Growth Performance and Nutrient Digestibility of Cirrhinus mrigala Fingerlings Fed Phytase Supplemented Sunflower Meal Based Diet
Growth Performance and Nutrient Digestibility of Cirrhinus mrigala Fingerlings Fed Phytase Supplemented Sunflower Meal Based Diet
Syed Makhdoom Hussain1,*, Tasneem Hameed1, Muhammad Afzal2, Arshad Javid3, Nosheen Aslam4, Syed Zakir Hussain Shah6, Majid Hussain5, Muhammad Zubair ul Hassan Arsalan1 and Muhammad Mudassar Shahzad1
1Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad, Pakistan
2Fish Nutrition Lab, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad, Pakistan
3Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore, Pakistan
4Department of Biochemistry, Government College University, Faisalabad, Pakistan
5Department of Zoology, University of Gujrat, Gujrat, Pakistan
6Department of Zoology, University of Sargodha, Sargodha, Pakistan
ABSTRACT
A feeding trial was conducted to study the effects of graded levels (0, 500, 1000, 1500 and 2000 FTU kg-1) of microbial phytase on growth performance and nutrient digestibility of Cirrhinus mrigala fingerlings fed sunflower meal based diet. The chromic oxide was added as inert marker in the diets. Three replicate groups of 15 fish (Average weight 5.05±0.013 g fish-1) were fed at the rate of 5% of live wet weigh and feces were collected twice daily. The results of present study showed improved growth performance of Cirrhinus mrigala fingerlings in response to phytase supplementation. Maximum growth performance was observed by the fish group fed on test diet-III having 1000 FTU kg-1 level. Similarly, nutrient digestibility was also significantly increased (p<0.05) by 1000 FTU kg-1 level of phytase supplementation. Digestibility coefficients for sunflower meal based diet increased 15.76%, 17.70% and 12.70% for crude protein, crude fat and apparent gross energy as compared to the reference diet, respectively at 1000 FTU kg-1 level. It was concluded that the phytase supplementation to sunflower meal based diet at 1000 FTU kg-1 level is optimum to release adequate chelated nutrients for maximum growth performance of C. mrigala fingerlings. Our results also suggested that phytase supplementation to sunflower meal based diet can help in the development of sustainable aquaculture by reducing the feed cost and nutrient discharge through feces into the aquatic ecosystem.
Article Information
Received 24 November 2015
Revised 03 October 2016
Accepted 03 May 2017
Available online 05 September 2017
Authors’ Contribution
This study is part of M.Phil thesis of TH who collected data and compiled the results. SMH planned the research and provided facilities for conducting the research work. MA helped in writing, revising and improvement of manuscript. AJ and MH analysed the data statistically and interpreted the results. NS provided help and facilities for chemical. MZHA collected and analysed samples. MMS helped in experiments and date collection.
Key words
Sunflower meal, Cirrhinus mrigala, Growth, Nutrient digestibility, Phytase.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1713.1724
* Corresponding author: drmakhdoom90@gmail.com
0030-9923/2017/0005-1713 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Cirrhinus mrigala is bottom feeder fish species, feed on decaying organic matter and vegetable debris (Britz et al., 1997; Azeredo et al., 1998). Aquaculture industry has been developing more efficiently than any other food producing sector (Yildirım et al., 2014). Fish meal is being used by aqua feed industry as a potential source of basic nutrients such as amino acids, fatty acids, vitamins, minerals and growth factors (Zhou et al., 2004; Rahim et al., 2017). It is major source of protein in fish feeds (Drew et al., 2007). However, increasing demand, limited, unstable supply and high cost of fish meal with the expansion of aquaculture made it necessary to search for alternative protein sources (Pham et al., 2008; Lim et al., 2011). Plant by-products are considered as best alternative protein and energy sources for fish growth (Gatlin et al., 2007; Hussain et al., 2011a, b, 2015). Plant meal based aqua-feed has anti-nutritional factors and these factors affect the morphology and physiology of digestive tract and disturbs the overall fish growth, one of them is phytate or phytic acid (Baruah et al., 2004). Phytate is a major storage form of phosphorus and it is estimated that 80% of total phosphorus is chelated in plant seeds, which is practically not available for agastric fish species (NRC, 1993). Phytic acid is a strong chelator and forms complexes with lipids and its derivatives along with other nutrients (Vohra and Satanarayana, 2003). It can chelate with proteins and reduce its availability for fish (Helland et al., 2006). Phytase is an enzyme chemically known as myo-inositol hexa-phosphate phosphohydrolase can be obtained from microbes and may be from some plant ingredients. This enzyme hydrolyzes the non-digestible phytic acid contents present in plant based fish feeds. Mono-gastric fish lack the ability to hydrolyze the phytate present in plant based diet due to absence of intrinsic phytase enzyme. This enzyme has been used as supplement in fish feed to increase the bioavailability and utilization of phosphorus (Baruah et al., 2007; Cao et al., 2007). It is now widely being used as supplement in plant based diets for increasing the digestibility and availability of protein which results in the better fish growth and reduce aquatic pollution (Farhangi and Carter, 2007; Lin et al., 2007; Soltan, 2009; Hussain et al., 2015).
Sunflower meal is considered as most promising alternative to fishmeal and an economical source of important nutrients. In all over the world, it is the fourth largest plant based source of protein contents after soybean meal, cottonseed meal and canola meal (Anjum et al., 2014). It has approximately 40% protein content that mainly depends on the oil extraction and de-hulling process (Mushtaq et al., 2006). It has been recognized that the disruption of cell wall matrix of sunflower meal by supplementation of exogenous microbial enzymes result in endogenous pro-teolytic enzymes to digest the chelated proteins (Choct and Kocher, 2000). It was also noted that it has more ability of degradation than soybean meal and canola meal. It contains 8-10% lignin, 20-25% cellulose and 30-34% protein (NRC, 1996). The present study was designed to search out the better and cost effective protein sources for Cirrhinus mrigala fingerlings to improve the fish growth performance and to reduce the aquatic pollution caused by phytic acid.
Materials and Methods
The experiment was conducted in the Fish Nutrition Lab, Department of Zoology, Government College University, Faisalabad, Pakistan.
Experimental design
Sunflower meal was selected as test ingredient to formulate basal diet which comprised of 30% sunflower meal and 70% reference diet. Basal diet was then further divided into one reference diet and five test diets and sprayed with graded levels of phytase (0, 500, 1000, 1500 and 2000 FTU kg-1). These five test diets and one reference diet were fed to six fish groups in three replicates, stocked in specially designed V-shaped tanks. The experiment lasted for 60 days till the collection of 4-5 g fecal material from each replicate separately.
