (day-1) and at 15, 30 and 45 days of storage and the grains were analyzed for total fungal and Fusarium count, zearalenone and trichothecenes concentration.
Total fungal and fusarium count
Total fungal count of the grain sample was determined by the method of Alam et al. (2014) using malt extract agar media. One gram well ground sample was taken in Universal glass bottle that contained 9 ml sterilized distill water and 0.01 % Tween 80. A dilution series ranged from 101-10-5 was made by using one in 9 ml after mixing the contents with Worley mix. An aliquot of 100 µl the sample suspension was spread on the media in Petri plates using sterilized bent Pasteur pipettes. The petri dishes were kept in the dark for one week at 25°C. After the incubation period, fungal colonies were counted and the total fungal count per gram of sample was calculated by using the following formula:
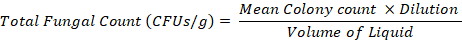
Similar procedure was adapted for the calculation of total Fusarium count of the samples where only the Fusarium colonies were counted excluding the colonies of other fungal species.
Determination of zearalenone and trichothecenes
The determination of mycotoxins i.e. ZON, DON, 3-ac DON, 15-ac DON, T-2 and HT-2 was carried out by modified procedures of Ostry and Skarkova, (2000) and Hanif et al. (2006) using High Performance Thin Layer Chromatography (HPTLC).
Chemicals and reagents
The standards of the above-mentioned mycotoxins were acquired from Biopure, Austria. The solvents used for HPTLC were of gradient grade and were obtained from Merck, Germany. Immunoaffinity cleanup columns ZON, DON, 3-ac DON, 15-ac DON, T-2 and HT-2 TestTM from Vicam (Watertown, MA, USA) were used for the sample clean-up.
Sample extraction and cleanup
The grains were finely grounded using laboratory grinder to pass through a 20-mesh size. Mycotoxins extraction from the sample was carried with a mixture of water and acetonitrile (16:84 v/v). About 20 g well ground sample was mixed with 50 ml of the extraction mixture and blended in high speed laboratory blender for 5 minutes. The suspension was then passed through Whatman filter paper to separate the filtrate. Aliquots of the filtrates (2 ml each) were loaded on immunoaffinity cleanup columns designated for each mycotoxin. The columns were washed with purified water (5 mL) and then vacuum dried for 3 minutes. The target mycotoxins were eluted with methanol (1 ml) into a 1.8 ml vial.
High performance thin layer chromatography
An aliquot of 20μl of cleaned sample extract was applied to silica gel thin layer chromatographic plates along with diluted calibration standards of mycotoxins. The TLC plates were eluted and developed with a mixture of propanol-acetone-chloroform (1:1:8 v/v) in dark in a 10 x 20 cm well saturated horizontal chamber by HPTLC. The plated were dried with gentle blow of cold air and then sprayed with solution of aluminum chloride in ethanol (20 %) and heated at 105°C for 10 min. After developing the plates, a fluorescence densitometry with a Camag TLC Scanner II having mercury lamp and K 400 secondary filter was used to determine the concentration of mycotoxins. The excitation and emission wavelengths were 366 and 420 nm, respectively.
Statistical analysis
The data were statistically analysed using two factor Completely Randomized Design (CRD) by statistical software Statistix 8.1. The significant differences among treatment means were calculated by Least Significant Difference (LSD) test for the main as well as interaction effect (Steel et al., 1996).
Results and Discussion
Total fungal count of maize grains
The data presented in Figure 2 show the total fungal count of maize grains samples at different aw levels and at storage duration of 0, 15, 30 and 45 days. It was observed that a significant (p<0.05) increase in total fungal count was occurred with increase in water activity and storage time. The lowest total fungal counts (12.26×103 CFUs/g) were recorded in control sample at the start of storage time (i.e. day-1), whereas the highest total viable fungal count i.e. 18.01×103 CFUs/g were documented in the sample modified to 0.95aw at day 45 of storage.
