Influence of Feeding Rate on the Growth, Feed Efficiency and Carcass Composition of the Giant Gourami (Osphronemus goramy)
Influence of Feeding Rate on the Growth, Feed Efficiency and Carcass Composition of the Giant Gourami (Osphronemus goramy)
Netti Aryani1, Azrita2, Ainul Mardiah3 and Hafrijal Syandri2*
1Department of Aquaculture, Faculty of Fisheries and Marine Science, Riau University, Pekanbaru, Indonesia
2Department of Aquaculture, Faculty of Fisheries and Marine Science, Bung Hatta University, Padang, Indonesia
3Department of Aquaculture, Faculty of Marine and Fisheries Science, Nahdlatul Ulama University of West Sumatera, Padang, Indonesia
ABSTRACT
Aquaculture feeding rate is an important factor affecting the growth of giant gourami. The aims of the study were to investigate the effect of different feeding rates on the growth, and carcass composition of giant gourami (initial weight 14.17±0.15 g and length 9.88±1.11 cm). The nutrition content of the diet was a cross energy of 3,340.50 kcal/kg, made up of 30% crude protein, 7% crude lipid, 6% crude fiber, 12% ash, and 12% moisture as a percentage of fish body weight, with three replicates per treatment. Fish were fed three times per day at 09:00, 14:00, and 18:00. The experiment was carried out for 120 days. Each month, 30 fish were removed from each of the floating net cages to be measured and weighed. The biomass of fish was calculated, and the amount of feed was adjusted. The feeding rate significantly (p< 0.05) influenced the final fish weight, net weight gain (NWG), average daily growth (ADG), specific growth rate (SGR), and feed conversion ratio (FCR). The maximum growth of giant gourami was found at 6% feeding rate. However, the best feed conversion ratio (1.34±0.18) was obtained at the 4% feeding rate. The carcass composition (crude protein and crude lipid) of giant gourami with different feeding rates showed a significant increase (p<0.05) after 120 days of the experiment. Based on growth performance, feed efficiency, and carcass composition, a 6% feeding rate showed the best result for the growth of giant gourami in Maninjau Lake.
Article Information
Received 17 June 2016
Revised 22 November 2016
Accepted 27 January 2017
Available online 08 September 2017
Authors’ Contributions
NA, A, AM and HS surveyed the location and collected the data. NA and A analyzed the data. HS and AM wrote the manuscript.
Key words
Perciformes, Giant gourami, Nutrition, Aquaculture, Fingerlings, Feeding rate.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1775.1781
* Corresponding author: syandri_1960@yahoo.com
0030-9923/2017/0005-1775 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Maninjau Lake is tecto-volcanic, and has a surface area 99.5 km². The lake has very important roles, such as being a tourism destination, as housing power plants with capacity 64 MW, and serving a fishery capture and aquaculture area with floating net cage farming (Syandri, 2003, 2004; Syandri et al., 2014). The total amount of floating net cages varied in the years of 2011 (15,000 units), 2012 (15,860 units), 2013 (16,120 units), 2014 (16,580 units) and 2015 (20,608 units) (Syandri et al., 2014, 2015; Junaidi et al., 2014). Based on the pollution loading capacity, the number of floating net cages recommended for aquaculture is 1,600 units (Syandri et al., 2016).
In this decade, the water quality of Maninjau Lake has decreased due to the loading of organic matter from carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) fish farming in the floating net. cages areas (Junaidi et al., 2014; Syandri et al., 2016). Furthermore, aquaculture activity in the floating net cages areas causes annual mass mortalities due to upwelling conditions, which decrease the oxygen capacity and increase the levels of ammonia and sulfide in the water. These data are presented in Table I.