In this study, phytase with different levels of treatments was used to check its efficacy on growth and nutrient digestibility of fish by using Completely Randomized Design (CRD). Due to slow growth rate and less amount of fecal matter collection, both growth and digestibility trials were conducted simultaneously. The trial consisted of two overlapping sections: (i) the first section was comprised of assessment of growth performance in terms of weight gain and feed conversion ratio (FCR) (ii) Second section was consisted of assessment of the nutrient digestibility of the test and reference diets which was determined indirectly by using chromic oxide as an inert marker.
Fish and experimental conditions
C. mrigala fingerlings were procured from a local public sector hatchery and allowed to acclimate for two weeks in V-shaped tanks (70 L capacity), designed for the collection of fecal material. During acclimation period the fingerlings were fed on reference diet (Allan and Rowland, 1992). Dissolved oxygen, pH and electrical conductivity were monitored through pH meter (Jenway 3510), D.O. meter (Jenway 970) and electrical conductivity meter (HANNA: HI. 8633) respectively. The range of water quality parameters such as temperature was 24.9-28.7ºC, pH 7.4-8.6, dissolved oxygen 5.8-7.3 mg L-1 and electrical conductivity 1.30-1.52 dSm-1. Compressed air was supplied from an air compressor through capillary system and air stones in the form of micro bubbles to all the experimental tanks. The fingerlings were treated with 0.5% saline solution for 1-2 min to kill and remove any pathogen if present (Rowland and Ingram, 1991).
Table I.- Chemical composition (%) of feed ingredients (dry matter basis).
| Ingredients | Dry matter (%) | Crude protein (%) | Crude fat (%) | Crude fiber (%) |
Ash (%) |
Carbohydrates (%) |
Gross energy (kcalg-1) |
| Fish meal | 91.63 | 48.15 | 7.16 | 1.07 | 26.73 | 16.89 | 2.69 |
|
Wheat flour |
92.45 | 10.10 | 2.35 | 2.65 | 2.08 | 82.82 | 2.96 |
| Corn gluten 60% | 92.59 | 59.12 | 4.96 | 1.19 | 1.58 | 33.15 | 4.23 |
| Rice polish | 94.09 | 12.35 | 13.54 | 12.70 | 10.18 | 51.23 | 3.33 |
|
Sunflower meal (test ingredient) |
93.80 | 41.93 | 3.74 | 1.97 | 10.83 | 37.99 |
3.54 |
Table II.- Ingredients composition (%) of reference and test diets (as fed basis).
| Ingredients | Reference diet | Test diets |
| Fish meal | 20.0 | 14.0 |
| Wheat flour | 24.0 | 16.8 |
| Corn gluten 60% | 20.0 | 14.0 |
| Rice polish | 25.0 | 17.5 |
| Fish oil | 7.0 | 4.9 |
|
Vitamin premix |
1.0 | 0.7 |
| Minerals | 1.0 | 0.7 |
| Ascorbic acid | 1.0 | 0.7 |
| Chromic oxide | 1.0 | 0.7 |
|
Sunflower meal (Test ingredient) |
- |
30.0 |
Feed ingredients and formulation of experimental diets
The feed ingredients were obtained from the local poultry feed market and analyzed chemically following the standards methods (AOAC, 1995) prior to the diet preparation (Table I). The compositions of reference and basal diet have been given in Table II. Reference diet and sunflower meal based basal diets were prepared by mixing appropriate amount of finely ground (< 0.5 mm particle size) ingredients in electric mixer for 10 minutes. Later on, fish oil was added gradually while mixing was continued for further five minutes. Afterwards, 10-15% water was also added to prepare suitable dough (Lovell, 1989). The diets were extruded into pellets (3mm) through Lab Extruder (model SYSLG30-IV Experimental Extruder). The required concentrations (0, 500, 1000, 1500 and 2000 FTU kg-1) of phytase (Phyzyme® XP 10000 FTU g-1; Danisco Animal Nutrition, Fin-65101 Vaasa, Finland) were prepared in 25 ml distilled water and sprayed on 1 kg of each test diet (Robinson et al., 2002). The reference diet was also sprayed with a similar amount of distilled water to maintain an equal level of moisture. The diets were stored at 4°C till further use.
Growth study
C. mrigala fingerlings were fed twice daily (morning and afternoon) at the rate of 5% of live wet weight on their prescribed diet and subsequently adjusted on daily feed intake. For each test diet three replicates were assigned and in each replicate fifteen fish (average weight: 5.05±0.013g fish-1) were stocked. After the feeding session of two hours, the uneaten diet was collected and water was drained out from each tank by opening the valves of the tanks. The tanks were washed completely to remove the particles of diets and refilled with water. Fish in each tank were bulk weighed every 15th day during experiment to assess the growth performance of C. mrigala fingerlings. Weight gain (%) and feed conversion ratio (FCR) of fingerlings was evaluated based on standard formulae:
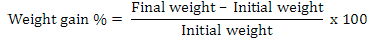
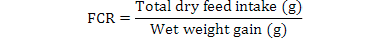
Chemical analysis of feed and feces
The samples of feed ingredients, test diets and feces were homogenized using a motor and pestle and analyzed by standard methods (AOAC, 1995). Moisture was determined by oven-drying at 105oC for 12 h. Crude protein (N × 6.25) was determined by Micro Kjeldahl Apparatus and crude fat by Petroleum Ether Extraction Method through Soxtec HT2 1045 system. Crude fiber, as loss on ignition of dried lipid-free residues after digestion with 1.25% H2SO4 and 1.25% NaOH whereas ash by ignition at 650oC for 12 h in electric furnace (Eyela-TMF 3100) to constant weight. Total carbohydrate (N-free extract) was calculated by difference, i.e., Total carbohydrate % =100- (CP%+ EE%+CF%+Ash %). Gross energy was determined with the help of Oxygen Bomb Calorimeter.
Digestibility studies
Chromic oxide was used as an inert marker at 1% inclusion level in reference diet assuming that the amount of the marker in the feed and feces remains constant throughout the experimental period and that all of the ingested marker will appear in the feces. After the completion of feeding session, feces were collected from the fecal collecting tube of each tank. Care was taken to avoid breaking the thin fecal strings in order to minimize the nutrient leaching. Fecal material of each replicated treatment was dried in oven, grinded and stored for chemical analysis. Chromic oxide contents in diets and feces were estimated after oxidation with molybdate reagent (Divakaran et al., 2002) using UV-VIS 2001 Spectrophotometer at 370 nm absorbance. The apparent nutrient digestibility of test diets was determined indirectly at the end of the experiment using chromic oxide as inert marker.
