Evaluation of Potato Genotypes for Yield, Baked and Organoleptic Quality
Evaluation of Potato Genotypes for Yield, Baked and Organoleptic Quality
Arifa Khan1*, Shazia Erum2, Naveeda Riaz1, Abdul Ghafoor2 and Farhat Ali Khan3
1International Islamic University, Islamabad, Pakistan; 2Bio Resources Conservation Institute, National Agriculture Research Center, Islamabad, Pakistan; 3Department of Botany Hazara University Mansehra, Pakistan.
Abstract | An experiment comprising of 11 exotic genotypes were evaluated for yield and baking quality traits at National Agriculture Research Center Islamabad, Pakistan during autumn 2016-2017. The experiment was carried out in randomized complete block design with three replications. Data were recorded on sprouting, plant height, number of tubers per plant, average tuber weight per plant, tuber shape, skin, flesh color, number of eyes per tuber, sensory evaluation and proximate analysis. Results showed significant differences in all yields and phenotypic quality traits. High sprouting (100%) and plant height was observed in CIP8 (31.60 cm). CIP12 produced more number of tubers/plant (23.4 tubers) with low tuber weight while better average tuber weight was observed in CIP22 followed by CIP13 genotypes (95%) while CIP7 showed minimum sprouting (70%).CIP28 genotypeproduced maximum plant height (70.00 cm) while minimum CIP28 and CIP34 genotypes (494.5 and 488.3 g respectively). Genotype CIP7 had more eyes (7.0 eyes per tuber) while CIP19 and CIP28 produced less eyes (4.0 to 4.4 eyes per tuber). Six genotypes produced round shaped tuber potatoes whereas CIP13 and CIP31 produced oval shaped tubers. Most of the genotypes (CIP3, CIP8, CIP12, CIP17, CIP19, CIP22 and CIP31) produced yellow to light yellow tubers skin color. CIP28 showedbetter color, flavor and taste acceptance point. In overall acceptability of baked potatoes, CIP28 and CIP17 genotypes recorded the best results. In short, genotypes CIP17 and CIP28 recorded suitable plant parameters, baked quality traits and overall acceptability as well. These genotypes are recommended for baking industry of Pakistan.
Received | April 15, 2019; Accepted | September 14, 2019; Published | November 25, 2019
*Correspondence | Arifa Khan, International Islamic University, Islamabad, Pakistan; Email: arifa.phdbt55@iiu.edu.pk
Citation | Khan, A., S. Erum, N. Riaz, A. Ghafoor and F.A. Khan. 2019. Evaluation of potato genotypes for yield, baked and organoleptic quality. Sarhad Journal of Agriculture, 35(4): 1215-1223.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.4.1215.1223
Keywords | Baking, Genotypes, Potato, Quality, Yield
Introduction
Potatoes are an important non-cereal largest staple food crop after rice, wheat and maize worldwide and more than 100 million people of the worldwide consume potatoes daily (IPC, 2016). Potatoes are grown in an estimated 125 countries throughout the world including China, India, Java, Ukraine, Pakistan etc. (IPC, 2016; Zhang et al., 2017). Potatoes provide different types of nutrients and vitamins (Kärenlampi and White, 2009). They are source of different minerals like iodine (I), copper (Cu), iron (Fe), potassium (K), manganese (Mn), phosphorous (P), zinc (Zn), magnesium (Mg) and calcium, (Ca) (USDA, 2014).
Based on USD Anutrient profile data, baked potatoesare good source of vitamin B6, vitamin C, folate and niacin (USDA, 2014; USDA, 2015). However, the quantity of vitamin Cand other nutrients may differ greatly depending on cultivars, soil condition, environment and storage conditions (Singh et al., 2009). Various investigations showed that there is more retention of fat soluble vitaminsin potatoes during heat-processing. Augustin et al. (1978) recorded more retention of vitamin C, riboflavin, thiamin, folic acid, niacin and vitamin B6 in whole baked, boiled and baked in microwave.
