Fluorescence Analysis, Extractive Values and Cytotoxic Screening of Crataeva adansonii DC Leaf and Bark through Brine Shrimp Larvae Assay
Syeda Farzana Bibi* and Siraj-ud-Din
Department of Botany, University of Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Crataeva adansonii is a medicinal plant and many folklore uses of this plant are well known to us. Pharmacognostic studies like finding extractive values and nutritional values play a vital role in the determination of adulterated or exhausted drugs. It lays down parameters for authentication and standardization of drugs. The cytotoxic study helps in the evaluation of a drug for its toxicity, which is determined by the prevention of growth and replication. Such drugs can be used to treat cancer, multiple sclerosis and rheumatoid arthritis. During the present study ethanolic extracts of leaf and bark parts of C. adansonii were evaluated through cytotoxic activity. Brine shrimp larvae assay was conducted for this purpose. Extractive values of leaf and bark parts were estimated using n-hexane, chloroform, ethanol, methanol, water, and ether as a solvent. 10g of plant powder was dissolved in 200 ml solvent during various extract preparations Fluorescence study was conducted after treating the powder drug with different reagents including ethanol, methanol, acetic acid, n-hexane, HCl, nitric acid, carrageenan, glacial acetic acid, diethyl ether and picric acid and change in coloration was observed. Distinct fluorescence was exhibited by the drug under the normal daylight and UV-radiations of short wavelength (256nm) and long wavelength (365nm). Both the leaf and bark parts showed maximum extractive values in water (23% and 15% respectively) followed by methanol (15% and 6% respectively. The leaf part showed greater extractive values as compared to the bark. The cytotoxic study revealed that the ethanolic extract crude drugs of each part dose-dependently exhibited cytotoxic activity. At higher concentration (100-1000 µg/ml) the leaf and bark ethanolic extracts showed cytotoxicity and at lower concentration (10 µg/ml) very low cytotoxic effect was observed (Figure 4). The LC50 values for crude leaf extract were found to be 5.34 and for that of stem bark extract, it was 7.44 ug/ml. This study manifests that the selected plant can be helpful in the preparation of novel pharmaceuticals. The pharmacognostic evaluation of this important medicinal drug will be supportive in the detection of adulteration and drug quality control.
Received | July 13, 2020; Accepted | August 18, 2020; Published | September 01, 2020
*Correspondence | Syeda Farzana Bibi, Department of Botany, University of Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: dr.syedafarzana@yahoo.com
Citation | Bibi, S.F. and S. Din. 2020. Fluorescence analysis, extractive values and cytotoxic screening of Crataeva adansonii DC leaf and bark through brine shrimp larvae assay Sarhad Journal of Agriculture, 36(3): 949-957.
DOI | http://dx.doi.org/10.17582/journal.sja/2020/36.3.949.957
Keywords | Crataeva adansonii, Fluorescence study, Extractive values, Cytotoxicity analysis, Physicochemical characters, Pharmacognostic study
Introduction
Crataeva adansonii is a moderate-sized deciduous tree belonging to family Capparaceae (Ryan and Ray, 2004). It is commonly known as Varun, temple plant and sacred garlic pear. It grows well on sandy loam in arid areas. In Pakistan, it is found on the Sub-Himalayan track. Its leaves are trifoliate compound (Figure1; Hoang, 2013). The fruit is globose in shape having a 4-5cm diameter. The flowers are showy and white, bearing long purplish stamens (Figure 2; Jeevan, 2016). Due to the elongated appearance of stamens, the plant is often called a spider tree.
Traditionally, this plant is used as diuretic, astringent, antipyretic, anti-inflammatory, appetizer, liver stimulant, contraceptive, stomachic, demulcent and tonic. Use of C. adansonii against bronchitis, calculi, asthma, cough, renal and urinary troubles, dyspepsia and uterine infections is also well known in traditional medicine (Kumar et al., 2012; Gitte et al., 2012). Previously anticancer, antimicrobial, antihypertension, analgesic, antiparasitic, antidiabetic and antioxidant activities of C. adansonii have been reported (Zingue et al., 2016; Adjagba et al., 2017). Cytotoxic studies have been carried out in various genera of the family Capparaceae including C. nurvala (Sinha et al., 2013; Ali et al., 2014; Hade et al., 2016; Parvin et al., 2011) and C. nurvala (Da Silva Ferreira et al., 2013).