Calculation of apparent nutrient digestibility coefficients (ADC %) of test diets
Apparent nutrient digestibility coefficients (ADC %) of experimental diets were calculated by using standard formula (NRC, 1993):
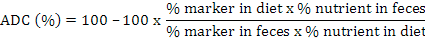
| Experimental diets |
Phytase levels (FTU kg-1) |
Initial weight (g) |
Final weight (g) |
Weight gain (g) |
Weight gain (%) |
Weight gain (fish-1day-1)g |
Feed intake (fish-1day-1)g |
FCR |
| Reference diet | - |
5.05± 0.010 |
10.58± 0.332 |
5.53± 0.326cd |
109.50± 6.342cd |
0.08± 0.005 |
0.12± 0.014 |
1.50± 0.10bcd |
| Test diet I | 0 |
5.05± 0.015 |
10.40± 0.103 |
5.35± 0.095d |
105.87± 1.783d |
0.08± 0.001 |
0.13± 0.012 |
1.72± 0.17cd |
| Test diet II | 500 |
5.04± 0.012 |
10.92± 0.095 |
5.88± 0.087bc |
116.74± 1.587bc |
0.08± 0.001 |
0.12± 0.006 |
1.42± 0.05abc |
| Test diet III | 1000 |
5.04± 0.012 |
12.25± 0.049 |
7.21± 0.044a |
142.96± 0.792a |
0.10± 0.001 |
0.12± 0.005 |
1.15± 0.04a |
| Test diet IV | 1500 |
5.05± 0.017 |
11.11± 0.075 |
6.06± 0.078b |
120.07± 1.694b |
0.09± 0.001 |
0.12± 0.007 |
1.34± 0.07ab |
| Test diet V | 2000 |
5.06± 0.012 |
10.48± 0.042 |
5.41± 0.046d |
106.91± 1.055d |
0.08± 0.001 |
0.14± 0.011 |
1.77± 0.13d |
Means within rows having different superscripts are significantly different. Data are means of three replicates.
Statistical analysis
The growth and nutrient digestibility data for each variable was statistically analyzed by using one-way analysis of variance (ANOVA) followed by Tukey’s Honesty Significant Difference Test (Snedecor and Cochran, 1991) by using COSTAT (Version 6.303, PMB 320, Monterey, CA, 93940 USA) software package.
Table IV.- Analyzed nutrients composition (%) of reference and sunflower meal-based test diets.
| Experimental diets |
Phytase levels (FTU kg-1) |
Crude protein |
Crude fat |
Apparent gross energy |
| Reference diet | - |
31.20±0.16 |
5.64±0.02 |
4.71±0.02 |
| Test diet I | 0 |
30.45±0.31 |
4.61±0.02 |
4.14±0.01 |
| Test diet II | 500 |
30.32±0.09 |
4.58±0.04 |
4.17±0.02 |
|
Test diet III |
1000 |
30.34±0.07 |
4.59±0.02 |
4.13±0.01 |
| Test diet IV | 1500 |
30.43±0.01 |
4.59±0.01 |
4.13±0.02 |
| Test diet V | 2000 |
30.33±0.15 |
4.64±0.02 |
4.14±0.01 |
Data are means of three replicates.
Results
A significant (P<0.05) increase in weight gain was commensurate with increase in phytase concentration to a level of 1000 FTU kg-1 after which further increase in phytase level decreased the weight gain of fish. The maximum weight gain of Cirrhinus mrigala fingerlings was observed when fingerlings were fed sunflower meal based test diet having 1000 FTU kg-1 level of phytase supplementation and this value was significantly different (P<0.05) from the weight gain on reference diet and other phytase supplementation based test diets. The best FCR value (1.15±0.04) was also observed in the diet containing phytase level of 1000 FTU kg-1. It was significantly (P<0.05) different from the FCR values noted at 0, 500 and 2000 FTU kg-1 levels of phytase supplementation. However, it did not differ significantly when compared with 1500 FTU kg-1 phytase supplemented diet. The comparatively lower values of FCR with sunflower meal based diet showed that fish palatability was improved by phytase supplementation. The minimum value of FCR at 1000 FTU kg-1 revealed that at this level maximum feed was converted into flesh.
Table V.- Analyzed nutrients composition (%) of feces of Cirrhinus mrigala fingerlings fed on reference and sunflower meal-based test diets.
| Experimental diets |
Phytase levels (FTU kg-1) |
Crude protein |
Crude fat |
Apparent gross energy |
| Reference diet | - |
14.33±0.34 |
2.60±0.11 |
2.32±0.15 |
| Test diet I | 0 |
14.18±0.15 |
2.22±0.10 |
1.97±0.14 |
| Test diet II | 500 |
13.33±0.52 |
1.93±0.12 |
1.88±0.04 |
| Test diet III | 1000 |
10.10±0.02 |
1.49±0.12 |
1.68±0.11 |
| Test diet IV | 1500 |
12.78±0.20 |
1.80±0.20 |
1.75±0.11 |
| Test diet V | 2000 |
14.26±0.07 |
2.22±0.19 |
1.97±0.06 |
Data are means of three replicates.
The data presented in Table VI makes it clear that phytase enzyme supplementation played a significant role in increasing nutrient digestibility and minimum amount of nutrients was discharged through feces at 1000 and 1500 FTU kg-1 phytase levels (Table V). It is also obvious from the results that in comparison with reference diet and phytase supplemented sunflower meal-based diets, 1000 FTU kg-1 level showed maximum values of crude protein (74%), crude fat (77%) and gross energy (68%) digestibility (Table VI). It was observed that gross energy digestibility value at 1000 FTU kg-1 level did not differ significantly from phytase level of 1500 FTU kg-1 but varied (P<0.05) from the reference diet and remaining phytase supplemented diets. In general, the results showed that nutrient digestibility started increasing from 500 FTU kg-1 level of phytase supplementation and reached to its maximum at 1000 FTU kg-1 level.
