Evaluation of Air-Borne Trace Metals in Orthopaedic Sections of Health Care Facilities
Evaluation of Air-Borne Trace Metals in Orthopaedic Sections of Health Care Facilities
Afzal Nimra1, Zulfiqar Ali1*, Safdar Sidra 2, Rida Ahmad1
1Environmental Health and Wildlife, Department of Zoology, University of the Punjab, 54600, Lahore, Pakistan
2Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore, Pakistan
Abstract | The airborne levels of carcinogenic trace metals lead (Pb), cadmium (Cd), arsenic (As) and nickel (Ni) were evaluated in the Orthopaedic Operation Theater (OOT), Orthopaedic Wards (OW) and Orthopaedic Emergency Rooms (OER) of six hospitals, in Lahore, Pakistan. Overall, the average levels of Cd, As and Ni (31, 20, and 37 ng/m3) were lower indoors as compared to outdoors (39, 21, and 51 ng/m3) except Pb. The high indoor levels of Pb (113 ng/m3) as compared to outdoors (85 ng/m3) suggested pronounced indoor sources. The average indoor-outdoor (I/O) ratio for the Pb, Cd, As and Ni were 1.34, 0.94, 0.99 and 0.79 respectively. The results showed that indoor air in the hospitals were affected by the common effects of indoor (wall paints, indoor equipment, environmental tobacco smoke) and outdoor (dust, soil and fuel combustion) sources. The hospitals were located on busy roads, where high vehicular emissions probably emit trace metal pollutants.
Novelty Statement | This study discusses the concentration and possible sources of airborne trace metals in sensitive areas of hospitals, which is to our knowledge, is the first study contributed from Pakistan and its understanding is important in order to improve law enforcement against causative factors.
Article History
Received: March 01, 2020
Revised: May 01, 2020
Accepted: May 11, 2020
Published: June 09, 2020
Authors’ Contributions
AN carried out the research work, and wrote the manuscript. ZA supervised the research work and improved the research design. SS helped with the statistical analysis. RA helped in field sampling and analysis.
Keywords
Trace metals, Hospital, Air quality, Indoor air, Pakistan, I/O ratio
Corresponding author: Dr. Zulfiqar Ali
zali.zool@pu.edu.pk
To cite this article: A. Nimra, Ali, Z., Safdar, S. and Ahmad, R., 2020. Evaluation of air-borne trace metals in orthopaedic sections of health care facilities. Punjab Univ. J. Zool., 35(1): 91-98. https://dx.doi.org/10.17582/journal.pujz/2020.35.1.91.98
Introduction
Poor air quality in health care facilities is one of the major detrimental factors that can compromise the health of the patients. It is hence essential to maintain a healthy pollution-free environment in hospitals with regular monitoring to exclude any potential contamination from multiple sources. While these sources may be located both indoors and outdoors, ventilation plays a major role apart from various routine activities within the hospitals. Among the major classes of pollutants identified in hospitals, particulate matter and bioaerosols are considered more harmful while gaseous pollutants are also present to some extent. Another major physical pollutant of concern is the presence of trace metals in the air released from different sources. The trace metals are the essential constituent of airborne total suspended particulate matter (TSPM) (Aziakpono et al., 2013). The TSPM consist of broad range of particles including super coarse (PM>10), coarse (PM2.5-10) and fine (PM2.5) fractions (Thomson et al., 2016). As a component of airborne particulate matter, the most concerning metals related to toxicity in humans include arsenic (As), antimony (Sb), chromium (Cr), cadmium (Cd), mercury (Hg), zinc (Zn), cobalt (Co), copper (Cr) and lead (Pb). These metals are mostly used as stabilizers in vinyl-based plastic materials, roof resilient flooring, and wall coverings. The trace elements such as cadmium (Cd), lead (Pb) and zinc (Zn) are mainly contributed by anthropogenic sources than natural sources (Shah and Shaheen, 2008; Okuda et al., 2008). The main anthropogenic sources include vehicular emissions (Cd, Pb, Cr, Ba), construction and industrial emissions (Mn, Al, Si) and combustion processes (Ni, Cr,) (Fang et al., 2010; Okuda et al., 2008; Susaya et al., 2010). Indoors, the abundance of trace metals are attributed to infiltration from outdoor sources (Habil et al., 2013) and various indoor sources such as paints, equipment, and tobacco smoke (Chattopadhyay et al., 2003; Paoletti et al., 2006; Taner et al., 2013). The trace metals mostly occur in the solid particle phase in the atmosphere and are ubiquitous in both coarse (PM2.5-10) and fine (PM2.5) fractions (Hu et al., 2012).