The extractive value of a crude drug refers to its soluble or extractable material in a suitable solvent. The solution obtained after the extraction of a drug contains different phytoconstituents. The composition of those constituents depends on the nature of the solvent and nature of a drug, it also gives an idea of whether the crude drug is genuine or exhausted (Tatiya et al., 2012). Extractive values play an imperative role in crude drug evaluation. During extraction, the use of different solvents assures exhausted material and adulteration e.g water and alcohol are used to detect poor quality signs, defective processing and presence of adulterants in the drug. Lipid content of the crude drug is indicated by extractive values, using petroleum ether as a solvent (Kokate, 1994; Madhavan et al., 2009). The physicochemical standards and Preliminary phytochemical analysis help in the detection and identification of adulteration in the drug. Closely related species of the same family and genus can also be distinguished through these findings (Gavit and Patel, 2019). The values of the total yield of extracts are resultants of the effects of the extraction condition. With the increase in the polarity of the solvents the extraction yield is increased (Ranjith, 2018).
Fluorescence analysis helps in the determination of constituents and it is effectively sensitive (Prakash and Vedanayaki, 2019). Many drug constituents show fluorescence in the visible range of daylight while others exhibit it under ultra-violet light. The same material appears to be dissimilar in light of different wavelengths, some substances are not luminescence in visible light but they show fluorescence when observed under UV light. The fluorescence quality of any drug supports characteristic highlights for drug assurance due to its exceptional specificity (Sharma and Dhanawat, 2019). If fluorescence is not shown by a drug then the application of different reagents to their derivatives or decomposition products may make them fluorescent. This analysis can be used as a qualitative assessment for drug standardization. During the identification of various crude drugs, fluorescence can serve to be a fingerprint (Ansari et al., 2006; Reddy and Chaturvedi, 2010). UV light qualitative evaluation of crude drugs is performed which is a distinguished parameter regarding the pharmacognostic evaluation of drugs (Reddy and Chaturvedi, 2010). Different plant material gives different coloration when treated with various chemicals and solvents (Sumithra and Kumar, 2016; Suriyavathana et al., 2018). The fluorescence quality of any drug supports characteristic highlights for drug assurance due to its exceptional specificity (Sharma and Dhanawat, 2019).
Toxic compounds are found in the extracts of some medicinal plants (Iqbal, 2016). For safety assessment, the toxicity profiling of these plants is very effective (Chan et al., 2016). Artemia salina (Brine shrimps) are widely used during the cytotoxicity experiments to check the potential of various chemical entities. Phytomedicine is used against cancer and other diseases (Amara et al., 2008). Almost half of the anticancer drugs are derived from plants (Newman and Cragg, 2007). Brine shrimp’s larvae are used for evaluating the cytotoxic potential of plant-derived drugs during anticancerous activity. Shrimp’s larvae can respond in quite a similar manner to the mammalian’s carcinoma (Solis et al., 1993). Therefore, cell lines assay can be replaced by brine shrimp assay for cytotoxicity as it is easily achievable and less costly (Piccardi et al., 2000). Plant-derived biologically active compounds show toxicity towards Artemia salina. This quick and economic activity is established for fractionation and screening process (Carballo et al., 2002). The role of Artemisia salina in a marine ecosystem cannot be neglected. It is an invertebrate, also known as brine shrimp. Brine shrimp lethality experiment helps find medium lethality concentration LC50 calculated for toxins and plant extracts (Lagadic and Caquet, 1998). The lethality experiment of brine shrimps as described in the literature is considered as a useful tool to detect cytotoxicity caused by heavy metals, plant extracts and toxins produced by cyanobacteria (Sharififar et al., 2009).
In the present research, the ethanolic extract of leaf and bark parts of C. adansonii was investigated to find its cytotoxic potential against brine shrimp larvae. Powder drug of the plant was analyzed during the study of fluorescence and extractive values.