| Exp. diets | Phytase levels |
Crude protein |
Crude fat |
Apparent gross energy |
| Reference diet | - |
58.33±1.57c |
59.15±0.89c |
55.63±2.20c |
| Test diet I | 0 |
54.62±1.92c |
53.05±0.41d |
53.71±1.45c |
| Test diet II | 500 |
59.62±2.92bc |
61.38±1.13c |
58.66±1.57bc |
| Test diet III | 1000 |
74.09±1.93a |
76.85±2.21a |
68.33±2.04a |
| Test diet IV | 1500 |
64.05±1.63b |
66.57±2.78b |
63.84±2.45ab |
| Test diet V | 2000 |
59.43±1.90bc |
58.72±1.58c |
59.03±2.32bc |
Means within rows having different superscripts are significantly different. Data are means of three replicates
Discussion
In present study, Cirrhinus mrigala fed on sunflower meal based diets supplemented with graded levels (0, 500, 1000, 1500 and 2000 FTU kg-1) of phytase showed improved weight gain, weight gain (%) and feed conversion ratio (FCR) as compared to reference diet. It indicates that phytase has potential to be an alternative protein source of fish meal. The highest increase in weight gain was observed at 1000 FTU kg-1 phytase supplementation level. It was 33.46% higher over the weight gain of fingerlings fed on reference diet. Similar growth performance was also reported in Nile tilapia when fed on plant based diet supplemented with 1000 FTU kg-1of phytase compared with the phytase control diet (Riche and Garling, 2004; Ashraf and Goda, 2007; Cao et al., 2008). After reviewing the literature related to the dose response studies, it was concluded that the optimal responses of phytase supplementation ranged between 250-1500 FTU kg-1 levels depending upon the sources and origin of phytases, experimental fish species, diet formulation technology and studied response parameters. Thus the level of phytase supplementation should have been adjusted based on considerations of earlier impactors (Cao et al., 2007; Kumar et al., 2011). However, there were limited data available for comparative studies on phytase supplementation in different fish diets, mainly in plant-based diets. Liebert and Portz (2005) compared nutrient utilization of Nile tilapia (Oreochromis niloticus) fed plant based low phosphorous diets supplemented with graded levels (500, 750, 1000 and 1250 FTU kg-1) of two different sources of phytase were used: phytase A (SP 1002 CT) and phytase B (Ronozyme P5000). It was found that phytase A supplementation of at least 750 FTU kg-1 diet was adequate to improve growth, FCR, protein deposition, while supplementation of at least 1000 FTU kg-1 from phytase B resulted in intermediate growth as compared to supplementation of phytase A. This result indicated that different phytase sources might lead to different effects. On the other hand, Furuya et al. (2001) found that supplementation of phytase at 700 FTU kg-1 level was sufficient to maintain growth of Nile tilapia fed on plant-based diets. The effect of phytic acid on growth depends largely on its quantity in the diet (Sajjadi and Carter, 2004). Spinelli et al. (1983) also concluded increase in phytate concentration decreases the growth rate and feed efficiency in rainbow trout. The findings of the present study provide evidence that phytase supplementation at 1000 FTU kg-1 level was sufficient for minimizing the effect of phytic acid and releasing the chelated nutrients of plant based diets. A significant increase in growth performance of fingerlings was proportionate with the increase in phytase supplementation to a level of 1000 FTU kg-1, after which it decreased. The next higher weight gain was noted at 1500 FTU kg-1 level. The present results of growth performance of C. mrigala fingerlings on plant based diets were in agreement with the findings of Baruah et al. (2007). However, Biswas et al. (2007) recorded significant increase in growth performance in red sea bream (Pagrus major), when compared to the control diet, at 2000 FTU kg-1 level of phytase supplementation. It indicated that phytase supplementation at 2000 FTU kg-1 diet might be effective in reducing an anti-nutritional factor or adverse consequences of phytate from soybean meal (Liu, 1997). Phytase dosage higher than 2000 FTU kg-1 level, however, did not further increase the growth performance in red sea bream. Similar patterns of phytase activity on growth performance have also been observed in many studies such as those by Forster et al. (1999), Yoo et al. (2005), Liebert and Portz (2007) and Baruah et al. (2007b). When canola protein concentrate was used for rainbow trout (Oncorhynchus mykiss) with different levels (500, 1500 and 4500 FTU kg-1) of phytase, Forster et al. (1999) found a higher specific growth rate (SGR) in rainbow trout fed on phytase supplemented diet at 500 FTU kg-1 level. Phytase supplementation, higher than 500 FTU kg-1 level did not further increase the SGR. In Korean rockfish, although supplementation of phytase in soybean meal diets at 1000 and 2000 FTU kg-1 levels did not increase growth performance, inferior results were observed when phytase was supplemented at 2000 FTU kg-1 level as compared to 1000 FTU kg-1 level (Yoo et al., 2005). Liebert and Portz (2007) reported that supplementation of phytase (SP 1002) at 750 FTU kg-1 was sufficient for maximum degradation of phytate resulting higher growth performance of Nile tilapia (Oreochromis nilotics). Nwanna and Schwarz (2008) also documented an increase in growth parameters at 750 and 1000 FTU kg-1 levels of phytase (RonozymeP(CT) Lot HB 950009) supplementation in diets of common carp (Cyprinus carpio). Baruah et al. (2007b) observed that the growth promoting effect was higher in Labeo rohita juveniles fed with a sub-optimum protein (25%) diet containing both 3% citric acid and 500 FTU kg-1 level of phytase supplementation than those which were fed with 35% crude protein diets. For their better results, they argued that citric acid provided the most favorable environment for phytase activity by lowering pH of the fish intestine.
Table VII.- Comparison of different fish species to show the optimum level of phytase supplementation.
|
Optimum dose of Phytase (FTU kg-1) |
Fish species | Phytase supplementation in fish diets improved following parameters | References |
| 250 |
Channel catfish |
Growth performance | |
| 250 | Channel catfish | Improved weight gain, feed intake and FCR | |
| 400 | Rainbow trout | Nutrient and mineral digestibility | |
| 500 |
Labeo rohita
|
Nutrient and mineral digestibility | |
| 500 | Rainbow trout | Amino acids, protein and mineral digestibility | |
| 500 | Rainbow trout | Protein, gross energy and mineral digestibility | |
| 500 | Cyprinus carpio (L.) | Growth, nutrient and mineral digestibility | |
| 500 | P. pangasius) | Growth and protein digestibility | |
| 500 | Rainbow trout | Growth and nutrient digestibility | |
| 500-1000 | Common carp | Weight gain | |
|
700
|
Nile tilapia | Growth performance improved | |
| 750 |
Oreochromis niloticus |
Growth performance, Protein effciency ratio (PER), phosphorous (P) and energy utilization | |
| 750 | Labeo rohita | Growth, protein and mineral digestibility | |
| 750 | Clarias garipinus | Growth performance | |
| 750 | Labeo rohita | Growth Performance, Nutrient digestibility, Minerals availability | |
| 750 | Labeo rohita | Growth Performance, Nutrient digestibility, Minerals availability | |
| 750 | Labeo rohita | Growth Performance, Nutrient digestibility, Minerals availability | |
| 800 | Cyprinus carpio | Growth performance | |
| 1000 | Carassius carassius | Growth performance | |
| 1000 | Oreochromis niloticus | PER, P digestibility | |
| 1000 |
Oreochromis niloticus |
Growth performance and mineral digestibility |
|
| 1000 | Red tilapia | Serum phosphorous (P) digestibility | |
| 1000 | Channel catfish | Growth parameters and phosphorus digestibility | |
| 1000 | Chanos chanos | Growth rate and nutrient digestibility | |
| 1000 | Striped bass | Growth and mineral digestibility | |
| 1000-2000 | Sebastes schlegeli | P digestibility | |
| 4000 | sex-reversed red tilapia | Growth performance | |
| 4000 |
Colossoma macropomum |
Growth, protein and fat digestibility | |
|
Phytase supplementation in fish diets could not improve following parameters |
|||
| 0-1200 | Oncorhynchus mykiss | No effect was observed on weight gain and feed efficiency of fish | |
| 2000-3000 | Oncorhynchus mykiss |
Protein digestibility increased, SGR and FCR was not improved, but negative effect on lipid digestibility |
|
| 500-8000 | Channel catfish | No effect on weight gain and protein digestibility | |
This inconsistency in the outcome could be attributed to differences in phytic acid content in different feed ingredients, nutritional quality of plant ingredients, water quality, fish species, size and culture and varying experimental conditions (Ashraf and Goda, 2007).