Total fusarium count of grains
The data regarding the total Fusarium count of maize grains samples stored at aw levels of 0.85, 0.90 and 0.95 aw for 45 days are shown in Figure 3. It was observed that significant increase (p<0.05) occurred in the total Fusarium count with increase in water activity and storage time. The total Fusarium count ranged from 4.20×102 CFUs/g to 5.40×102 CFUs/g in control and 0.95 aw samples, respectively. The data concerning storage time showed that total Fusarium count ranged from 4.20×102 CFUs/g to 9.40×102 CFUs/g; the lowest in control sample at the start of storage time while the highest in 0.95 aw sample at day 45.
Zearalenone content of maize grains
Table 1 represent the data regarding the ZON contents of maize grain samples at storage interval of 0, 15, 30 and 45 days in response to water activity modification at 0.85, 0.90 and 0.95 aw levels. Statistical analysis of the data showed that both aw and storage duration had significant influence on ZON production in maize grains. The highest content of ZON (928.55 µg/kg) was recorded at 0.95 aw at 45 days interval, whereas the lowest concentration (855.50 µg/kg) was analyzed in the control sample at the start of storage. An increasing trend of ZON contents was observed with respect to increasing aw and storage time whereas the interactive impact of aw and incubation time was non-significant (p>0.05).
Trichothecenes mycotoxins
There are two types of trichothecenes mycotoxins i.e. type-A and type-B. The type-A trichothecenes includes HT-2 and T-2 whereas the type-B consists of DON, 3-ac DON and 15-ac DON.
HT-2 content
Table 2 presents the HT-2 contents of maize grain samples stored at 0.85, 0.90 and 0.95aw for 45 days. The data showed that the HT-2 contents of maize samples significantly (p<0.05) varied with aw levels and storage time. The control sample exhibited the lowest HT-2 contents (221.24 µg/kg) at day-1 of storage time while the highest content (303.80 µg/kg) was recorded at 0.95 aw after 45 days of storage. The data regarding the effect of storage time showed that an increase in HT-2 content occurred from an average value of 244.98 at day-1 to 277.17 µg/kg at day 45 of storage. Likewise, the HT-2 content of the grains steadily increased with increase in water activity.
T-2 content
Table 3 represents the data pertaining to T-2 mycotoxin content of maize samples in response to aw modification and storage for a period of 45 days. The data concerning T-2 contents in maize grains varied significantly (p<0.05) with water activity alteration and storage time. The lowest T-2 contents (221.15 µg/kg) was recorded in control sample at the onset of storage time which progressively increased to 251.13 µg/kg
Table 1: Zearalenone contents (µg/kg) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 855.50±1.50 | 875.47±2.39 | 892.29±2.30 | 899.28±3.40 | 880.64 ±4.20d |
| 0.85 | 875.21±2.50 | 887.59±2.08 | 903.22±2.10 | 908.84±2.90 | 893.72±4.04c |
| 0.90 | 891.18±2.25 | 896.94±2.25 | 909.78±2.15 | 920.48±3.12 | 904.60±3.95b |
| 0.95 | 905.71±2.75 | 909.22±2.30 | 923.19±2.34 | 928.55±3.42 | 916.67±4.12a |
| Mean | 881.90±2.75d | 892.31±2.40c | 907.12±2.38b | 914.29±3.34a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
Table 2: HT-2 contents (µg/kg) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 221.24±1.45 | 231.23±1.88 | 241.16±1.98 | 251.14±2.04 | 236.19±2.33d |
| 0.85 | 236.25±1.85 | 247.17±2.05 | 258.13±1.94 | 268.04±2.12 | 252.40±2.35c |
| 0.90 | 251.29±1.75 | 262.58±1.95 | 275.20±2.23 | 285.71±1.99 | 268.69±2.23b |
| 0.95 | 271.16±1.54 | 282.40±2.06 | 293.81±2.12 | 303.80±2.11 | 287.79±2.14a |
| Mean | 244.98±2.34d | 255.85±2.43c | 267.07±2.45b | 277.17±2.55a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
Table 3: T-2 contents (µg/kg) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 221.15±1.54 | 230.95±1.66 | 241.15±1.78 | 251.13±1.68 | 236.10±1.68d |
| 0.85 | 231.00±1.74 | 239.02±1.58 | 249.83±1.89 | 259.12±1.79 | 244.74±1.87c |
| 0.90 | 236.78±1.86 | 245.76±1.65 | 254.65±1.95 | 263.71±1.93 | 250.23±1.96b |
| 0.95 | 240.96±1.95 | 251.14±1.96 | 260.92±2.06 | 271.03±1.87 | 256.01±1.99a |
| Mean | 232.47±1.98d | 241.72±2.14c | 251.64±2.21b | 261.25±2.18a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
after 45 days of storage. Similarly, the samples which were modified to 0.95aw showed 240.96 µg/kg T-2 mycotoxin that steadily increased to 271.03 µg/kg after 45 days of storage.