Currently, there is an increase awareness of consumers regarding nutrition and health and they demand better food with high quality in short time. But the major challenge is to reduce as much as possible acrylamide levels in fried potatoes and maintaining intact their sensorial properties and low oil content. Similarly, better quality intern of organoleptic properties like taste, color, flavors are also important parameters among researchers (Akilen et al., 2016).
Among several methods of potatoes, baking is known as a reliable and alternative to frying because it has good potential like other potatoes product. Baked potato products like chips are being sold by different manufacturers.If an individual requires quick meal that has little to no maintenance, baked potatoes are good option. They are low-priced, delicious, and a wellsubstitute to hamburgers and French fries (Blessington et al., 2010).
Keeping this in view, the present study was carried out where 11 exotic genotypes were grown to evaluate their yield performance and better baking quality.
Materials and Methods
The experiment was carried out at Plant Genetic Resource Institute (PGRI), National Agriculture Research Centre (NARC) Islamabad, Pakistan during November 4, 2016 to July 2017. Eleven exotic genotypes imported from International Potato Centre, Peru were sown in a RCBD (Randomized Complete Block Design) with three replications where plant×plant and row×row distance was kept at 25 and 65cm, respectively. The recommended dose of fertilizers i.e. nitrogen 250 kg, phosphorus 125 kg and potassium 125 kg ha-1was applied. All the phosphorus, potassium and half dose of nitrogen were applied at the time of sowing while remaining was used at 1st and 2nd earthing up. Crop was visited regularly during growing season. Irrigation and plant protection measures were carried out when required.
Potato baking method
Medium size, healthy skinned, without sprouted, non-green potato tubers were selected, washed, dried and tagged potato tubers were wrapped in aluminum foil and put it in baking tray in baking oven at 400°C for almost 30 mints for perfect baking. Bearable hot potatoes were served to the panelist.
Observations
Following observations were recorded during experimental trials.
Sprouting percentage
It was calculated by following formula:
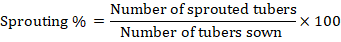
Plant height (cm)
For plant height, 5 plants of each genotype were selected randomly in each replication and their height was measured from base to top with the help of a meter rod and then averaged.
Number of tubers per plant
To count the number of tuber per plant, 5 plants from each genotype selected randomly in each replication were selected and their tubers were counted and then averaged.
Average tuber weight per plant (g)
For tuber weight, 5 plants of each genotype from each replication were selected randomly and their tubers weight was noted with the help of digital electrical balance and then averaged.
Shape of the tuber
For shape of tuber, tubers of 5 plants of each genotype from each replication were observed visually and shape of tuber recorded.
Tuber skin type
For tuber skin characteristics, tubers of 5 plants of each genotype from each replication were observed carefully and their skin type was noted.
Tuber flesh color
For tuber flesh color, tubers of 5 plants of each genotype from each replication were observed carefully and their flesh color was noted.
Number of eyes
For number of eyes, tubers of 5 plants of each genotype from each replication were observed carefully and their eyes were noted and then averaged.
Sensory evaluation
Sensory evaluation for color, taste, flavor, texture and overall acceptability were checked.
For this, 10 expert of food technologist from department of Food Science Research Institute (FSRI), NARC, Islamabad assessed the baked potato samples. The food panelists were served with 100 g of each sample of baked potatoes and instructed to taste and evaluate the sample using an evaluation sheet. Sensory attributes assessed included color, taste, flavor, texture and overall acceptability. A 9-point Larmond scale with 1 representing ‘dislike extremely’ and 9 representing ‘like extremely’ was used for the evaluation. Panelists were also given the option to make general comments about the samples. Unsalted cracker and water were supplied to panelists for refreshing their palates before tasting subsequent samples.
Proximate analysis
Proximate analysis was carried out for moisture %, dry matter, specific gravity, ash %, total sugar, reducing sugar and non-reducing sugar by following standard procedures.
Statistical analysis
For yield and yield traits, data were analyzed statistically by applying a computer package program MSTAT-C (Freed and Eisensmith, 1989) and comparison was made using Duncan’s Multiple Range (DMR) test at 5% probability level. For quality analysis, data were analyzed usingChi square testat 5% probability level (O’Mahony,1982).