Sunflower meal is a rich source of protein. It has been successfully used as major ingredient of feed for various fish species. However, its very high content of phytic acid limits its use as major ingredient in diets stomach less fish species (Farhangi and Carter, 2007). The growth performance of C. mrigala fingerlings in terms of weight gain and feed conversion ratio (FCR) was significantly improved in sunflower meal-based diets supplemented with phytase. The maximum weight gain and best FCR were observed at 1000 FTU kg-1 phytase supplementation level. A steadily increase in growth performance was observed with the increase in phytase supplementation dose up to 1000 FTU kg-1, however interestingly, higher doses (2000 FTU kg-1) causes decrease in growth performance. The findings of the present study provide evidence that phytase supplementation at 1000 FTU kg-1 level is sufficient to minimize the effect of phytic acid while using sunflower meal as major feed ingredient in diet of C. mrigala. The present results of growth performance of C. mrigala fingerlings fed on phytase supplemented diets are similar to the findings of Baruah et al. (2007) and Hussain et al. (2011b).
Phytic acid can non-selectively chelates to proteins and also inhibit enzymes (pepsin, trypsin and alpha-amylase) activities (Liener, 1994) resulting in reduced protein digestibility (Kumaret al., 2011). The treatment of fish feed with phytase resulted in the improvement of protein digestibility and retention in fishes (Cheng and Hardy, 2002; Vielma et al., 2004; Debnath et al., 2005; Baruah et al., 2005; Ai et al., 2007; Liebert and Portz, 2007). In present study, the maximum apparent crude protein digestibility (%) of Cirrhinus mrigala fingerlings fed on sunflower meal was observed at 1000 FTU kg-1 of phytase. It was also evident that further increase in phytase level resulted in a decline in the digestibility of nutrients. In a comparative study with two different types of phytase enzyme (Wang et al., 2009) determined that 750 FTU kg-1 level of phytase A (SP 1002) supplementation was enough for increasing nutrient digestibility resulting in optimally increased fish growth. Similarly, the phytase B (Ronozyme® P) supplemented at 1000 FTU kg-1 diet was found adequate for maximum phytate degradation resulting in increased nutrient digestibility. However, Baruah et al. (2007) found highest digestibility of crude protein for Labeo rohita juveniles fed soybean meal based diet at 750 FTU kg-1 level whereas it decreased at 1000 FTU kg-1 level. Protein digestibility was also increased in rainbow trout fed on soybean meal based diet supplemented with phytase at 500-1000 FTU kg-1 levels (Dalsgaard et al., 2009). The maximum value of crude fat digestibility of sunflower meal was also observed at 1000 FTU kg-1 level and this value was significantly different (p<0.05) from digestibility values of reference diet and left over test diets. Further higher phytase supplementation levels resulted in significant decrease in fat digestibility. Ashraf and Goda (2007) also observed similar trend. They found that phytase supplementation increased the apparent digestibility coefficient of lipid up to 88.3% at 1000 FTU kg-1 level and at higher levels the lipid digestibility started decreasing (87% to 84%). In contrast, Wang et al. (2009) reported reduced lipid digestibility by phytase supplementation. They claimed that phytase supplementation might inhibit lipase activity which in turn minimized the lipase hydrolysis efficiently for lipid, resulted in reduced lipid digestibility in phytase supplemented diets. However, Dalsgaard et al. (2009) found no significant effect on fat digestibility in rainbow trout (Oncorhynchus mykiss) fed on plant based test diets supplemented with phytase. Higher digestibility of gross energy (68.33%) was observed at 1000 FTU kg-1 level. The present study is in accordance with Ashraf and Goda (2007). They found maximum gross energy digestibility (73%) on 1000 FTU kg-1 level and after this optimum level, it decreased to 71.8% on 1500 FTU kg-1and 71% on 2000 FTU kg-1 level. Portz and Liebert (2004) determined significant improvement in gross energy digestibility in diets supplemented with 1000 and 2000 FTU kg-1 levels. In general, phytase supplementation improves the protein bioavailability and this may lead to further increases in gross energy digestibility resulting in maximum fish growth performance (Liebert and Portz, 2005). Cheng and Hardy (2002) reported that phytase supplementation in diets having canola protein concentrate enhanced apparent gross energy digestibility for rainbow trout, while Lanari et al. (1998) did not find any positive response for rainbow trout fed soybean meal based test diets. Differences in results of different researchers may be due to changes in phytic acid contents in feed ingredients, fish species used and a range of other inherent characteristics of feed ingredients (Vielma et al., 2004).
Conclusion
In conclusion, the present study confirmed that sunflower meal-based diet supplemented with 1000 FTU kg-1 level of phytase significantly released chelated nutrients and increased the nutrient digestibility for improving growth performance of Cirrhinus mrigala fingerlings. It was also found that phytase supplementation played a significant role in developing environment friendly and economical feed for indigenous culture able major carp Cirrhinus mrigala.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Ai, Q., Mai, K., Zhang, W., Xu, W., Tan, B., Zhang, C. and Li, H., 2007. Effects of exogenous enzymes (phytase, non-starch polysaccharide enzyme) in diets on growth, feed utilization, nitrogen and phosphorus excretion of Japanese seabass, Lateolabrax japonicus. Comp. Biochem. Physiol. Part A, 147: 502-508. https://doi.org/10.1016/j.cbpa.2007.01.026
Anjum, M.A., Hussain, Z., Khan, S.H. Ahmad, N., Amer M.Y. and Iftikhar, N., 2014. Assessment of poultry feed ingredients used in commercial compound feed. Pak. J. Life Soc. Sci., 12: 69-73.