The success of fish farming activity depends on proper food, management of water quality, aquaculture technology, stocking density, and genetics (Turnbull et al., 2005; Narejo et al., 2005; North et al., 2006; Effendi et al., 2006; Masiha et al., 2013; Aryani et al., 2013; Paray et al., 2015; Abdullo et al., 2015), well as on the cultured species (Mukai and Lim, 2011; Ramaswamy et al., 2013) and feeding rate (Graig and Helfrich, 2002; Du et al., 2006; Shomoushaki et al., 2012; Al Zahrani et al., 2013). Between1992 and 2015, the aquaculture activitiy in floating net cages was dominated by carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus), in contrast, giant gourami have never been cultured in Maninjau Lake. Giant gourami is a species with a low growth rate, however, this species is resistant to poor water quality has a large market in Indonesia. This species also has a high price in the market. Aquaculture activity of giant gourami depends on the feeding rate which is important for the growth, feed conversion, nutrient retention efficiency, and chemical composition of fish carcasses (Du et al., 2006; Marzuqi et al., 2012). The effects of feeding rate on fish growth and feed conversion efficiency have been determined for several species, including Tilapia nilotica (Teshima et al., 1987), Ctenopharyngodon idella (Du et al., 2006), Cyprinus carpio (Shamoushaki et al., 2012), and Epinephelus polyphekadion (Al Zahrani et al., 2013). In this study, fish were fed on the same purified diet during 120 days at three level of feeding rates. The effect of feeding rate was evaluated on growth, feed efficiency, protein efficiency ratio and carcass composition.
Table I.- Composition of diet for feeding of giant gourami reared in floating net cages.
|
Ingredients |
Gross energy (Kcal) |
Protein (%) |
Lipid (%) | Crude fiber (%) | Amount (g/kg) |
|
Fish meal |
2,7292.52 |
43.92 |
2.50 |
6.45 |
460.52 |
| Soybean cake |
3,956,70 |
35.9 |
1.32 |
5.80 |
155.10 |
| Coconut cake |
4,250.90 |
12.60 |
15.20 |
3.5 |
126.50 |
| Fine rice bran |
4,101,25 |
15.03 |
12.51 |
12.43 |
235.88 |
| Wheat starch |
3,828,40 |
1.5 |
1.30 |
- |
10.00 |
| Topioca starch |
3,560,17 |
0.95 |
0.76 |
- |
10.00 |
| Vitamin premix |
- |
0.1 |
- |
- |
1.00 |
| Salt |
- |
0.2 |
- |
- |
1.00 |
Analysis result of Animal Science Laboratory Bung Hatta University.
Materials And Methods
Giant gourami fingerlings were collected from a private hatchery in the Luak District, specificaly the Lima Puluh Kota Region of West Sumatra Province, and transported to the Research Center, for the Faculty of Fisheries and Marine Science of Bung Hatta University in Maninjau Lake. Fish were treated with a prophylactic formalin bath (100 mg Lˉ¹) for 1 h to remove external parasites and were acclimatized to floating net cages (4x4x2 m) for one month prior to the experiment. Twelve units of floating net cages, each with a size of 2x2x2 m were, were used for culturing giant gourami fingerlings. The water depth in each floating net cages was 1.5 m. The average initial length and weight of the fish were 14.17±0.15 g and 9.88±1.11 cm, respectively. Three hundred giant gourami fingerlings were cultured in each floating net cage. During the experiment, fish were feed three times per day at 09:00, 14:00 and 18:00 hours.
The feed was prepared from fish meal, soybean cake, coconut cake, fine rice bran, wheat starch, tapioca starch, vitamin premix, and salt. The ingredients were ground thoroughly and sieved to pass through 0.5 mm mesh size. An experimental feed was formulated to contain 30% grude protein. All the ingredients were mixed according to the formula composition (comparison) of pelleted feed shown in Table I and then put into a manually operated pellet machine to make pelleted feed 1 mm in size.
The chemical composition of feed was a gross energy of 3,340.50 kcal/kg, made up of 30% crude protein, 7% crude lipid, 6% crude fiber, 12% ash, and 12% moisture content. Three feeding levels (2, 4 and 6% body weight per day) were evaluated, each with three replicates. The experiment was carried out for 120 days. Each month 30 individual fish were taken from each floating net cage, anesthetized with MS-222 Sigma-Aldrich Chemical St Louis, MO (40 mg/L) (Yanto, 2009). Each fish was measured and weighed. Fish were returned to their floating net cage after evaluation, and no mortality was observed. Fish were fasted 24 h before being analysis. The biomass of fish was calculated, and the amount of feed was adjusted.