Results and Discussion
Plants emergence is a significant factor of any crop that affects stand establishment, population dynamics of crop and helps towards the final yield. Maximum sprouting were recorded ingenotype CIP22 (100%) followed by CIP13 (95%), howeverCIP3, CIP8, CIP17, CIP19, CIP22 and CIP30 genotypes were also statistically similar to CIP22Figure 1. While genotype CIP7 showed minimum sprouting (70%), which was statistically similar to CIP12, CIP28 and CIP34 genotypes (80%) (Figure 1). In case of plant height, CIP28 genotypeproduced maximum plant height (70.00 cm) followed by CIP17 genotype (Figure 2). Minimum plant height was observed in CIP8 (31.60 cm) followed by CIP13 and CIP19 genotypes. The results regarding number of tubers/plant revealed that followed by CIP7 (18.0 tubers) (Figure 3). Genotypes CIP3 and CIP17produced minimum number of tubers/plant (8.0 and 8.1 tubers, respectively).
In potatoes, tubers weight has an important role in yield. It is revealed that maximum average tuber weight was obtained from CIP28 and CIP34genotypes (494.5 and 488.3 g respectively) followed by CIP7 and CIP17 (Figure 4). While minimum tubers weight was recorded in CIP12 (39.8 g) followed by CIP3. Results regarding the number of eyes per tuber showed that more number of eyes per tuber was recorded in CIP7 genotype (7.0 eyes per tuber) followed by CIP3, CIP13, CIP17 and CIP22 genotypes (Figure 5). While CIP19 and CIP28 produced minimum number of eyes (4.0 to 4.4 eyes per tuber). Results showed that round shaped tuber potatoes were recorded in CIP3, CIP7, CIP17, CIP22, CIP28 and CIP34 (Table 1) Oval shaped tuber potatoes were observed in CIP13 and CIP31. While, CIP8 genotype produced oblongshaped tubers. Most of the genotypes (CIP3, CIP8, CIP12, CIP17, CIP19, CIP22 and CIP31) produced yellow to light yellow tubers skin color (Table 1). CIP7 had red skin color while CIP13 brown color. CIP28 and CIP34 produced tubers of cream color skin. The results of study showed that white flesh color tubers were recorded in CIP3, CIP7 and CIP34 genotype. Yellow to light yellow flesh color tubers were noted in CIP8, CIP12, CIP13, CIP17 and CIP31. While cream flesh colored potato tubers were produced by CIP19, CIP22 and CIP28 (Table 1).
Sensory evaluation is some essential criteria for quality judgment in product development and to congregate the consumer requirements. Among 11 genotypes, CIP28 obtained maximum acceptance point (7.1 out of 8) followed by CIP7, CIP13and CIP22 genotypes (Table 2). Minimum acceptance points were recorded for CIP19, CIP8 and CIP31 genotypes. In baked potato flavor, CIP28 produced the best flavor followed by CIP13, CIP17, CIP19, CIP22, CIP31 and CIP34 genotypes (Table 1). Less umber of acceptance points in flavor was noted in CIP3, CIP7, CIP8 and CIP12 genotypes (Table 2).
Table 1: Tuber color, flesh color and tuber shape.
| Genotypes | Tuber color | Tuber flesh color | Tuber shape |
| CIP 3 | light yellow | white | round |
| CIP7 | red | white | round |
| CIP8 | light yellow | light yellow | oblong |
| CIP12 | light yellow | light yellow | rein form |
| CIP13 | brown | yellow | oval |
| CIP17 | light yellow | yellow | round |
| CIP19 | yellow | cream | round oval |
| CIP22 | yellow | cream | round |
| CIP28 | cream | cream | round |
| CIP31 | cream | white | round |
| CIP34 | light yellow | light yellow | oval |
Table 2: Color, taste, flavor, texture and overall acceptability of potatoes genotypes.