Ashraf, M. and Goda, A.S., 2007. Effect of dietary soybean meal and phytase levels on growth, feed utilization and phosphorus discharge for Nile tilapia (Oreochromis niloticus L.). J. Fish. aquat. Sci., 2: 248-263.
AOAC, 1995. Official methods of analysis, 15th edition, Association of Official Analytical Chemists, Washington DC, pp. 1994.
Azeredo, P.A., Cho, C.Y., Leeson, S. and Bureau, D.P., 1998. Effect of feeding level and water temperature on growth, nutrient and energy utilization and waste outputs of rainbow trout (Oncorhynchus mykiss). Aquacult. Living Res., 11: 227-238. https://doi.org/10.1016/S0990-7440(98)89005-0
Baruah, K., Pal, K.A.K., Narottam, P.S. and Debnath, D., 2007. Microbial Phytase supplementation in rohu, Labeo rohita, diets enhances growth performance and nutrient digestibility. J. World Aquacult. Soc., 38: 129-137. https://doi.org/10.1111/j.1749-7345.2006.00081.x
Baruah, K., Sahu, P.N., Pal, K.A., Jain, K.K., Debnath, D. and Mukherjee, C.S., 2007b. Dietary microbial phytase and citric acid synergistically enhances nutrient digestibility and growth performance of Labeo rohita (Hamilton) juveniles at sub-optimal protein level. Aquacult. Res., 38: 109-120. https://doi.org/10.1111/j.1365-2109.2006.01624.x
Baruah, K.P., Sahu, A.K., Jain, N.P., Mukherjee, K.K. and Debnath, S.C., 2005. Dietary protein level, microbial phytase, citric acid and their interactions on bone mineralization of Labeo rohita (Hamilton) juveniles. Aquacult. Res., 36: 803-812. https://doi.org/10.1111/j.1365-2109.2005.01290.x
https://doi.org/10.1016/j.aquaculture.2007.01.014
Britz, P.J., Hecht, I. and Mangold, S., 1997. Effect of temperature on growth, feed consumption and nutritional indices of Haliotis midae fed a formulated diet. Aquaculture, 152: 191-203. https://doi.org/10.1016/S0044-8486(97)00002-1
Cao, L., Wang, W., Yang, C., Yang, Y., Diana, J., Yakupitiyage, A., Luo, Z. and Li, D., 2007. Application of microbial phytase in fish feed. Enz. Microb. Technol., 14: 342-362. https://doi.org/10.1016/j.enzmictec.2007.01.007
Cao, L., Yang, Y., Wang, W.M., Yakupitiyage, A., Yuan D.R. and Diana, J.S., 2008. Effects of pretreatment with microbial phytase on phosphorous utilization and growth performance of Nile tilapia (Oreochromis niloticus). Aquacult. Nutr., 14: 99-109. https://doi.org/10.1111/j.1365-2095.2007.00508.x
Cheng, Z.J. and Hardy, R.W., 2002. Effect of microbial phytase on apparent nutrient digestibility of barley, canola meal, wheat and wheat middlings, measured in vivo using rainbow trout (Oncorhynchus mykiss). Aquacult. Nutr., 8: 271-277. https://doi.org/10.1046/j.1365-2095.2002.00219.x
Cheng, Z.J. and Hardy, R.W., 2003. Effects of extrusion and expelling processing and microbial phytase supplementation on apparent digestibility coefficients of nutrients in full-fat soybeans for rainbow trout (Oncorhynchus mykiss). Aquaculture, 218: 501-514. https://doi.org/10.1016/S0044-8486(02)00458-1
Cheng, Z.J. and Hardy, R.W., 2004. Effects of microbial phytase supplementation and dosage on apparent digestibility coefficients of nutrients and dry matter in soybean product-based diets for rainbow trout Oncorhynchus mykiss. J. World Aquacult. Soc., 35: 1-15. https://doi.org/10.1111/j.1749-7345.2004.tb01054.x
Dalsgaard, J., Ekmann, K.S., Pedersen, P.B. and Verlhac, V., 2009. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus depleted juvenile rainbow trout (Oncorhynchus mykiss) and on the magnitude and composition of phosphorus waste output. Aquaculture, 286: 105-112. https://doi.org/10.1016/j.aquaculture.2008.09.007
Debnath, D., Pal, A.K., Narottam, P.S., Jain, K.K., Yengkokpam, S. and Mukherjee., S.C., 2005. Effect of dietary microbial phytase supplementation on growth and nutrient digestibility of Pangasius pangasius (Hamilton) fingerlings. Aquacult. Res., 36: 180-187. https://doi.org/10.1111/j.1365-2109.2004.01203.x
Divakaran, S., Leonard G.O. and Ian, P.F., 2002. Note on the methods for determination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467. https://doi.org/10.1021/jf011112s
Drew, M.D., Borgeson, T.L. and Thiessen, D.L., 2007. A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim. Feed Sci. Tech., 13: 118-136. https://doi.org/10.1016/j.anifeedsci.2007.06.019
Farhangi, M. and Carter, C.G., 2007. Effect of enzyme supplementation to dehulledlupin-based diets on growth, feed efficiency, nutrient digestibility and carcass composition of rainbow trout, Oncorhyncus mykiss (Walbaum). Aquacult. Res., 38: 1274-1282. https://doi.org/10.1111/j.1365-2109.2007.01789.x
Forster, I., Higgs, D.A., Dosanjh, B.S. and Rowshandeli, M., 1999. Potential for dietary phytase to improve the nutritive value of canola protein concentrate and decrease phosphorus output in rainbow trout (Oncorhynchus mykiss) held in 11°C fresh water. Aquaculture, 179: 109-125. https://doi.org/10.1016/S0044-8486(99)00156-8
Furuya, W.M., Goncalves, G.S., Furuya, V.R.B. and Hayashi, C., 2001. Phytase as feeding for Nile tilapia, Oreochromis niloticus. Performance and digestibility. Rev. Brasil. Zootec., 30: 924-929. https://doi.org/10.1590/S1516-35982001000400003
Gatlin III, D.M., Barrows, F.T., Brown, P., Dabrowski, K., Gaylord, T.G., Hardy, R.W., Herman, E., Hu, G., Krogdahl, A., Nelson, R., Overturf, K., Rust, M., Sealey, W., Skonberg, D., Souza, E.J., Stone, D., Wilson, R. and Wurtele, E., 2007. Expanding the utilization of sustainable plant products in aqua feeds: a review. Aquacult. Res., 38: 551-579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
Hassan, S., Altaff, K. and Satyanarayana, T., 2009. Use of soybean meal supplemented with cell bound phytase for replacement of fish meal in the diet of juvenile milkfish, Chanos chanos. Pak. J. Nutr., 8: 341-344. https://doi.org/10.3923/pjn.2009.341.344
Helland, S., Denstadli, V., Witten, P.E., Hjelde, K., Strobakken, T., Skrede, A., Asgard, T. and Baeverfjord, G., 2006. Hyper dense vertebrae and mineral content in Atlantic salmon (Salmo salar L.) fed diets with graded levels of phytic acid. Aquaculture, 261: 603-614. https://doi.org/10.1016/j.aquaculture.2006.08.027
Husain, S.M., Rana, S.A., Afzal, M. and Shahid, M., 2011a. Efficacy of phytase supplementation on mineral digestibility in Labeo rohita fingerlings fed on corn gluten meal (30%) based diets. Pak. J. agric. Sci., 48: 237-241.