Carcass compositions of giant gourami from different treatments were analyzed. The proximate compositions of carcass samples were analyzed based on the AOAC (2000) method. The data were statistically analyzed using SPSS 16 software. Analysis of water quality parameters (temperature, pH, dissolved oxygen, ammonia, nitrite, water transparency, total alkalinity, and water hardness) was carried out every 30 days following a standard protocol (APHA, 1995).
All data were analyzed using one-way ANOVA Minitab statistical software for Windows (release 12, 1998). Standard deviation (±SD) was calculated to identify the range of means. The following parameters were analyzed according to the formula below:
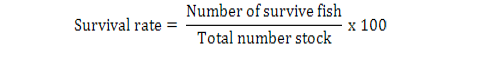
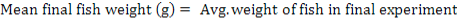
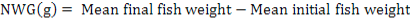
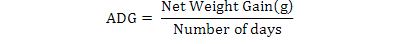
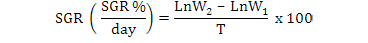
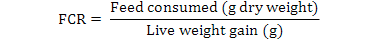
Results
Growth and feed efficiency
Giant gourami were fed commercial feed at feeding rates of 2%, 4% and 6% of biomass body weight per day. The average body weight of giant gourami was significantly increased (p<0.05) after every treatment (Fig. 1).
The feeding rates did not affect the survival of giant gourami, although final fish weight, NWG, ADG, SGR, and FCR were significantly (p<0.05) affected by feeding three levels of feding rates (Table II). The feeding rate of 6 % had significantly increased (p<0.05) growth rate and feed conversion ratio compared to 2 % and 4 % feeding rate.
Table II.- Performance of giant gourami with three different feeding rates during 120 days.
|
Parameters |
Feeding rate (% body weight per day) |
||
|
2% |
4% |
6% |
|
| Survival percentage |
91.55± 1.67a |
90.88± 1.01a |
92.54± 1.92a |
| Mean initial fish weight (g) |
14.37± 0.15a |
14.17± 0.25a |
14.23± 0.21a |
| Mean final fish weight (g) |
202.52± 15a |
261.67± 15.33b |
310.38± 4.81c |
|
Net weight gain (NWG) (g) |
187.63± 14.91a |
247.5± 15.09b |
296.15± 4.65c |
| Average daily growth (ADG) |
1.56± 0.13a |
2.06± 0.13b |
2.46± 0.04c |
|
Specific growth rate (SGR %/day) |
2.20± 0.3a |
2.43± 0.02 b |
2.57± 0.06 c |
| Feed conversion ratio (FCR) |
0.75± 0.12 a |
1.34± 0.18 b |
1.78± 0.10 c |
Different superscript on the same row represent significantly differences among the feeding rate.
Carcass composition
Table III shows carcass compositions (% mean wet weight basis) of the giant gourami at the three levels of feeding rates during the 120 days experiment.
Crude protein and crude lipid show a significant increase (p<0.05) after 120 days of the experiment. The carcass dry matter, the moisture, carbohydrate, crude fibre and ash remained unchanged throughout the experimental period.
|
Parameters (%) |
Initial |
Observations after 120 days of experiment |
||
|
2% / day |
4% / day |
6% / day |
||
| Dry matter |
20.53± 0.04 |
23.31± 0.14a |
23.38± 0.16a |
22.95± 0.5a |
| Moisture |
79.47± 0.02 |
76.69± 0.13a |
76.62± 0.44a |
77.05± 0.21a |
| Crude protein |
18.05± 0.03 |
19.02± 0.16a |
22.47± 0.56b |
25.23± 0.46c |
| Crude lipid |
0.57± 0.01 |
3.21± 0.07a |
3.31± 0.03b |
3.47± 0.08c |
| Carbohydrate |
1.65± 0.02 |
0.77± 0.03a |
0.79± 0.04a |
0.76± 0.01a |
| Crude fiber |
0.68± 0.03 |
0.03± 0.005a |
0.04± 0.01a |
0.036± 0.01a |
| Ash |
0.40± 0.04 |
0.34± 0.01a |
0.34± 0.01a |
0.34± 0.01a |
Different superscript letters on the same row represent significantly differences among the feeding rate.