| Genotypes | Color | Taste | Flavor | Texture | Overall Acceptability |
| CIP 3 | 6.5 | 6.0 | 5.6 | 5.7 | 6.0 |
| CIP7 | 6.8 | 6.1 | 5.9 | 5.5 | 5.8 |
| CIP8 | 5.7 | 5.9 | 5.9 | 5.6 | 5.7 |
| CIP12 | 6.6 | 5.8 | 5.8 | 5.9 | 6.1 |
| CIP13 | 6.9 | 6.5 | 6.5 | 6.1 | 6.4 |
| CIP17 | 7.4 | 6.5 | 6.6 | 6.8 | 6.5 |
| CIP19 | 5.6 | 5.9 | 6.1 | 6.1 | 6.0 |
| CIP22 | 6.8 | 6.5 | 6.3 | 6.0 | 6.4 |
| CIP28 | 7.1 | 6.8 | 7.1 | 6.8 | 6.9 |
| CIP31 | 5.8 | 6.3 | 6.4 | 6.4 | 5.9 |
| CIP34 | 6.3 | 6.1 | 6.4 | 6.1 | 6.1 |
In case of baked potatoes taste, good taste was observed in CIP28 followed by CIP13and CIP17 genotypes (Table 2). Poor taste was observed in CIP3, CIP12 and CIP7genotypes. While in case of baked potatoes texture, CIP17and CIP28 genotypes showed good texture followed by CIP31 genotype (Table 2). CIP7 and CIP8 genotypes recorded inferior texture followed by CIP3 genotype. Data regardingproximate analysis of potato genotypes showed that CIP34 genotype had lowest moisture contents compared to all other genotypes. Maximum moisture percentage was recorded in CIP12 (Table 3). Alike, lowest dry matter content was recorded in CIP19 while more dry matter was observed in CIP17 and CIP28 and same trend was observed in case of ash content. In potatoes quality parameters, the specific gravity is very important because it shows the dry matter or solid contents of potatoes. More specific gravity was recorded in CIP17 and CIP28.
Table 3: Proximate analysis of potato genotypes.
| Genotype | Moisture % | Dry mater (%) | Specific gravity | Ash % | Total sugar (%) | Reducing sugar (%) | Non reducing sugar (%) |
| CIP 3 | 77.00 | 18.80 | 1.09 | 1.22 | 1.77 | 0.94 | 0.95 |
| CIP 7 | 78.21 | 81.59 | 1.09 | 0.93 | 3.09 | 1.18 | 2.81 |
| CIP 8 | 81.22 | 18.78 | 1.07 | 0.88 | 3.08 | 1.58 | 2.17 |
| CIP 12 | 87.11 | 12.76 | 1.05 | 0.78 | 3.03 | 1.14 | 2.45 |
| CIP 13 | 81.56 | 18.33 | 1.06 | 0.88 | 1.82 | 0.95 | 0.88 |
| CIP 17 | 82.54 | 20.44 | 1.35 | 1.02 | 2.98 | 0.90 | 1.6 |
| CIP 19 | 82.56 | 17.33 | 1.09 | 0.74 | 2.22 | 1.68 | 0.62 |
| CIP 22 | 81.15 | 18.74 | 1.06 | 0.98 | 3.06 | 2.28 | 1.05 |
| CIP 28 | 79.07 | 20.91 | 1.38 | 1.03 | 2.98 | 0.93 | 1.7 |
| CIP 31 | 81.66 | 18.34 | 1.07 | 0.89 | 1.66 | 0.48 | 1.08 |
| CIP 34 | 70.06 | 18.85 | 1.13 | 1.06 | 1.45 | 0.85 | 0.6 |
Total sugar percentage was more in CIP7 and CIP8 followed by CIP12 and CIP22 (Table 3). While minimum total sugar was observed in CIP31. The appropriate level of reducing sugar level was noted in CIP17 and CIP28 while lowest was recorded in CIP34 genotypes. While in case of non-reducing sugar, CIP12 showed highest percentage of non-reducing sugar while lowest was observed in CIP19 genotype. In overall acceptability of baked potatoes, CIP28 and CIP17 genotypes recorded the best results followed by CIP22, CIP12, CIP13and CIP34 genotypes (Table 2). Genotypes CIP7, CIP8 and CIP31 showed lower overall acceptability.