Hussain, S.M., Afzal, M., Javid, A., Hussain, A.I., Ali, Q., Mustafa, I., Chatha, S.A.S., Shah, S.Z.H., Hussain, M. and Irfan-Ullah, M., 2015. Efficacy of phytase supplementation on growth performance and mineral digestibility of Labeo rohita fingerlings fed on cottonseed meal based diet. Pakistan J. Zool., 47: 699-709.
Hussain, S.M., Afzal, M., Rana, S.A., Javid, A. and Hussain, M., 2011c. Impact of phytase supplementation on nutrient digestibility for Labeo rohita fingerlings fed on sunflower meal based diets. Pak. J. Life Soc. Sci., 9: 85-90.
Hussain, S.M., Afzal, M., Rana, S.A., Javid, A. and Iqbal, M., 2011b. Effect of phytase supplementation on growth performance and nutrient digestibility of Labeo rohita fingerlings fed on corn gluten meal-based diets. Int. J. Agric. Biol., 13: 916-922.
Hussain, S.M., Hameed, T., Afzal, M., Mubarik, M.S., Asrar, M., Shah, S.Z.H., Ahmad, S., Arsalan, M.Z.H., Riaz, D., Tahir, N., Amber, F., Shahzad M.M. and Khichi, T.A.A., 2014. Effects of phytase supplementation on mineral digestibility in Cirrhinus mrigala fingerlings fed on sunflower meal-based diets. Int. J. Biol., 5: 173-181.
Jackson, L., Li, M.H. and Robinson, E.H., 1996. Use of microbial phytase in channel catfish Ictalurus punctatus diets to improve utilization of phytate phosphorus. J. World Aquacult. Soc., 27: 309-313. https://doi.org/10.1111/j.1749-7345.1996.tb00613.x
Kumar, V., Makkar, H.P.S. and Becker, K., 2011. Detoxified Jatrophacurcas kernel meal as a dietary protein source. Growth performance, nutrient utilization and digestive enzymes in common carp (Cyprinus carpio L.) fingerlings. Aquacult. Nutr., 17: 313-326. https://doi.org/10.1111/j.1365-2095.2010.00777.x
Lanari, D., Agaro, E.D. and Turri, C., 1998. Use of nonlinear regression to evaluate the effects of phytase enzyme treatment of plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquaculture, 161: 345-356. https://doi.org/10.1016/S0044-8486(97)00282-2
Li, M.H. and Robinson, E.H., 1997. Microbial phytase can replace inorganic phosphorus supplements in channel catfish Ictalurus punctatus diets. J. World Aquacult. Soc., 28: 402-406. https://doi.org/10.1111/j.1749-7345.1997.tb00287.x
Liebert, F. and Portz, L., 2005. Nutrient utilization of Nile tilapia Oreochromis niloticus fed plant based low phosphorus diets supplemented with graded levels of different sources of microbial phytase. Aquaculture, 248: 111-119. https://doi.org/10.1016/j.aquaculture.2005.04.009
Liebert, F. and Portz, L., 2007. Different sources of microbial phytase in plant based low phosphorus diets for Nile Tilapia Oreochromis niloticus may provide different effects on phytate degradation. Aquaculture, 267: 292-299. https://doi.org/10.1016/j.aquaculture.2007.01.023
Liener, I.E., 1994. Implications of anti-nutritional components in soybean foods. Crit. Rev. Fd. Sci. Nutr., 34: 31-67. https://doi.org/10.1080/10408399409527649
Lim, S.J., Kim, S., Ko, G., Song, J., Oh, D., Kim, J. and Lee, K., 2011. Fish meal replacement by soybean meal in diets for Tiger puffer, Takifu gurubripes. Aquaculture, 313: 165-170. https://doi.org/10.1016/j.aquaculture.2011.01.007
Lin, S., Mai, K., and Tan, B., 2007. Effects of exogenous enzyme supplementation in diets on growth and feed utilization in tilapia, Oreochromis niloticus × O. aureus. Aquacult. Res., 38: 1645-1653. https://doi.org/10.1111/j.1365-2109.2007.01825.x
Liu, K., 1997. Chemistry and nutritional value of
soybean components. In: Soybeans: Chemistry, technology and utilization. Chapman and Hall, International Thomson Publishing, Singapore, pp. 532. https://doi.org/10.1007/978-1-4615-1763-4
Lovell, R.T., 1989. Fish nutrition and feeding. Van Nostrand Reinhold Co., New York. https://doi.org/10.1007/978-1-4757-1174-5
Mushtaq, T., Sarwar, M., Ahmad, G., Nisa, M.U. and Jamil, J., 2006. The influence of exogenous multi-enzyme preparation and graded levels of digestibility sine on the performance of young broiler chicks two weeks post hatching in sunflower meal based diets. Poult. Sci., 85: 2180-2185. https://doi.org/10.1093/ps/85.12.2180
NRC, 1993. Nutrient Requirements of Fish114. National Research Council, National Academy Press, Washington DC.
NRC, 1996. Nutrient requirements of beef cattle, 7th Revised Edition. National Academy of Sciences, Washington DC.