Materials and Methods
Collection of plant material
C. adansonii DC. leaf and bark were collected from the University of Peshawar campus and identified by plant taxonomist Prof. Dr. Siraj-ud-Din. A voucher specimen was submitted in the herbarium of the University of Peshawar (PUP).
Preparation of crude extract
Leaf and bark parts of C. adansonii were collected and cleaned thoroughly, this material was shade dried and ground to make a fine powder. One kilogram plant powder was soaked in 5-6 liters of ethanol. The mixture was kept in an airtight jar for 12-15 days at room temperature and occasional vigorous shaking was ensured. This solution was filtered and then it was concentrated in a rotary evaporator to get a thick crude extract. After repeating this process 3 times the collected plant extracts were preserved in a cold and dry place (Miliauskas et al., 2004).
Extractive values
Various solvents can be used to extract chemical constituents of a powder drug, for example, alcohol extracts resins and tannins; water extracts mucilages and glycosides; ether extracts oily and fatty substances. The amount of an extract is the approximate measure of its chemical constituents. The number of adulterants can be calculated by these values (Kokate, 1994).
Procedure
Extractive values were calculated for the drug, using standard protocols (Ansari et al., 2006; Yadav et al., 2007). 200 ml each of various solvents were used to dissolve 10 g of crude powder drug and kept in airtight bottles for 7 days with occasional vigorous shaking. The solution was strained through a muslin cloth and then filtered using a filter paper. A rotary evaporator was operated to evaporate the filtrate. After repeating the process three times, extracts were combined and weighed, their percent extractive values were calculated using the following formula:
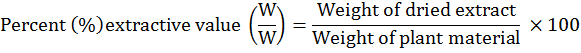
Fluorescence study
Certain drugs can show different kinds of fluorescence when they are exposed to daylight and also under ultraviolet radiation, so the fluorescence technique can be used for crude drugs as an identification tool (Jarald and Jarald, 2007). During this research study, the methodology of Kokoshi et al. (1958) was followed to study the fluorescence of C. adansonii leaf and bark.
Procedure
Fluorescence analysis was carried out using crude powder drugs as such and also after treating it with different reagents including ethanol, methanol, acetic acid, n-hexane, HCl, nitric acid, carrageenan, glacial acetic acid, diethyl ether and picric acid. The short and long wavelengths of UV light and daylight were used to observe the samples (Brain and Turner, 1975; Chase and Pratt, 1949; Evans, 2002).
Cytotoxicity
Ethanolic extracts of C. adansonii DC. leaf and bark were used to conduct brine shrimp assay to determine their preliminary cytotoxic potential by following the methodology proposed by Atta-ur-Rhman et al. (2001).
Technique for hatching
A hatching tray (22 x 32 cm) was partitioned with the help of a perforated plate into two chambers; brine solution was poured into the tray to half fill it. In one chamber 25 mg Artemisia salina eggs were sprinkled and covered with black paper. A lamp was suspended over the other chamber of the tray to illuminate it and the apparatus was kept at room temperature. After a hatching period of almost 60 hours, brine shrimp napualli were mature, and they moved from the dark chamber towards the enlightened one across the perforated partition.
Preparation of sample
A stock solution was prepared by dissolving 20 mg of plant extract in a 2 ml solvent. 5, 50 and 500 µl of this solution was transferred to glass vials using a micropipette. In this way, 3 replicates were prepared for each concentration i.e. 10, 100 and 1000 µg/ml. Each vial was kept open overnight to evaporate it and then a 5ml sea salt solution was poured into it. 10 mature napualli were shifted to each vial using Pasteur pipette. For positive and negative control, reference cytotoxic drug and brine solution were used respectively. Vials were kept at room temperature in light.
Calculations and statistical analysis
The number of dead and alive napualli was recorded after 24 hours. LC50 values were determined considering a 95% confidence interval with the help of the Finney computer program (Ibrar and Muhammad, 2011; Khan et al., 2010).