The improvement in sprouting shows better vigor of potato tubers. Bugarcic et al. (1997) and Abbasi et al. (2004) reported diverse sprouting among various varieties of potatoes. In potato tubers, the process of sprouting is influenced by exterior (temperature and moisture) as well as interior factors (physiological maturity and dormancy) present within the seed tuber (Abbasi et al., 2004). As external factors were alike but the variations recorded here in sprouting, might be due to internal factors. This dissimilarity in emergence can be ascribed to the tubers dormancy controlling factors that might have played a key role in expressing the least values of emergence (Abbasi et al., 2004). Eaton et al. (2017) who reported difference in plant height of different potatoes genotypes and might be due to plant genetic makeup and environmental effects. Similar results were also reported by Luthra et al. (2005), Schittenhelm et al. (2006). It is presumed that the differences in plant height among various genotypes may be due to combined effects of plant genetics, nutrient status of soil and agro-environmental conditions, under which the plants were grown. It seems that CIP28 and CIP17such genetic character that enabled them to took energetic start and absorbed more food resources in form of mineral nutrients, moisture and solar radiations during early and entire growing season and resulted more height (Figure 2).
The difference in number of tubers per plant depends on genetic makeup of plant, canopy development and environmental conditions. Our results are in line with (Subarta and Upadhya, 1997) and Eaton et al. (2017) who described that number of tubers depends on plantgeneticmakeup and environmental conditions. Stolen and tuberilisation processes is affected by genetic makeup and environmental factor (Subarta and Upadhya, 1997). Tubers weight might be because their genetic inheritability. Kumar et al. (2004) reported that different cultivars had various traits and differed greatly from each other (Kumar et al., 2004). Furthermore, large size/weight tubers may be due to fast emergence of plantsand improved growth of plants (Patel et al., 2008). In tuber potatoes, a set of external quality traits like tuber shape, eye depth, skin and flesh color are crucial aspects for consumers, as they are immediatelyobvious while making the purchase and may also impact processing quality (Werij, 2011).
Tuber shape is control by large number of characters, which consider the ration between length and width; it differs round to oblong, oval etc. (van Eck, 2007; Werij, 2011). The most easily visible traits of potato tubers are the tuber skin and flesh color. Similarly, the skin color of potatoes is affected by or controlled by numbers of genetic factors that (van Eck et al., 1994). The flesh color of potato also plays an important role in its evaluation. Likewise, color of potato flesh differs significantly but normally purple to white. It is because of two different pigments (carotenoids and anthocyanins) that accumulate and give color to potato (Lewis et al., 1998). If potato has blue, red or purple color, it has more concentration of anthocyanin (Hung et al., 1997). Acylated glucosides gives purple to radish color in potatoes (Brown, 2005; Lachman et al., 2009). Sensory assessment is an indispensablecriterion for superiorityof any product to congregate the customer requirement. Any product must offer satisfaction and pleasure to the clients if it has to be a part of their eating behavior.
Color is one of the most important physical parameters to select the food, which influences consumer perception and can be the reason for the rejection of particular product. Pritchard and Adam (1994) and Rodriguez et al. (1997) recorded somewhat relationship between non-reducing sugars and potato tubers fries color. Percentage of sugar present in potatoes significantly affects the color of potato fries (Coffin et al., 1989; Cipar et al., 1990). Potato tubers showed dark fries at high reducing sugar levels which is disliked by the consumers. Alike, Pandey et al. (2004) a stated that the potatoes color was affected by reducing sugar content and dry matter of potato tubers. The difference in color of genotypes in this study might be due to genetic characters of genotypes. Kumar et al. (2004) said that the quality of potato tubers is also affected by the level of sugars, because at high temperature Maillard reaction occur which leads to interaction between reducing sugars (glucose and fructose) and free amino acid which ultimately affect the color, flavor and it has also been related to acrylamide formation in fried potato products. Amount of sugar in potato tubers depends upon several factors which include genotype, environmental conditions, agronomical practices and several post-harvest factors including storage (Kumar et al., 2004). The difference among genotypes for flavor is might be due to starch content in tubers. Potato flavor is controlled by starch present in potatoes; and potatoes varieties having more starch showed good flavor (Zaehringer et al. 1967). But Sayre et al. (1975) argued that reducing sugar and dry matter also affect the processed products of potatoes. Genetic characters of genotypes also affect the flavor of products of potatoes. Pardo et al. (2000), Jansky (2008) and Abong et al. (2009) stated that varietal sensory traits including flavor significantly varied among genotypes due to their genetic makeup.