Deoxynivalenol content
The data related to the DON mycotoxin content of maize grains samples stored at 0.85, 0.90 and 0.95 aw levels for 45 days are shown in Table 4. Analysis of variance indicated that both aw and storage duration alone and in combination significantly (p<0.05) affected DON production maize grains. Generally, an increasing trend of the mycotoxin production was noted with increasing level of aw and storage time. The lowest mean value (1246.45 µg/kg) was recorded in control sample, while the highest (1377.47 µg/kg) was documented at 0.95aw. The data concerning the storage time indicated that lowest mean value of DON contents (1230.02 µg/kg) in maize samples was recorded at day-1 followed by 15 days (1281 µg/kg), 30 days (1358.7 µg/kg) and the highest (1397.51 µg/kg) was recorded at 45 days of storage.
3-ac DON content
The data regarding 3-ac DON in maize samples at different water activity levels and different storage time showed increasing trend as the water activity and storage time increased (Table 5). Significant (p<0.05) variation was observed in 3-ac DON contents at different water activity levels. The maximum value (271.71 µg/kg) was recorded at 0.95 aw and the minimum (234.41 µg/kg) was noted in control sample.
Table 4: DON contents (µg/kg) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 1151.48±2.98 | 1211.48±3.18 | 1291.50±3.45 | 1331.33±3.46 | 1246.45±3.23d |
| 0.85 | 1211.14±2.15 | 1260.95±3.65 | 1341.10±3.65 | 1381.25±3.73 | 1298.61±3.45c |
| 0.90 | 1261.18±3.18 | 1306.05±3.22 | 1386.18±2.98 | 1426.21±3.98 | 1344.91±3.65b |
| 0.95 | 1296.28±3.25 | 1345.99±3.24 | 1416.34±3.55 | 1451.25±4.04 | 1377.47±3.98a |
| Mean | 1230.02±4.86d | 1281.12±4.67c | 1358.78±4.78b | 1397.51±4.54a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
Table 5: 3-ac DON contents (µg/kg) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 211.25±1.56 | 231.26±1.76 | 241.95±1.87 | 253.16±1.79 | 234.41±185d |
| 0.85 | 233.04±1.65 | 243.26±1.89 | 254.28±1.96 | 264.98±1.98 | 248.89±1.97c |
| 0.90 | 244.22±1.88 | 255.32±1.99 | 266.43±1.78 | 276.98±2.03 | 260.74±2.08b |
| 0.95 | 257.26±1.99 | 267.16±2.11 | 276.45±1.94 | 285.98±2.32 | 271.71±2.43a |
| Mean | 236.44±2.14d | 249.25±2.35c | 259.78±2.09b | 270.28±2.56a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
Table 6: 5-ac DON contents (µg kg-1) of maize grains at water activity level of 0.85, 0.90, 0.95aw at 1, 15, 30 and 45 days of storage.
| Water activity (aw) | Time Interval (days) | Mean | |||
| 1 | 15 | 30 | 45 | ||
| Control | 80.98±1.44 | 87.24±1.58 | 93.98±1.68 | 100.11±1.65 | 90.58±1.78d |
| 0.85 | 87.55±1.65 | 93.88±1.66 | 100.65±1.58 | 107.92±1.76 | 97.50±1.94c |
| 0.90 | 94.84±1.78 | 101.64±1.87 | 109.03±1.76 | 114.92±1.58 | 105.11±1.76b |
| 0.95 | 100.85±1.89 | 107.91±1.98 | 114.72±1.67 | 120.88±1.87 | 111.09±2.12a |
| Mean | 91.05±2.01d | 97.67±1.99c | 104.60±2.23b | 110.96±1.96a | |
Means in each row and column followed by different letters are significantly different at p≤0.05.