Water quality parameters
Water quality parameters were recorded during the experiments along with their tolerable limits (Table IV).
Table IV.- Water quality parameters.
|
Variables |
Mean ± SD (Min - Max) |
Tolerable limits |
|
Temperature (oC) |
28.5±1.29 (27-31) |
25-30 |
| pH |
7.86±0.16 (7.70-8.10) |
6.5-8.5 |
|
Dissolved oxygen (mg L-1) |
6.805±0.308 (6.37-7.08) |
5-12 |
|
Ammonia (mg L-1) |
0.46±0.05 (0.40-0.51) |
1 |
|
Nitrite (mg L-1) |
0.165±0.047 (0.10-0.21) |
0.15 |
|
Water transparency (m) |
1.91±0.10 (1.90-2.00) |
> 6 |
|
Alkalinity (mg L-1 CaCO3) |
66.86±37.17(80.1-112.3) |
75-200 |
|
Water Hardness (mg L-1) |
60.61±27.88 (30.8-90.08) |
63-250 |
DISCUSSION
Growth and feed efficiency
Our results showed that the greatest growth of giant gourami was observed when fish were fed by submerged feed with at a 6% feeding rate. The average of giant gourami body weight increased with increasing levels of feeding rate. According to Graig and Helfrich (2002), both types of feed commercial (submerged and floating) can produce optimum growth of fish, with the exception of shrimp species. Clarias gariepinus fed commercial feed at various with feeding rates 2, 3 and 4% body weight per day showed an increase in body weight every 7 days during the 28 day experiment (Marimuthu et al., 2011). Furthermore, Cyprinus carpio reared for 42 days with feeding rates 2.5, 5.0 and 7.5% and showed an increase in body weight every 7 days (Shamoushaki et al., 2012).
After 120 days, the maximum growth of giant gourami was recorded. Giant gourami was fed the 6% feeding rate had the following results; mean final fish weight 310.38±4.81 g, NWG 296.15±4.65 g, ADG 2.46±0.04 g and SGR 2.57±0.06%/day. The highest growth of giant gourami was obtained using the 6% feeding rate during the research period. The optimum feeding rate is an important factor for promoting the highest growth (Mihelakakis et al., 2001; Cho et al., 2007). The optimal growth of giant gourami (at the 6% feeding rate) was high compared to subtropical species such as Sparus aurata (Mihelakakis et al., 2001), Paralichthys olivaceus (Kim et al., 2007), Limanda ferruginea (Puvanedran et al., 2003), Ctenopharyngodon idella (Du et al., 2006), Epinephelus polyphekadion (Al Zahrani et al., 2013), Tilapia nilotica (Teshima et al., 1987) and Parachanna obscura (Bassey and Ajah, 2010). The feeding rate of giant gourami seems to be close to tropical fish, including Epinephelus fuscoguttatus (7% body weight per day) (Haryanto and Ariyati, 2014), Clarias gariepinus (8% body weight per day) (Marimuthu et al., 2011), and Colossoma macropomum (10% body weight per day) (Silva et al., 2007). However, the optimal feeding rate of Epinephelus fuscoguttatus is inconsistent in the literature (1.5% body weight per day) (Marzuqi et al., 2012).