Texture is also controlled by starch content of potatoes (Van Marle et al., 1997). It gives firmness to texture of processed products of potatoes. Poor texture of baked potatoes may also be affected by sugar content of potatoes as reported by Adams (2004) who described that potatoes having high sugar content showed poor/soft texture after cooking. But Pandey et al. (2004) and Marwaha (1998) described that texture of fried potatoes were also influenced by reducing sugar content and dry matter of tubers. Potatoes having high dry matter showed mealiness when processed (Mehdi, et al., 2008). The low overall acceptability might be due to total sugars in these genotypes. Kabira and Lemaga (2003) confirmed that more content of sugar in potato genotypes resulted in dark frying color and these were bitter in taste, which is unacceptable to consumer.
Conclusions and Recommendations
Genotypes CIP17 and CIP28 recorded suitable plant height, more tuber with average tuber weight, baking quality, yield, yield related traits and overall proximate quality as well. These genotypes can be cultivated and processed for baked potatoes.
Acknowledgements
Authors acknowledge the support from Potato Program and Bio-conservation Institute of National Agriculture Research Centre, Islamabad, Pakistan.
Novelty Statement
The study highlights determination of two new genotypes of potato for baking industry in Pakistan
Author’s Contribution
Arifa Khan, Principal author, conceived the idea, conducted the research and prepared the 1st draft. NaveedaRiaz, Planned and supervised the research and experiments. Shazia Erum, Co-supervised the research. Abdul Ghafoor, Helped in field and guided inwriting up. Farhat Ali Khan, Help in data recording.
References
Abong, G.O., M.W. Okoth, E.G. Karuri, J.N. Kabira and F.M. Mathocko. 2009. Influence of potato cultivar and stage of maturity on oil content of French fries (chips) made from eight Kenian potato cultivars. African J. Food Agric. Nutri. 9: 8-19.
Adams, J.B. 2004. Raw materials quality and the texture of processed vegetables. In: Texture in Foods, Solid Foods. (Ed.): D. Kilcast, Woodhead Publ. Ltd.: Cambridge, 2: 342- 363.
Ahmad, N., M.A. Khan, N.A. Khan, R. Binyaminand and M.A. Khan 2011. Identification of resistance source in potato germ plasm against PVX and PVY. Pak. J. Bot. 43: 2745-2749.
Akilen, R., N. Deljoomanesh, S. Hunschede, C.E. Smith, M.U. Arshad, R. Kubant, G.H. Anderson, B.J. Lamont, M.F. Waters and S. Andrikopoulos. 2016. The effects of potatoes and other carbohydrate side dishes consumed with meat on food intake, glycemia and satiety response in children. Nutr. Diabetes. 6: 34-42.
Amdie, A., A. Tekalig and B. Tekile. 2017. Adaptability study of improved Potato (Solanumtuberosum L.) varieties in highlands of Guji zone, Southern Oromia. Acad. Res. J. Agric. Sci. Res. 5: 186-191.
Augustin, J., G.K. Johnson, C. Teitzel, R.H. True, J.M. Hogan and R.M. Deutsch, 1978. Changes in nutrient composition of potatoes during home preparation. Am. Potato J. 55: 653-662.
Blessington T., M.N. Nzaramba, D.C. Scheuring, A.L. Hale, L. Reddivari and J.C. Miller. 2010. Cooking methods and storage treatments of potato: Effects on carotenoids, antioxidant activity, and phenolics. Am. J. Potato Res. 87: 479-491.