Results and Discussion
Extractive values
Extractive values are valuable for uncovering exhausted or adulterated drugs. Extraction with different solvents may give a clue about the adulteration of different kinds of exhausting materials (Madhavan et al., 2009). Extractable values with alcohol and water are indicative of the existence of adulterants, in addition to any defects in processing and low quality of the drug. On the other hand, extraction with petroleum ether shows the presence of lipid contents (Kokate, 1994).
During this research, percent extractive values were determined for bark and leaf of C. adansonii using various solvents, including n-hexane, ethanol, chloroform, distilled water, acetone and butanol. Maximum extractive value for the leaf part was found in water (23 %) followed by methanol (15 %), similarly, in the case of the bark part, it was water (15 %) followed by methanol (6 %). Extractive values in other solvents were variable (Table 1; Figure 3).
Table 1: Percent extractive values of Crataeva adansonii leaf and stem bark with different solvents.
|
S. No |
Plant part |
Solvent |
Extractive value (%) |
|
1 |
Leaf |
Methanol |
15 |
|
Ethanol |
11 |
||
|
N-hexane |
4 |
||
|
Water |
23 |
||
|
Chloroform |
6 |
||
|
Ether |
3 |
||
|
2 |
Bark |
Methanol |
6 |
|
Ethanol |
3 |
||
|
N-hexane |
3 |
||
|
Water |
15 |
||
|
Chloroform |
4 |
||
|
Ether |
2 |
Prakash and Vedanayaki (2019) used various solvents like ethyl acetate, aqueous, acetone, hexane, methanol and chloroform to extract Zephyranthes citrine plant powder. The highest quantity of yield was shown by methanol i.e. 4.00g. During the physicochemical analysis of Amaranthus blitum maximum extractive value was found in water (24.43 ± 0.983). Ethanol and methanol also showed considerable extractive values of 10.66 ± 0.666 and 10.46 ± 0.290 respectively (Gavit and Patel, 2019). Chloroform, ethyl acetate, petroleum ether, methanol, ethanol, water and acetone were used for the maceration process and extraction of Murraya koenigii Psidium guajava and Curcuma longa. The yield of aqueous extract was maximum (26.46%) due to the increased polarity of water. The extractive value of methanolic extract was slightly lower (16.42%) than ethanolic extract.
Fluorescence study
The presence of diverse chemical constituents in the plants accounts for variable fluorescence phenomena under UV light and in regular daylight. In agreement with the previous work, C. adansonii stem bark and leaf powder were subjected to fluorescence analysis using several reagents including ethanol, methanol, acetic acid, n-hexane, HCl, nitric acid, carrageenan, glacial acetic acid, diethyl ether and picric acid (Table 2). The powder drug was treated with the reagents and then exposed to ordinary daylight, UV- light of short wavelength (255nm) and long wavelength (366nm). Leaf and bark powders manifested different colors when they were exposed to the radiation. The crude methanol extract and powder drug of Zephyranthes citrina were analyzed during a fluorescence study after mixing with various reagents (Prakash and Vedanayaki, 2019) and various colors were recorded. Sharma and Dhanawat (2019) studied the fluorescence of Euphorbia neriifolia Linn. leaf and stem and declared it as an important parameter for herb standardization. Similarly, Azhagumadhavan et al. (2019) evaluated fluorescence analysis and other Physico-chemical parameters of Costus spicatus rhizome and described various diagnostic characters. Under UV and ordinary light, the powdered drug and extract showed reproducible and distinctive color change. Fluorescence analysis of Murraya koenigii Psidium guajava and Curcuma longa was performed after treating with distilled water, ethanol acetone, diethyl ether, benzene, methanol, chloroform, glacial acetic acid, nitric acid, sulphuric acid, hydrochloric acid, 1N NaOH, 5% picric acid, 5% FeCl3, 1N NaOH + methanol, and the solutions were observed for their characteristic colors under the visible daylight and long and short wavelength UV light (Ranjith, 2018). Fluorescence analysis is one of the diagnostic tools used for the detection of adulterants in whole plants as well as in their powder forms.