The best results were obtained with feeding rates of 4% and 6% (body weight per day). However, the 2% feeding rate was good, but was ranked lower due to insufficient growth of giant gourami. The FCR was low for the 2% feeding rate, and the growth of giant gourami was lower when compared to the 6% feeding rate. The best FCR at 6% body weight per days is optimal feeding rate on growth of giant gourami. However, the increase in feeding rate significantly improved the SGR for all the treatments. The same result was obtained by Haryanto and Ariyati (2014) for the juvenile tiger grouper (Epinephelus fuscoguttatus), Marimuthu et al. (2011) for African catfish (Clarias gariepinus) fingerlings, and Al Zahrani et al. (2013) for camouflage grouper (Epinephelus polyphekadion) fingerlings. In general, the maximum FCR and feed efficiency ratio did not occur at the same feeding rate (Du et al., 2006). Furthermore, greater growth followed by a higher FCR is an indicator of overfeeding (Kim et al., 2007), which results in higher production costs, water pollution, and feed wastage (Syandri et al., 2016).
Carcass composition
In this study, the crude protein and crude lipid levels significantly increased in all treatments compared to the initial giant gourami at different levels of feeding rates. Giant gourami had moisture contents ranging from 76 to 77%. Similar results were obtained by Ahmad et al. (2012) for common carp (Cyprinus carpio) fingerlings, Ramaswamy et al. (2013) for Indian major carp (Catla catla) fingerlings, Masiha et al. (2013) for rainbow trout (Oncorhynchus mykiss) fingerlings, Soto and Novoa (2015) for the four-sided sea cucumber (Isostichopus badionotus) and Mateen et al. (2016) for Hypophthalamichthys molitrix, Labeo rohita and Cirrhinus mrigala. However, Orire and Ozoadibe (2015) have reported contrasting results for Clarias gariepinus, and Denji et al. (2015) for Oncorhynchus mykiss juveniles. Carcass composition is known to be influenced by many factors, such as geographic location, age, sex, maturity and feeding conditions. Among these factors, formulated feed, type and feed ingredients are considered the most important (Du et al., 2006; Ahmad et al., 2013; Ramaswamy et al., 2013). Several studies have been done on the effects of different levels of protein and lipid on carcass composition of Verasper variegatus (Yunyun et al., 2015), Isostichopus badionotus (Soto and Novoa, 2015), Ctenopharyngodon idella (Chen et al., 2012) and Clarias nieuhofii (Kiriratnikom and Kiriratnikom, 2012).
Water quality parameters
The physico-chemical parameters of water play a significant role in the growth of fish. These parameters remained within the favorable range required for giant gourami (Effendi et al., 2006). The average water temperatures recorded during the experiment ranged from 27 to 31°C, which falls within the tolerance limits of fish (26 to 30°C). Other species such as Trichogaster trichopterus, Osphronemidae have the temperature 31°C as tolerance limit of water quality (Geheber et al., 2010). High temperatures may have contribute to fish mortality during the experiment.
Fish can only breathe normally in an environment with sufficient oxygen. The oxygen needs of fish vary with different species. Dissolved oxygen (DO) is a critical factor in aquaculture. The success or failure of fish farming depends on the availability of DO. Alabaster and Lloyd (1980) indicated that a 50% reduction of DO in water could depress air saturation and reduce the appetite of fish, causing a disruption of fish growth. The DO levels recorded during the experiment ranged from 6.37 to 7.08 mg Lˉ¹. Certain fish species may depend on DO, including Cyprinidae. The oxygen demand for carp is 5 mg Lˉ¹, however they can withstand as low level as 3 mg Lˉ¹ (Cholik et al., 2005). Giant gourami are facultative air breathers, meaning it can tolerance low DO.
The effect of pH on pond fish, as illustrated by Boyd (1982), indicates that fish cultured in water more acidic than a pH 6.5 or more alkaline than pH from 9-9.5 for long time periods the growth of fish is diminished. Howerver, in our study, the water pH ranged from 7.70 to 8.10 and had no effect on growth of giant gourami. Sulawesty et al. (2011) also reported that the water quality in several floating nets cages in Maninjau Lake areas had pH 7.5 to 8.0, DO 4.0 to 6.0 mg/L and ammonium 0.04 to 1.00 mg/L. Huet and Timmersmens (1986) stated that pH with the level between neutral and alkaline are the best conditions for a fish pond.