The trace metals associated to particulate matter such as Pb, Cd, Cr, Ni and As can be severely toxic and carcinogenic upon inhalation in higher quantities (Panne et al., 2001; Valavanidis et al., 2008; Pandey et al., 2013; Lawrence and Khan, 2020). According to International Agency for Research on Cancer (IARC) classification, arsenic (As), nickel (Ni) and cadmium (Cd) are carcinogenic to humans (IARC, 2012), whereas the inorganic lead (Pb) is classified as a probable carcinogen (IARC, 2006). Even in the minor levels, the existence of PM metals may significantly contribute to PM induced severe health implications (Dreher et al., 1997; Xia et al., 2007).
Hospitals are the buildings where these trace metals can be found throughout (IARC, 1972) and particle emission processes are the main drivers of many elements in the atmosphere. Although the airborne concentration of the trace metals in the urban environments have been reported in several countries such as Brazil (Sella et al., 2004), Denmark (Yang et al., 2002) and India (Khillare et al., 2004), limited studies are available on the elemental composition of PM in hospitals (Wang et al., 2006b; Brown et al., 2012; Loupa et al., 2016; Li et al., 2017; Slezakova et al., 2012, 2014). The current study aims to determine the airborne concentration of carcinogenic trace metals in the orthopaedic sections of hospitals of Lahore, Pakistan.
Materials and Methods
Sampling sites
Lahore is the capital of Punjab and the second largest city of Pakistan with respect to population i.e. 7,684,000 inhabitants (Shirazi and Kazim, 2014). According to the Punjab Specialized Health Care and Medical Education Department, there are 11 teaching, 2 district headquarters, 4 tehsil headquarters, 6 rural health centers and 37 basic health units working in the vicinity of district Lahore.
The health care sector in Pakistan can be divided into different categories depending on the governing bodies i.e. public hospitals run by the government, privately owned hospitals, and trust hospitals being run by different welfare trusts for the benefit of the general public. For the current study, six hospitals were selected, two from each category. Following a preliminary study in different operation theatres of two hospitals (Nimra et al., 2015), orthopaedic sections (orthopaedic operation theater, orthopaedic ward, and emergency room: where airborne injuries are treated initially) of each hospital were further selected owing to the reportedly higher levels of dust and infections in these areas. The selected hospitals were categorized and labeled as follows:
Category A= Government Hospitals (H1 and H2)
Category B= Trust Hospitals (H3 and H4)
Category C= Private Hospitals (H5 and H6)
From each hospital, the following sites were selected and labeled thus:
- Orthopaedic Operation Theatre (OOT)
- Orthopaedic Ward (OW)
- Orthopaedic Emergency Room (OER)
- Outdoor (OD)
Brief information on the characteristics of the hospitals is described in Table 1 composed with the help of hospital administrative staff and hospital’s official websites. All of the studied hospitals were located in urbanized areas of Lahore near main roads where the traffic influx was very high.
Air sampling
Air sampling was conducted to collect TSPM using a high-volume air sampler fitted with pre-weighed glass fiber microfilters (EPM 2000-Whatman England) at a rate of 35 l/min for 8 hours at each site. The sampling campaigns were organized at an interval of three months for a year to determine the airborne levels of Pb, Ni, Cd and As. After sampling, the filters were carefully removed and stored in sterilized Petri plates for further analysis.
Metal extraction
For the extraction of trace metals, each filter was heated at 50oC with 5ml HNO3 and 5ml of HCLO4 for 30 minutes and then heated in a digestion block Teckam PTC-2 under a fume hood at 170 ºC for 4 hours to solubilize the metals in ionic form. After digestion, the samples were cooled down and 5ml of deionized H2O was added to wash out a residual solution. The solution was filtered and the final solution was made up to 25ml with deionized water and was analyzed by ICP-AES (Arcous model, Germany) (Kermani et al., 2016). Filter blanks were also digested and analyzed in a similar way to test samples. The filter blanks were subtracted from the exposed filters to get the actual trace metal concentration in the TSPM.