Table 2: Fluorescence analysis of Crataeva adansonii leaf and stem bark.
|
S. No |
Part |
Solvent |
Daylight |
UV-255 |
UV-366 |
|
1 |
Leaf |
Powder |
Olive green |
Brown |
Blackish |
|
Ethanol |
Green |
Medium brown |
Brownish black |
||
|
Methanol |
Mustard green |
Brown |
Bluish |
||
|
Acetic acid |
Olive |
Green |
Purplish green |
||
|
N-hexane |
Medium olive |
Light green |
Purplish green |
||
|
HCl |
Olive |
Olive green |
Purplish brown |
||
|
Nitric acid |
Brown |
Mustard |
Reddish-brown |
||
|
Carrageenan |
Green |
Dark brown |
Blackish |
||
|
Glacial acetic acid |
live |
Green |
Chocolate brown |
||
|
Diethyl ether |
Olive |
Purple Green |
Dark purple green |
||
|
Picric acid |
Pale olive |
Yellowish green |
Dark brown |
||
|
2 |
Bark |
Powder |
Yellowish-brown |
Brown |
Dark brown |
|
Ethanol |
Pale brown |
Brown |
Brown |
||
|
Methanol |
Yellowish-brown |
Brown |
Dark brown |
||
|
Acetic acid |
Light pale brown |
Mustard |
Purplish brown |
||
|
N-hexane |
Light pale brown |
Olive green |
Light brown |
||
|
HCl |
Light brown |
Light brown |
Light purplish brown |
||
|
Nitric acid |
Reddish-brown |
Yellowish green |
Dark reddish-brown |
||
|
Carrageenan |
Light brown |
Brown |
Dark brown |
||
|
Glacial acetic acid |
Light brown |
Yellowish green |
Brown |
||
|
Diethyl ether |
Brown |
Brown |
Dark brown |
||
|
Picric acid |
Yellow |
Pale |
Light brown |
Cytotoxic study
The cytotoxic activity of C. adansonii crude extract was compared with standard drug etoposide (standard cytotoxic drug). At higher concentration (100-1000 µg/ml) the leaf and bark ethanolic extracts showed cytotoxicity and at lower concentration (10 µg/ml) very low cytotoxic effect was observed (Figure 4). The LC50 values for crude leaf extract were found to be 5.34 and for that of stem bark extract, it was 7.44 ug/ml. Stem bark extracts of Albizia lebbeck were evaluated for cytotoxic activities of the synthesized nanoparticles on human breast cancer cell lines, the extract was found to be effective (Umar et al., 2019). The methanolic stem and leaf extracts of Cynometra ramiflora were tested on brine shrimp nauplii, as a result strong lethality was demonstrated in preliminary cytotoxicity assay (Afrin et al., 2019). During a similar cytotoxic activity, the crude methanolic extract of Bougainvillea glabra leaves was screened in a lethality bioassay by using brine shrimp nauplii (Artemia salina) and it was revealed that even at low doses the extracts were toxic (Dokuparthi et al., 2018). Cytotoxicity screening data offer significant primary data to help choose plant extracts with possible antineoplastic attributes for future work (Itharat et al., 2004). The cytotoxicity results obtained from the study on leaf and bark of C. adansonii may offer potential avenues for the development of cytotoxic drugs.
Conclusions and Recommendations
From this study, it is concluded that C. adansonii leaf and bark ethanolic extracts show significant cytotoxicity in a dose-dependent manner. Evaluation of standardizing parameters for drug preparation was an important part of this study. Extractive values and fluorescence study is supportive in the detection of adulteration and drug quality control. The extractive value of the formulation helped find the most effective solvent for extraction and to determine the characteristics of its chemical constituents. It is recommended that further studies may be carried out to check the pharmacological potential of the drug.
Novelty Statement
This research work discovers the cytotoxic potential of ethanolic crude extract of C. adansonii and highlights that the drug can be helpful in the preparation of novel pharmaceuticals. Moreover, the parameters for authentication and standardization of drugs are laid down through finding extractive values and fluorescence study of the drug.