The alkalinity levels recorded during the experiment ranged from 80.1-112.34 mg/L CaCO3. An increase in pH can occur in water with low alkalinity (20 to 50 mg Lˉ¹ CaCO3) in water with moderate to high alkalinity (75 to 200 mg Lˉ¹ CaCO3), or in water with alkalinity less than 25 mg Lˉ¹ (Boyd, 1979). The water did not widely fluctuate between moderate or high alkalinity levels (good buffering capacity) and had similar hardness levels and a neutral or slightly basic pH (7.0 to 8.3). The water hardness for the cultured giant gourami was 30.85 to 90.08 mg Lˉ¹. Water hardness is important in aquaculture and is a commonly reported aspect of water quality. Water hardness can be influenced by a mixture of divalent salts; however, calcium and magnesium are the most common sources of water hardness. During the experiment of giant gourami culturing, water quality parameters reflected the best environmental conditions. All water quality parameters were measured within the optimal range for growth of giant gourami and the acceptable ranges that are recommended for tropical fish aquaculture (Boyd, 1982; Beveridge, 1996).
Conclusion
In conclusion, based on growth and feed efficiency, we can concluded that a feeding rate of 6% body weight per day results in the best growth of giant gourami in maninjau lake, west sumatera, Indonesia. The carcass composition (crude protein and crude lipid) of giant gourami was recorded to be highest at the 4% feeding rates.
Acknowledgements
This study was supported under study grant Lembaga Pengelola Dana Pendidikan, Ministry of Finance, Republic of Indonesia, No. PRJ-1042/LPDP/2015.
Conflict of interest statement
We declare that we have no conflict of interest.
References
Ahmad, M., Qureshi, T.A., Singh A.B., Manohar, S., Borana, K. and Chalko, S.R., 2012. Effect of dietary protein, lipid and carbohydrate contents on the growth, feed efficiency and carcass composition of Cyprinus carpio communis fingerlings. Int. J. Fish. Aquacult., 4: 30-40.
Alabaster, J.S. and Lloyd, R., 1980. Water quality criteria for freshwater fish. Butterworth and Company Limited, London-Boston, pp. 297.
Al Zahrani, A.W., Mohamed, A.H., Serrano, A.E. and Traifalgar, R.F.M., 2013. Effects of feeding rate and frequency on growth and feed utilization efficiency in the camouflage grouper (Epinephelus polyphekadion) fingerlings fed a commercial diet. Eur. J. exp. Biol., 3: 596-601.
AOAC, 2000. Official methods of analysis, 13th ed. Association of Official Analytical Chemists, Washington, DC, USA.
APHA, 1995. Standard methods for the examination of water and wastewater, 17th ed. APHA, New York, USA.
Aryani, N., Nuraini, and Suharman, I., 2013. Morphological characterization of baung fish (Hemibagrus nemurus) aquatic habitat on the different method based truss morfometrics. J. Fish. Aquacult., 4: 139-142.
Bassey, A.U. and Ajah, P.O., 2010. Effect of three feeding regimes on growth, condition factor and food conversion rate of pond cultured Parachanna obscura (Gunther, 1861) (Channidae) in Calabar, Nigeria. Turk. J. Fish. aquat. Sci., 10: 195-202.
Beveridge, M.C.M., 1996. Cage aquaculture. 2nd ed. Fishing News, Oxford, pp. 346.
Boyd, C.E., 1979. Water quality in warm water fish ponds. Alabama Agriculture Experiment Station, Auburn University, Auburn, AL, pp. 482.
Boyd, C.E., 1982. Water quality management for pond fish culture. Elsevier, Amsterdam, pp. 318.