The data concerning the 3-ac DON contents with respect to time storage showed highest (270.28 µg/kg) value at days 45 and lowest (236.44 µg/kg) was recorded at the start of storage.
15-ac DON content
Table 6 represents the data regarding 15-ac DON in maize grain samples at different water activity levels and at different time storage. The data recorded for water activity showed a range of average values from 90.58 µg/kg to 111.09 µg/kg. The minimum value was recorded in control sample and the maximum was noted at 0.95 aw. Similarly, the mean highest value (110.96 µg/kg) for time storage was recorded at 45 day of storage and lowest (91.05 µg/kg) was noted for 15-ac DON contents in maize samples at the start of storage time.
To study the effect of water activity and storage time, the samples of maize were modified to various water activity levels (0.85, 0.90 and 0.95 aw) and stored for 45 days under ambient room temperature of 25°C. The samples were analyzed for total fungal count, total Fusarium count and mycotoxins ZON, HT-2, T-2, DON, 3-ac DON, and 15-ac DON at different storage intervals i.e. 0, 15, 30 and 45 days of storage.
The results regarding total fungal counts in maize grain samples showed that the lowest total viable fungal counts i.e. 12.26×103 were recorded at the start of experiment (day-1 of storage) in control (aw not modified) samples. The total fungal count increased with increase in aw and storage duration. The highest values for total fungal population was recorded for the samples of maize at incubation period of 45-day and water activity level 0.95aw. The present results are fairly in line with findings of Marin et al. (2000) and Suhr and Nielsen, (2004). They demonstrated that sufficient availability of water enhanced the total fungal population of stored maize grains. In other studies, conducted by Beg et al. (2006), Zinedine et al. (2007) on broiler feed having corn grains as vital ingredient reported that fungal colonization and ultimately mycotoxins accumulation was a serious issue that was affected by various factors including water availability and length of storage time. Fungal infection in cereal grains is one of the serious issues that are responsible for the reduction of quality and quantity of grains as well as cause huge economic losses to human societies. Fungal infestation of food and feed also results in the production of a number of mycotoxins and threatens humans and animals’ health (Manizan et al., 2018). Reasonably warm and humid climatic environment, poor post-harvest management and insufficient storage practices facilitate the fungal growth and mycotoxins production (Hell et al., 2000; Klich, 2007). There are several methods which can be used for the control of fungal growth in cereals e.g. thermal inactivation, irradiation, enzymatic and microbial degradation, use of chemical preservatives and reducing the water activity of the substrate (Byun and Yoon, 2003; Haque et al., 2009; Alam et al., 2014).
The results of our study exhibited that as the water activity of the grains increased from control to 0.95 aw, the total Fusarium count also increased. The highest total Fusarium count i.e. 9.40×103 CFUs/g was recorded at 0.95 aw after 45 days of storage. Our results are in line with those of Jayas and Jeyamkondan, (2002) and Mannaa and Kim (2017) who studied the influence of aw and temperature on fungi and their mycotoxins secretions in stored grains. They reported that Fusarium, Alternaria and Epicaccum species are hygroscopic in nature and increase their mycelial growth at high water activity level i.e. 1.00 aw. Marin and Magan (1999), Sanchis and Magan (2004) and Waskiewicz et al. (2010) also reported that F. graminearum and Fusarium spp grow best at water activity level of 0.98 aw and produce maximum zearalenone, fumonisins and deoxynivalenol contents in cereal grains at higher aw levels and at temperature range of 18-25°C. Fusarium species are amongst the most common plant pathogens which attack the crops at various stages of crop cycle. Their preferred hosts include cereals like wheat, maize, rice, oat, barley and triticale. They infect different parts of the plant including seedlings, heads, roots and stems and cause a variety of diseases e.g. Fusarium head blight, Fusarium wilt and Fusarium root rot (Balmas et al., 2015). These diseases are the main cause of economic losses which are estimated in billions of dollars worldwide. In a study conducted in Europe the main diseases caused in cereal crops were due to the infection of different Fusarium species like F. graminearum, F. crookwellence, F.poae, F. sporotriochiodes, F. langsethiae, F. seudograminearum, and F. equiseti (Botallico, 2002; Vogelgsang et al., 2008). There are several reports which describe the infection of Fusarium species on winter corn with relation to climatic conditions and crop alternation (Xu et al., 2013; Czembor et al., 2015).