Author’s Contribution
This paper is a part of the major author’s contribution to the requirement of Ph.D. Syeda Farzana conducted the experimental work and Prof. Dr. Siraj-ud-Din guided her as a research supervisor during the entire research process, data compilation and analysis.
Conflict of interest
The authors have declared no conflict of interest.
References
Adjagba, M., B. Awede, R. Osseni, C. Hountondji, G. Dougnon, I. Lagnika, R. Darboux and A. Laleye. 2017. Antihypertensive effect of extracts from Crateva adansonii DC. ssp. adansonii in the Wistar rats. Int. J. Biol. Chem. Sci., 11(6): 2604-2615. https://doi.org/10.4314/ijbcs.v11i6.5
Afrin, S., R. Pervin, F. Sabrin, S. R. Rony, M. Sohrab, M. Islam and M. Billah. 2019. In vitro antioxidant activity, antimicrobial and preliminary cytotoxic activity of Cynometra ramiflora-a mangrove plant. Journal of Microbiology, Biotechnol. Food Sci., 6(2): 844-850. https://doi.org/10.15414/jmbfs.2016.6.2.844-850
Ali, M.S., A. Dey, A.A. Rahman, M.R. Kuddus and M.A. Rashid. 2014. In vivo sedative and cytotoxic activities of methanol extract of leaves of Crataeva nurvala Buch-Ham. Pak. J. Biol. Sci., 17(3): 439-442. https://doi.org/10.3923/pjbs.2014.439.442
Amara, A., M. El-Masry and H. Bogdady. 2008. Plant crude extracts could be the solution: extracts showing in vivo antitumorigenic activity. Pak. J. Pharm. Sci., 21(2): 159-171.
Ansari, M., J. Ahmad and S. Ansari. 2006. Pharmacognostic evaluation of the stem bark of Balanitesa egyptiaca Delile” Hingot. Hamdard Med., 50(1): 82-94.
Atta-ur-Rhman Choudhary, M. and W. Thomsen. 2001. Bioassay technique for drug development Harwood academic publishers. https://doi.org/10.3109/9780203304532
Azhagumadhavan, S., S. Senthilkumar, M. Padma, P. Sasikala, T. Jayaseelan and S. Ganesan. 2019. A study on establishment of phytochemical analysis of quality parameters and fluorescence analysis of Costus spicatus-rhizome extract medicinal plants a well known tropical folklore medicine. J. Drug Deliv. Ther., 9(1): 240-243. https://doi.org/10.22270/jddt.v9i1-s.2329
Brain, K.R. and T.D. Turner. 1975. The practical evaluation of phytopharmaceuticals (Vol. 1): Wright-Scientechnica Bristol.
Carballo, J.L., Z.L. Hernandez-Inda, P. Perez and M.D. Garcia-Gravalos. 2002. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol., 2(1): 17. https://doi.org/10.1186/1472-6750-2-17
Chan, C.K., G. Chan, K. Awang and H. Abdul-Kadir. 2016. Deoxyelephantopin from elephantopus scaber inhibits HCT116 human colorectal carcinoma cell growth through apoptosis and cell cycle arrest. Molecules, 21(3): 385. https://doi.org/10.3390/molecules21030385
Chase Jr, C.R. and R. Pratt. 1949. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. J. Am. Pharm. Assoc., 38(6): 324-331. https://doi.org/10.1002/jps.3030380612
Da Silva Ferreira, R., D. Zhou, J.G. Ferreira, M.C.C. Silva, R.A. Silva-Lucca, R. Mentele and A. Gustchina. 2013. Crystal structure of Crataeva tapia bark protein (CrataBL) and its effect in human prostate cancer cell lines. PLoS One, 8(6): e64426. https://doi.org/10.1371/journal.pone.0064426
Dokuparthi, S.K., G. Lakshmi, A. Anjana, S.F. Fatima, P. Ashwini, S. Kandagatla and S. Raj. 2018. Brine shrimp lethality bioassay of Bougainvillea glabra. J. Drug Deliv. Ther., 8(4): 244-246. https://doi.org/10.22270/jddt.v8i4.1780
Evans, W., 2002. Pharmacognosy. English Language Book: Society Baillere Tindall, Oxford University Press.