Chen, Y.J., Tian, L.X., Yang, H.J., Chen, P.F., Yuan. Y., Liu, Y.J. and Liang, G.Y., 2012. Effect of protein and starch level in practical extruded diets on growth, feed utilization, body composition, and hepatic transaminases of Juvenile Grass Carp, Ctenopharyngodon idella. J. World Aquacult. Soc., 43: 187-197. https://doi.org/10.1111/j.1749-7345.2012.00549.x
Cho, S.H., Lee, S.M., Park, B.H., Ji, S.C., Choi, C.Y. and Lee, J.H., 2007. Effect of daily feeding ratio on growth and body composition of sub adult olive flounder (Paralichthys olivaceus), fed an extruded diet during the summer season. J. World Aquacult. Soc., 38: 68-73. https://doi.org/10.1111/j.1749-7345.2006.00074.x
Denji, K.A., Mansour, M.R., Akrami, R., Ghobadi, S.H., Jafarpour, S.S. and Mirbeygi, S.K., 2015. Effect of dietary prebiotic mannan oligosaccharide (MOS) on growth performance, intestinal micro flora, body composition, hematological and blood serum biochemical parameters of rainbow trout (Oncorhynchus mykiss) juveniles. J. Fish. aquat. Sci., 10: 255-265.
Du, Z. Y., Liu, Y.J., Tian, L.X., He, J.G., Coa, J.M. and Liang, G.Y., 2006. The influence of feeding rate on growth, feed efficiency and body composition of juvenile grass carp (Ctenopharyngodon idella). Aquacult. Int., 14: 247-257. https://doi.org/10.1007/s10499-005-9029-7
Effendi, I., Bugri, H.J. and Widanarni, 2006. Effect of different rearing density on survival rate and growth of Giant gourami (Osphronemus goramy L.) fry at size of 2 cm in length. J. Akuakult. Indonesia, 5: 127-135. https://doi.org/10.19027/jai.5.127-135
Geheber, A.D., Mcmahan, C.D. and Piller, K.R., 2010. First record of the non-native three spot gourami, Trichogaster trichopterus (Pallas 1770) (Teleostei: Osphronemidae) in Jamaica. Aquat. Invas., 5(Suppl. 1): S13-S16. https://doi.org/10.3391/ai.2010.5.S1.004
Haryanto, P. and Ariyati, R.W., 2014. The influence of different feeding dose on growth of juvenile tiger grouper (Epinephelus fuscoguttatus). J. Aquacult. Manage. Technol., 3: 58-66.
Huet, M. and Timmersmens, J., 1986. Textbook of fish culture: Breeding and cultivation of fish, 2nd ed., Fishinf New Book, London, pp. 456.
Junaidi, A., Syandri, H. and Azrita, 2014. Loading and distribution of organic materials in Maninjau lake West Sumatra Province-Indonesia. J. Aquacult. Res. Develop., 5: 7.
Kim, K.D., Kang, Y.J. and Kim, K.W., 2007. Effects of feeding rate on growth and body composition of juvenile flounder (Paralichthys olivaceus). J. World Aquacult. Soc., 38: 169-173. https://doi.org/10.1111/j.1749-7345.2006.00086.x
Masiha, A., Ebrahimi, E., Soofiani, M.N. and Kadivar, M., 2013. Effect of dietary canola oil level on the growth performance and fatty acid composition on fingerlings of rainbow trout (Oncorhynchus mykiss). Iran. J. Fish. Sci., 14: 336-349.
Marimuthu, K., Umah, R., Muralikrishnan, S., Xavier, R. and Kathiresan, S., 2011. Effect of different feed application rate on growth, survival and cannibalism of African catfish (Clarias gariepinus) fingerlings. Emirates J. Fd. Agric., 23: 330-337.
Marzuqi, M., Astuti, N.W.W. and Suwirya, K., 2012. Effect on dietary protein and feeding rate on growth of Tiger grouper (Epinephelus fuscoguttatus) juvenile. J. Ilmu Teknol. Kelautan Trop., 4: 55-65
Mateen, A., Ghaffar, A., Abbas, G., Ferrando, S. and Gallu, L., 2016. Body composition and fatty acid profile of carps under the Influence of rice Polish and pond fertilization. Pakistan J. Zool., 48: 1263-1267.