Brown, C. 2005. Antioxidants in potato. American J. Potato Res. 82: 163-172. https://doi.org/10.1007/BF02853654
Bugarcic, Z., Z. Vesiljeic, A. Dokic, S. Jevtic and B. Lazic. 1997. Phenotype values, variability and productivity properties in Dutch potato varieties under different agro-ecological conditions. Proc. First Balkan Symp. Veg. Potatoes, Belgrade, Yugoslavia. 4–7 June, 1996 Acta Hort. 2: 921–927. https://doi.org/10.17660/ActaHortic.1997.462.147
Cipar, M.S., D. Hunter, P. Porter and G. Henderson. 1990. Norwis: a new potato variety combining chipping quality, wide adaptation, disease resistance and high yield. Am. Potato J. 67: 371-379.
Coffin, R., R. Chase, N. Thompson, G.R. Johnston, A. McKeown, M.K. Keenan, D. Craven, R. Kitohen, J. Wilson and R.Y. Yada. 1989. Saginaw gold: A yellowfleshed potato cultivar with medium high specific gravity and excellent chip and french fry quality after storage. Am. Potato J. 66: 303-313.
Devaux, A., P. Kromann and O. Ortiz. 2014. Potatoes for sustainable global food security. Potato Res. 57: 185-189. https://doi.org/10.1007/s11540-014-9265-1
Eaton, T.E., A. Kalam, K. Humayun and A.B. Siddiq. 2017. Evaluation of six modern varieties of potatoes for yield, plant growth parameters and resistance to insects and diseases. Agric. Sci. 8: 1315-1326. https://doi.org/10.4236/as.2017.811095
GOP. 2016-17. Govt. of Pak. Pakistan bureau of statistics.
Hung, C.Y., J.R. Murray, S.M. Ohmann and C.B.S. Tong. 1997. Anthocyanin accumulation during potato tuber development. J. Am. Soc. Hortic. Sci. 122: 20-23. https://doi.org/10.21273/JASHS.122.1.20
International Potato Center. 2016. Potato facts and figures. http://cipotato.org/potato/facts/ Accessed July 21, 2016
Kabira, J.N. and B. Lemaga. 2003. Potato processing: Quality evaluation procedures for research and food industries applications in East and Central Africa. Kenya Agric. Res. Pub. Nairobi, Kenya. 10 p.
Khan, M.F., T. Najma, L. Anila, A. Khali and M. Mansoor. 2013. Morphological characterization of potato (Solanumtuberosum L.) germplasm under rain-fed environment. J. Nairobi Kenya. 22: 3214-3223.
Kumar, D., B. Singh and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Ann. App. Biol. 145, 247-256. https://doi.org/10.1111/j.1744-7348.2004.tb00380.x
Lachman, J., K. Hamouz, M. Sulc, M. Orsák, V. Pivec A. Hejtmánková and P. Dvorák. 2009. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 114: 836-843. https://doi.org/10.1016/j.foodchem.2008.10.029
Lewis, C.E., J.R.L. Walker, J.E. Lancaster, K.H. Sutton. 1998. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanumtuberosum L. J. Sci. Food Agric. 77: 45-57. https://doi.org/10.1002/(SICI)1097-0010(199805)77:1<45::AID-JSFA1>3.3.CO;2-J
Li, L., Strahwald, J., H.R. Hofferbert, J. Lubeck, E. Tacke, H. Junghans, J. Wunder and C. Gebhardt. 2005. DNA Variation at the Invertase Locus inv GE/GF Is Associated with tuber quality traits in populations of potato breeding clones. Genet. 170: 813-821. https://doi.org/10.1534/genetics.104.040006
Manrique, L.A. and D.P. Bartholomew. 1991. Growth and yield performance of potato grown at three elevations in Hawaii: II. Dry matter production and efficiency of partitioning. Crop Sci. 31: 367-372. https://doi.org/10.2135/cropsci1991.0011183X003100020029x
Masarirambi, M.T., F.C. Mandisodza, A.B. Mashingaidzeand E. Bhebhe. 2012. Influence of plant population and seed tuber size on growth and yield components of potato (Solanumtuberosum). Int. J. Agric. Biol. 14: 545–549.