Fungal infestation also deteriorates the crop quality by producing mycotoxins which results in the degradation of the quality and quantity of cereal grains (Champeil et al., 2004; Wegulo et al., 2015). The Fusarium mycotoxins contamination in cereals is a major problem for food safety and security throughout the world. Our results showed that the samples of maize had an increased level of ZON from 35.32 µg/kg in control sample to 928 µg/kg in the sample at 0.95aw at 45-day of storage. Similarly, the results for DON had the highest value recorded in maize samples which was maximum (1397 µg/kg) at 45-day and 0.95 aw. The results of Milani (2013) and Neme and Mohammad (2017) are in close agreement to our findings. They also noted increase in ZON and DON contents with increase in aw levels. Likewise, increasing pattern was noted for HT-2, T-2, 3-ac DON and 15-ac DON with increase in water activity and incubation time in all the samples of maize. Sanchis and Magan (2004) and Alam et al. (2009) reported that the optimum temperature for growth and aflatoxin production by Aspergillus range from 25 to 30oC and best water activity level is 0.95 aw.
Conclusions and Recommendations
It was concluded that lower water activity level and shorter storage time restricted total fungal and Fusarium population, while increased level of water activity (0.95 aw) and longer storage time (45-days) provided conducive environment for total fungal and Fusarium growth which inevitably increased the production of mycotoxins zearalenone and trichothecenes in maize grains.
Novelty Statement
This is the first study of its type to investigate the combined effect of water activity and storage time on Fusaria population and their associated mycotoxins production in maize grains in Pakistan.
Author’s Contribution
Fazle Akbar conducted the experimental work, collected and analyzed the data and wrote the manuscript. Sahib Alam designed and supervised the study, provided overall inputs and conceived the idea of the research.
References
Abbas, H.K., R.M. Zablotowicz, H.R. Bruns and C.A. Abel. 2006. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus iso https://doi.org/10.1080/09583150500532477 lates. Biocontrol Sci. Tech. 16: 437-449.
Alam, S., H.U. Shah and N. Magan. 2009. Water availability affects extracellular hydrolytic enzyme production by Aspergillus flavus and Aspergillus parasiticus. World Mycotoxin J. 2: 313-322. https://doi.org/10.3920/WMJ2008.1108
Alam, S., H.U. Shah, N.A. Khan, A. Zeb, A.S. Shah and N. Magan. 2014. Water availability and calcium propionate affect fungal population and aflatoxins production in broiler finisher feed during storage. Food Addit. Contam. 31:1896-1903. https://doi.org/10.1080/19440049.2014.963699
Antonissen, G., A. Martel, F. Pasmans, R. Ducatelle, E. Verbrugghe, V. Vandenbroucke, S. Li, F. Haesebrouck, F. Van Immerseel and S. Croubels. 2014. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins. 6: 430-452. https://doi.org/10.3390/toxins6020430
Balmas, V., B. Scherm, A. Marcello, M. Beyer, L. Hoffmann, Q. Migheli and M. Pasquali. 2015. Fusarium species and chemotypes associated with Fusarium head blight and Fusarium root rot on wheat in Sardinia. Plant Path. 64: 972-979. https://doi.org/10.1111/ppa.12337
Beg, M.U., M. Al-Mutairi, K.R. Beg, H.M. Al-Mazeedi, L.N. Ali and T. Saeed. 2006. Mycotoxins in poultry feed in Kuwait. Arch. Environ. Contam. Toxic. 50: 594-602. https://doi.org/10.1007/s00244-005-2094-0
Bottalico, A. and G. Perrone. 2002. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 108: 611-624. https://doi.org/10.1007/978-94-010-0001-7_2