Nwanna, L.C., Oishi, C.A. and Filho, M.P., 2008. Use of phytase to improve the digestibility ofalternative feed ingredients by Amazon tambaqui, Colossoma macropomum. J. Sci. Asia, 34: 353-360. https://doi.org/10.2306/scienceasia1513-1874.2008.34.353
Papatryphon, E., Howell, R.A. and Soares, J.H., 1999. Growth and mineral absorption by striped bass Morone saxatilis fed a plant feedstuff based diet supplemented with phytase. J. World Aquacult. Soc., 30: 161-173. https://doi.org/10.1111/j.1749-7345.1999.tb00863.x
Pham, M.A., Lee, K.J., Dang, T.M., Lim, S.J., Ko, G.Y., Eo, J. and Oh, D.H., 2008. Improved apparent digestibility coefficient of protein and phosphorus by supplementation of microbial phytase in diets containing cottonseed and soybean meal for juvenile olive flounder (Paralichthys olivaceus). Asian-Australasian J. Anim. Sci., 21: 1367-1375. https://doi.org/10.5713/ajas.2008.80053
Portz, L. and Liebert, F., 2004. Growth, nutrient utilization and parameters of mineral metabolism in Nile tilapia Oreochromis niloticus (Linnaeus, 1758) fed plant based diets with graded levels of microbial phytase. J. Anim. Physiol. Anim. Nutr., 88: 311-320. https://doi.org/10.1111/j.1439-0396.2004.00486.x
Riche, M. and Garling, D.L., 2004. Effect of phytic acid on growth and nitrogen retention in tilapia Oreochromis niloticus L. Aquacult. Nutr., 10: 389-400. https://doi.org/10.1111/j.1365-2095.2004.00314.x
Robinson, E.H., Li, M.H. and Manning, B.B., 2002. Comparison of microbial phytase and dicalcium phosphate or growth and bone mineralization of pond-raised channel catfish, Ictalurus punctatus. J. appl. Aquacult., 12: 81-88. https://doi.org/10.1300/J028v12n03_08
Sajjadi, M. and Carter. C.G., 2004. Effect of phytic acid and phytase on feed intake, growth, digestibility and trypsin activity in Atlantic salmon (Salmo salar L.). Aquacult. Nutr., 10: 135-142. https://doi.org/10.1111/j.1365-2095.2003.00290.x
Sardar, P.H., Randhawa, S., Abid, M. and Prabhakar, S.K., 2007. Effect of dietary microbial phytase supplementation on growth performance, nutrient utilization, body compositions and haemato-biochemical profiles of Cyprinus carpio (L.) fingerlings fed soyprotein-based diet. Aquacult. Nutr., 13: 444-456. https://doi.org/10.1111/j.1365-2095.2007.00497.x
Schafer, A., Koppe, W.M., Meyer-Burgdorff, K.H. and Gunther, K.D., 1995. Effects of a microbial phytase on the utilization of native phosphorus by carp in a diet based on soybean meal. Water Sci. Tech., 31: 149-155. https://doi.org/10.1016/0273-1223(95)00434-O
Soltan, M.A., 2009. Effect of dietry fish meal replacement by poultry by-product meal with different grain source and enzyme supplementation on performance, feces recovery, body composition and nutrient balance of Nile tilapia. Pak. J. Nutr., 8: 395-407. https://doi.org/10.3923/pjn.2009.395.407
Spinelli, J., Houle, C.R. and Wekell, J.C., 1983. The effect of phytates on the growth of rainbow trout (Salmo gairdneri) fed purified diets containing varying quantities of calcium and magnesium. Aquaculture, 30: 71-83. https://doi.org/10.1016/0044-8486(83)90153-9
Van-Weerd, J.H., Khalaf, K.H.A., Aartsen, F.J. and Tijssen, P.A.T., 1999. Balance trials with African cat fish, Clarias gariepinus fed phytase-treated soybean meal-based diets. Aquacult. Nutr., 5: 135-142. https://doi.org/10.1046/j.1365-2095.1999.00100.x
Vielma, J., Lall, S.P. and Koskela, J., 1998. Effects of dietary phytase and cholecalciferol on phosphorus bioavailability in rainbow trout (Oncorhynchus mykiss). Aquaculture, 163: 309-323. https://doi.org/10.1016/S0044-8486(98)00240-3
Vielma, J., Makinen, T., Ekholm, P. and Koskela, J., 2000. Influence of dietary soy and phytase levels on performance and body composition of large rainbow trout Oncorhynchus mykiss and algal availability of phosphorus load. Aquaculture, 183: 349-362. https://doi.org/10.1016/S0044-8486(99)00299-9
Vielma, J., Ruohonen, K., Gabaudan, J. and Vogel, K., 2004. Top spraying soybean meal-based diets with phytase improves protein and mineral digestibilities but not lysine utilization in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacult. Res., 35: 955-964. https://doi.org/10.1111/j.1365-2109.2004.01106.x
Vohra, A. and Satyanarayana, T., 2003. Phytases: microbial sources, production, purification and potential biotechnological applications. Crit. Rev. Biotechnol., 23: 29-60. https://doi.org/10.1080/713609297
Wang, F., Yang, Y.H., Han, Z.Z., Dong, H.W., Yang, C.H. and Zou, Z.Y., 2009. Effects of phytase pre-treatment of soybean meal and phytase sprayed in diets on growth, apparent digestibility coefficient and nutrient excretion of rainbow trout (Oncorhynchus mykiss Walbaum). Aquacult. Int., 17: 143-157. https://doi.org/10.1007/s10499-008-9187-5
Yan, W. and Reigh, R.C., 2002. Effects of fungal phytase on utilization of dietary protein, minerals and dephosphorylation of phytic acid in the alimentary tract of channel catfish Zctulurus punctutus fed an all-plant-protein diet. J. World Aquacult. Soc., 33: 10-22. https://doi.org/10.1111/j.1749-7345.2002.tb00473.x
Yildirim, O., Acar, U., Turker, A., Sunar, M.C. and Kesbic, O.S., 2014. Effects of replacing fish meal with peanut meal (Arachis hypogaea) on growth, feed utilization and body composition of Mozambique Tilapia fries (Oreochromis mossambicus). Pakistan. J. Zool., 46: 497-502. https://doi.org/10.1016/j.aquaculture.2004.10.025
Yu, F.N. and Wang, D.Z., 2000. The effects of supplemental phytase on growth and the utilization of phosphorus by crucian carp Carassius carassius. J. Fish Sci. China, 7: 106-109 [in Chinese].
Zhou, Q.C., Tan, B.P., Mai, K.S. and Liu, Y.J., 2004. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture, 241: 441-451. https://doi.org/10.1016/j.aquaculture.2004.08.044
To share on other social networks, click on any share button. What are these?