Gavit, H.B. and N.M. Patel. 2019. Pharmacognostical, phytochemical and physicochemical evaluation of Amaranthus blitum L. leaves from south Gujarat. J. Pharm. Phytochem., 8(3): 2148-2155.
Gitte, T., M. Kare and A. Deshmukh. 2012. Ethno-medicinal studies on barks of some medicinal plants in Marathwada (MS), India. Recent Res. Sci. Technol., 4(10): 8-10.
Hade, S.N., P.A. Joshi, H.H. Pilley, V.P. Wadegaonkar and P.A. Wadegaonkar. 2016. Evaluation of Crataeva nurvala extracts as antioxidant, antiproteolytic and cytotoxic against hepato-carcinoma and mouse melanoma cell lines. J. Appl. Pharm. Sci., 6(09): 189-196. https://doi.org/10.7324/JAPS.2016.60928
Hoang, V., 2013. Capparidaceae- Crataeva adansonii. [Photograph]. Retrieved from https://www.flickr.com/photos/vanlaphoang1945/9800491433/
Ibrar, M. and N. Muhammad. 2011. Evaluation of Zanthoxylum armatum DC for in-vitro and in-vivo pharmacological screening. Afr. J. Pharm. Pharmacol., 5(14): 1718-1723. https://doi.org/10.5897/AJPP11.405
Iqbal, M., 2016. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere, 144: 785-802. https://doi.org/10.1016/j.chemosphere.2015.09.048
Itharat, A., P.J. Houghton, E. Eno-Amooquaye, P. Burke, J.H. Sampson and A. Raman. 2004. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharmacol., 90(1): 33-38. https://doi.org/10.1016/j.jep.2003.09.014
Jarald, E.E. and S.E. Jarald. 2007. Textbook of pharmacognosy and phytochemistry. CBS Publisher and distributers, New Delhi, India. pp. 6.
Jeevan, H., 2016. Barna- Crataeva adansonii. [Photograph]. Retrieved from https://www.flickr.com/photos/harajeevan/24190511335/in/photolist-
Khan, H., M. Saeed, M.A. Khan, A. Dar and I. Khan. 2010. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J. Ethnopharmacol., 127(2): 521-527. https://doi.org/10.1016/j.jep.2009.10.003
Kokoshi, C.J., R.J. Kokoshi, P.J. Sharma. 1958. Fluorescene of powdered vegetable drugs under UV radiation. J. Am. Pharm. Assoc., 47: 715-717. https://doi.org/10.1002/jps.3030471010
Kokate, C., 1994. Practical pharmacognosy 3rd ed Vallabh Prakashan New Delhi.
Kumar, D.G., V. Parvathi, P. Meenakshi, M.A. Rathi and V.K. Gopalakrishnan. 2012. Anticancer activity of the ethanolic extract of Crateva nurvala bark against testosterone and MNU-induced prostate cancer in rats. Chinese J. Natl. Med., 10(5): 334-338. https://doi.org/10.1016/S1875-5364(12)60067-3
Lagadic, L. and T. Caquet. 1998. Invertebrates in testing of environmental chemicals: are they alternatives? Environ. Health Perspect., 106(2): 593-611. https://doi.org/10.1289/ehp.98106593
Madhavan, V., H. Basnett, M. Gurudeva and S. Yoganarasimhan. 2009. Pharmacognostical evaluation of Drosera burmannii Vahl (Droseraceae).
Miliauskas, G., P.R. Venskutonis and T.A. VanBeek. 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem., 85: 231-237. https://doi.org/10.1016/j.foodchem.2003.05.007
Newman, D.J. and G.M. Cragg. 2007. Natural products as sources of new drugs over the last 25 years. J. Natl. Prod., 70(3): 461-477. https://doi.org/10.1021/np068054v
Parvin, S., M.A. Kader, M.A. Rahman, M.I.I. Wahed and M.E. Haque. 2012. Antibacterial activities and brine shrimp lethality bioassay of the chloroform extract of stem bark of Crataeva nurvala Buch Ham. Int. J. Pharm. Sci. Res., 3(3): 830.