Mihelakakis, A., Yoshimatsu, T. and Tsolkas, C., 2001. Effect of feeding frequency on growth, feed efficiency, and body composition in young common pandora. Aquacult. Int., 9: 197-204. https://doi.org/10.1023/A:1015345224537
Mukai, Y., and Lim, L.S., 2011. Larval rearing and feeding behavior of African catfish (Clarias gariepinus) under dark conditions. J. Fish. aquat. Sci., 6: 272-278. https://doi.org/10.3923/jfas.2011.272.278
North, B.P., Turnbull, J.F., Ellis, T., Porter, M.J., Migaud, H., Bron, J. and Bromage, N.R., 2006. The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture, 255: 466-479. https://doi.org/10.1016/j.aquaculture.2006.01.004
Paray, B.A., Al-Sadoon, M.K. and Haniffa, A., 2015. Impact of different feeds on growth, survival and feed conversion in stripped snakehead Channa striatus (Bloch 1793) larvae. Indian J. Fish., 62: 82-88.
Puvanendran, V., Boyce, D.L. and Brow, J.A., 2003. Food ratio requirements of yellowtail flounder (Limanda ferruginea, Storer) juveniles. Aquaculture, 220: 459-475. https://doi.org/10.1016/S0044-8486(02)00620-8
Shamoushaki, M.M., Khari, N. and EslamI, Z., 2012. Determination of optimum feeding rate for growth of Caspian carp (Cyprinus carpio, Linnaeus, 1758) fingerling. AACL Bioflux, 3: 136-141.
Silva, C.R., Gomes, L.C. and Brandão, F.R., 2007. Effect of feeding rate and frequency on tambaqui (Colossoma macropomum) growth, production and feeding costs during the first growth phase in cages. Aquaculture, 264: 135-139. https://doi.org/10.1016/j.aquaculture.2006.12.007
Soto, M.Z. and Novoa, M.A.O., 2015. Effect of different diets on body Biochemical composition of the four-sided sea cucumber (Isostichopus badionotus), under culture conditions. J. World Aquacult. Soc., 46: 45-52. https://doi.org/10.1111/jwas.12163
Syandri, H., 2003. Cage’s culture and problems in Maninjau Lake, West Sumatra Province. J. Perikanan Ilmu Kelautan, 8: 74-81.
Syandri, H., 2004. The use of Osteochilus vittatus and Puntius javanicus as biological agent in Maninjau Lake. J. Natur Indonesia, 6: 87-91.
Syandri, H., Junaidi, A. and Yunus, T., 2014. State of aquatic resources Maninjau lake West Sumatra Province, Indonesia. J. Ecol. environ. Sci., 1: 109-113.
Syandri, H., Elfiondri, A. and Junaidi, A., 2015. Social status of the fish-farmers of floating net cages in Maninjau Lake, Indonesia. J. Aquacult. Res. Devlop., 7: 1-5.
Syandri, H., Azrita, and Niagara, 2016. Trophic status and load capacity of water pollution waste fish culture with floating net cages in Maninjau Lake, Indonesia. Ecol. environ. Conserv., 22: 469-476.
Teshima, S., Kanazaw, A. and Koshio, S., 1987. Effects of feeding rate, fish size and dietary protein and cellulose levels on the growth of (Tilapia nilotica). Mem. Fac. Fish, 1: 7-15.
Turnbull, J., Bell, A., Adams, C., Bron, J. and Huntingford, F., 2005. Stocking density and welfare of cage farmed Atlantic salmon: application of a multivariate analysis. Aquaculture, 243: 121–132. https://doi.org/10.1016/j.aquaculture.2004.09.022
Yunyun L.V., Chang, Q., Chen, S., Yu, C., Qin, B. and Wang, Z., 2015. Effect of dietary protein and lipid levels on growth and body composition of Spotted Halibut, Verasper variegatus. J. World Aquacult. Soc., 46: 311-318. https://doi.org/10.1111/jwas.12196
To share on other social networks, click on any share button. What are these?