Byun, J.R. and Y.H. Yoon. 2003. Binding of aflatoxin G1, G2 and B2 by probiotic Lactobacillus spp. Asian Aus. J. Anim. Sci. 16: 1686-1689. https://doi.org/10.5713/ajas.2003.1686
Champeil, A., T. Doré and J.F. Fourbet. 2004. Fusarium head blight: Epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 166: 1389-1415. https://doi.org/10.1016/j.plantsci.2004.02.004
Czembor, E., L. Stępien and A. Waskiewicz. 2015. Effect of environmental factors on Fusarium species and associated mycotoxins in maize grain grown in Poland. PLoS One. 10: e0133644. https://doi.org/10.1371/journal.pone.0133644
Haque, M.N., R. Chowdhury, K.M.S. Islam and M.A. Akbar. 2009. Propionic acid is an alternative to antibiotics in poultry diet. Bangl. J. Anim. Sci. 38: 115-122. https://doi.org/10.3329/bjas.v38i1-2.9920
Hell, K., K.F. Cardwell, M. Setamou and H.M. Poehling. 2000. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. J. Stored Prod. Res. 36: 365-382. https://doi.org/10.1016/S0022-474X(99)00056-9
Hope, R. and N. Magan. 2003. Two-dimensional environmental profiles of growth, deoxynivalenol and nivalenol production by Fusarium culmorum on a wheat-based substrate. Lett. Appl. Microbiol. 37: 1-5. https://doi.org/10.1046/j.1472-765X.2003.01358.x
Jayas, D.S. and S. Jeyamkondan. 2002. Post-harvest technology: modified atmosphere storage of grains meats fruits and vegetables. Biosyst. Eng. 82: 235-251. https://doi.org/10.1006/bioe.2002.0080
Kaaya, N.A. and W. Kyamuhangire. 2006. The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. Int. J. Food Microbiol. 110: 217-223. https://doi.org/10.1016/j.ijfoodmicro.2006.04.004
Klich, M.A. 2007. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycosci. 48: 71-80. https://doi.org/10.1007/S10267-006-0336-2
Krnjaja, V., J. Levic, S. Stankovic, T. Petrovic and M. Lukic. 2013. Molds and mycotoxins in freshly harvested maize. Zbornik Matice srpske za prirodne nauke. 124: 111-119. https://doi.org/10.2298/ZMSPN1324111K
Liu, Z., J. Gao and J. Yu. 2006. Aflatoxins in stored maize and rice grains in Liaoning Province, China. J. Stored Prod. Res. 42: 468-479. https://doi.org/10.1016/j.jspr.2005.09.003
Magan, N. and M. Olsen. 2004. Mycotoxins in food: detection and control. Woodhead Publishing, Cambridge, England. https://doi.org/10.1201/9781439823361
Manizan, A.L., M. Oplatowska-Stachowiak, I. Piro-Metayer, K. Campbell, R. Koffi-Nevry, C. Elliott, D. Akaki, D. Montet, C. Brabet. 2018. Multi-mycotoxin determination in rice, maize and peanut products most consumed in côte d’ivoire by UHPLC-MS/MS. Food Control. 87: 22-30. https://doi.org/10.1016/j.foodcont.2017.11.032
Mannaa, M. and K.D. Kim. 2017. Influence of temperature and water activity on deleterious fungi and mycotoxin production during grain storage. Mycobio. 45: 240-254. https://doi.org/10.5941/MYCO.2017.45.4.240
Marin, S., N. Magan, M. Abellana, R. Canela, A.J. Ramos and V. Sanchis. 2000. Selective effect of propionates and water activity on maize mycoflora and impact on fumonisin B1 accumulation. J. Stored Pro. Res. 36: 203-214. https://doi.org/10.1016/S0022-474X(99)00043-0
Marın, S., N. Magan, N. Belli, A.J. Ramos, R. Canela and V. Sanchis. 1999. Two-dimensional profiles of fumonisin B1 production by Fusarium moniliforme and Fusarium proliferatum in relation to environmental factors and potential for modelling toxin formation in maize grain. Int. J. Food Mic. 51: 159-167. https://doi.org/10.1016/S0168-1605(99)00115-4
Milani, J.M. 2013. Ecological conditions affecting mycotoxin production in cereals: a review. Vet. Med. 58: 405-411. https://doi.org/10.17221/6979-VETMED
Multon, J.L. 1988. Preservation and storage of grains, seeds and their by-products. New York, Lavoisier Publishing Inc. 1095.