Piccardi, R., A. Frosini, M.R. Tredici and M.C. Margheri. 2000. Bioactivity in free-living and symbiotic cyanobacteria of the genus Nostoc. J. Appl. Phycol., 12(3): 543-547. https://doi.org/10.1023/A:1008106715148
Prakash, J. and S. Vedanayaki. 2019. Organoleptic, fluorescence, qualitative and quantitative analysis of bulb extract of Zephyranthes citrina. J. Pharm. Phytochem., 8(3): 2531-2536.
Ranjith, D., 2018. Fluorescence analysis and extractive values of herbal formulations used for wound healing activity in animals. J. Med. Plant Stud., 6(2): 189-92.
Reddy, M. and A. Chaturvedi. 2010. Pharmacognostical studies of Hymenodictyon orixence (Roxb.) Mabb. Leaf. Int. J. Ayurveda Res., 1(2): 103. https://doi.org/10.4103/0974-7788.64400
Ryan, K.J. and C.G. Ray. 2004. Medical microbiology. McGraw Hill. 4: 370.
Sharififar, F., M.H. Moshafi, G. Dehghan-Nudehe, A. Ameri, F. Alishahi and A. Pourhemati. 2009. Bioassay screening of the essential oil and various extracts from 4 spices medicinal plants. Pak. J. Pharm. Sci., 22(3): 317-322.
Sharma, G.K. and M. Dhanawat. 2019. Effectual qualitative chemical evaluation of Euphorbia neriifolia Linn. by using fluorescence analysis. J. Drug Deliv. Ther., 9(1): 44-47. https://doi.org/10.22270/jddt.v9i1-s.2248
Sinha, S., P. Mishra, H. Amin, B. Rah, D. Nayak, A. Goswami and S. Ghosal. 2013. A new cytotoxic quinolone alkaloid and a pentacyclic steroidal glycoside from the stem bark of Crataeva nurvala: Study of anti-proliferative and apoptosis inducing property. Eur. J. Med. Chem., 60: 490-496. https://doi.org/10.1016/j.ejmech.2012.12.017
Solis, P.N., C.W. Wright, M.M. Anderson, M.P. Gupta and J.D. Phillipson. 1993. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med., 59(03): 250-252. https://doi.org/10.1055/s-2006-959661
Sumithra, N. and S.R. Kumar. 2016. Phytochemical, physicochemical and fluorescence analysis of leaf extract of Syzygium calophyllifolium walp. Asian J. Pharm. Clin. Res., 9(1): 275- 278.
Suriyavathana, M. and M. Punithavathi. 2018. Phytochemical analysis and free radical scavenging potential activity of Vetiveria zizanioides Linn. J. Pharm. Phytochem., 7(2): 1955-1960.
Tatiya, A., S. Surana, S. Bhavsar, D. Patil and Y. Patil. 2012. Pharmacognostic and preliminary phytochemical investigation of Eulophia herbacea Lindl. Tubers (Orchidaceae). Asian Pac. J. Trop. Dis., 2: 50-55. https://doi.org/10.1016/S2222-1808(12)60123-6
Umar, H., D. Kavaz and N. Rizaner. 2019. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed., 14: 87. https://doi.org/10.2147/IJN.S186888
Yadav, N., N. Vasudeva, S. Sharma and S. Singh. 2007. Pharmacognostical and phytochemical studies on Chenopodium album Linn. root. Hamdard Medic., 50(1): 95-102.
Zingue, S., J. Cisilotto, A.B. Tueche, A. Bishayee, F.A. Mefegue, L.P. Sandjo and C.F. Awounfack. 2016. Crateva adansonii DC., an African ethnomedicinal plant, exerts cytotoxicity in vitro and prevents experimental mammary tumorigenesis in vivo. J. Ethnopharmacol., 190: 183-199. https://doi.org/10.1016/j.jep.2016.06.004
To share on other social networks, click on any share button. What are these?