Mehdi, M., T. Saleem, H.K. Rai, M.S. Mir and G. Rai. 2008. Effect of nitrogen and FYM interaction on yield and yield traits of potato genotypes under Ladakh condition. Potato J. 35: 126-129.
O’Mahony, M. 1982. Some assumptions and difficulties with common statistics for sensory analysis. Food Technol. 36: 71-82.
Pakistan Economic Survey. 2016-17. Economic advisor’s wing, finance division, GoP, Islamabad.
Pandey, S.K., S.V.D. Sing, P. Kumar and P. Manivel. 2004. Sustaining potato chipping industry from western and central Uttar Pardesh: Adoption of suitable varieties. Potato J. 31:119-127.
Pardo, J.E., A. alvarruz, J.I. Perez, R. Gomez and R. Varon. 2000. .Physical, chemical and sensory quality evaluation of potato varieties (Solanum tuborosum L.). J. Food Quality. 23: 149-160.
Patel, C.K., P.T. Patel and S.M. Chaudhari. 2008. Effect of physiological age and seed size on seed production of potato in North Gujarat, India. Potato J. 36: 18-23.
Pritchard, M.K. and L.R. Adam. 1994. Relationships between fry color and sugar concentration in stored Russet Burbank and Shepody potatoes. Am. Potato J. 71: 59-68.
Randhawa, K.S., B.S. Joyapa and K.S. Sandhu. 1980. A note on the performance of some new genotype (SolanumtuberosumL.) for qualitative and quantitative factors under Punjab condition. Haryana J. Hortic. Sci. 1: 84–87.
Rodriguez, L., E. Wrolstad, R.E. Pereira and C. Modeling. 1997. The contribution of sugars, ascorbic acid, chlorogenic acid and amino acids to non-enzymatic browning of potato chips. J. Food Sci. 62: 1001-1005.
Sayre, R.N., M. Nonaka and M.L. Weaver. 1975. French fry quality related to specific gravity and solids content, variation among potato strips within the same tuber. Am. Potato J. 52:73-82.
Schittenhelm, S., H. Sourell and F.J. Löpmeier. 2006. Drought resistance of potato cultivars with contrasting canopy architecture. Eur. J. Agron.24: 193-202. https://doi.org/10.1016/j.eja.2005.05.004
Subarta, M. and M.O. Upadhya. 1997. Potato production in western Bengal. Environ. Ecol. 15: 646–9.
USDA, 2014. United States Potato Board. 2014 https://www.usda.gov/nass/pubs/todayrpt/uscapo15
USDA, 2015. United States Potato Board. 2015. Potato nutrition handbook. 1-54 pp.
Van Eck, H.J. 2007. Genetics of morphological and tuber traits, in: D. Vreugdenhil, et al. (Eds.), Potato biology and biotechnology, Elsevier Sci B.V. Amsterdam. pp. 91-115. https://doi.org/10.1016/B978-044451018-1/50048-8
Van Marle, J.T., T.S. Smits, J. Donkers, C.V. Dijk, A.G.J. Voragen and K. Recourt. 1997. Chemical and microscopic characterization of potato (Solanum tuberosum L.) cell walls during cooking. J. Agric. Food Chem. 45: 50-58.
Werij, J. 2011. Genetic analyisis of potato tuber quality traits, Laboratory of plant breeding, Wageningen University, Wageningen. pp. 125.
Zaehringer, M.V., R.M. Reeves, E. Talley, D. Dinkel and R. Hyde. 1967. Specific gravity and composition of potatoes for various processing and cooking purposes. Potato Handbook, 1: 5. Academic Press, New York.
Zhang, H.F. Y. Xu, H. Wu, Hu and X.F. Dai. 2017. Progress of potato staple food research and industry development in China. J. Integ. Agric. 16: 2924-2932. https://doi.org/10.1016/S2095-3119(17)61736-2
To share on other social networks, click on any share button. What are these?