Neme, K. and A. Mohammed. 2017. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategy. A review. Food Contr. 78: 412-425. https://doi.org/10.1016/j.foodcont.2017.03.012
Nesci, A., M. Rodriguez and M. Etcheverry. 2003. Control of Aspergillus growth and aflatoxin production using antioxidants at different conditions of water activity and pH. J. App. Micro. 95: 279-287. https://doi.org/10.1046/j.1365-2672.2003.01973.x
Sanchis, V. and Magan, N., 2004. Environmental conditions affecting mycotoxins. In: Magan, N., Olsen, M. (Eds.), Mycotoxins in Food. CRC Press, LLC, USA, pp.177. https://doi.org/10.1201/9781439823361.ch8
Suhr, K.I. and P.V. Nielsen. 2004. Effect of weak acid preservatives on growth of bakery product spoilage fungi at different water activities and pH values. Int. J. food Micro. 95: 67-78. https://doi.org/10.1016/j.ijfoodmicro.2004.02.004
Taschl, I. and T. Jenkins. 2018. 2017 BIOMIN mycotoxin survey results. https://www.biomin.net/en/blog-posts/2017-biomin-mycotoxin-survey-results/. Accessed on March 15, 2019.
Trung, T.S., J.D. Bailly, A. Querin, P. Le Bars and P. Guerre. 2001. Fungal contamination of rice from south Vietnam, mycotoxinogenesis of selected strains and residues in rice. Rev. Med. Vet. 152: 555-560.
Vega-Mercado, H. and G.V. Barbose-Canovas. 1994. Prediction of water activity in food systems. A review on theoretical models. Revista Espanola de Ciencia y Technologia de Alimentos, 34: 368-388.
Vogelgsang, S., M. Sulyok, I. Bänziger, R. Krska, R. Schuhmacher and H.R. Forrer. 2008. Effect of fungal strain and cereal substrate on in vitro mycotoxin production by Fusarium poae and Fusarium avenaceum. Food Add. Contam. 25: 745-757. https://doi.org/10.1080/02652030701768461
Waskiewicz, A., P. Golinski, Z. Karolewski, L. Irzykowska, J. Bocianowski, M. Kostecki and Z. Weber. 2010. Formation of fumonisins and other secondary metabolites by Fusarium oxysporum and F. proliferatum: a comparative study. Food Addit. Contam. 27: 608-615. https://doi.org/10.1080/19440040903551947
Wegulo, S.N., P.S. Baenziger, J.H. Nopsa, W.W. Bockus and H. Hallen-Adams. 2015. Management of Fusarium head blight of wheat and barley. Crop Protect. 73: 100-107. https://doi.org/10.1016/j.cropro.2015.02.025
Xu, X., L.V. Madden, S.G. Edwards, F.M. Doohan, A. Moretti, L. Hornok and A. Ritieni. 2013. Developing logistic models to relate the accumulation of DON associated with Fusarium head blight to climatic conditions in Europe. Eur. J. Plant Path. 137: 689-706. https://doi.org/10.1007/s10658-013-0280-x
Zinedine, A., C. Juan, J. M. Soriano, J.C. Molto, L. Idrissi and J. Manes. 2007. Limited survey for the occurrence of aflatoxins in cereals and poultry feeds from Rabat, Morocco. Int. J. Food Micro. 115: 124-127. https://doi.org/10.1016/j.ijfoodmicro.2006.10.013
To share on other social networks, click on any share button. What are these?