Data analysis
The following formula was used to calculate metal concentration (US EPA, 2011).
Table 1: Brief profile of selected hospitals.
|
Type |
Category A |
Category B |
Category C |
|||
|
H 1 |
H 2 |
H 3 |
H 4 |
H5 |
H6 |
|
|
Teaching |
Teaching |
Teaching |
Teaching |
Non-teaching |
Non-teaching |
|
|
Building year |
1943 |
1958 |
1991 |
1974 |
1992 |
2000 |
|
Building age |
74 |
59 |
26 |
43 |
25 |
17 |
|
Patient visit /month |
60000 |
75000 |
45000 |
39000 |
9000 |
31830 |
|
Number of beds |
534 |
1096 |
580 |
350 |
40 |
260 |
|
Location |
Main road |
Main road |
Main road |
Main road |
Main road |
Main road |
|
Average surgeries/annum |
119000 |
159000 |
59,500 |
25,000 |
900 |
10,000 |
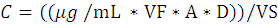
Where, C= Concentration in µg/m3; µg/mL= metal concentration in solution; VF=Total extraction solution volume; A= Area correction; D= Dilution factor if required; VS= Volume of air sampled.
The data analysis was done using IBM SPSS statistics 21. Kolmogorov- Simonov and Levene test confirmed data to be non-parametric, which was normalized for further analysis. Repeated measure One Way ANOVA with one factor was applied to compare indoor and outdoor value sets for each metal irrespective of sampling sites. A spearmen correlation test was applied to test the correlation between different trace metals. The average I/O ratio for each metal was calculated and graphically presented using Microsoft Excel (version, 2013). The I/O ratio > 1 indicates indoor sources of pollutants whereas, I/O ratio < 1 suggests the outdoor origin of indoor pollutants (Lomboy et al., 2015).
Results and Discussion
Trace metal concentration in the total suspended particulate matter (TSPM) collected at the different indoor and their respective outdoor (ambient) sites are represented in Table 2.
The average concentration of Pb, Cd, As, and Ni in indoor sites were 113 (± 32), 31 (± 15), 20 (± 10) and 37 (± 11) respectively. For outdoor environments, the recorded levels of Pb, Cd, As, and Ni were 85 (± 23), 39 (± 18), 21(± 08) and 51 (± 20) respectively (Table 2). The average levels of Pb were higher in indoor sites, while the Cd, As and Ni levels were comparatively high in outdoor environments.
Slezakova et al. (2012) have reported high levels of trace elements in hospital air while enrichment factor analysis revealed most of the carcinogenic metals (including Pb, Ni, As) to be anthropogenic rather from natural sources. Okuda et al. (2008) and Li et al. (2017) have also reported a majority of toxic metals to be anthropogenic. In the current study, the concentration of trace metals was found to be variable but still higher and observed to be in the following order for both indoor and ambient samples: Pb > Ni > Cd > As.
Table 2: Mean airborne trace metal composition of total suspended particulate matter sampled from indoor and outdoor sites of selected hospitals.
|
*N=216 |
Indoor sites (ng/m3) |
Outdoor sites (ng/m3) |
|||||||
|
Pb |
Cd |
As |
Ni |
Pb |
Cd |
As |
Ni |
||
|
Orthopaedic operation theatre (OOT) |
OOT1 |
149 |
17 |
19 |
39 |
77 |
62 |
18 |
82 |
|
OOT2 |
130 |
43 |
15 |
42 |
90 |
21 |
20 |
53 |
|
|
OOT3 |
106 |
24 |
55 |
24 |
91 |
57 |
19 |
32 |
|
|
OOT4 |
163 |
72 |
10 |
28 |
77 |
32 |
18 |
35 |
|
|
OOT5 |
151 |
38 |
11 |
31 |
85 |
39 |
20 |
32 |
|
|
OOT6 |
123 |
24 |
23 |
32 |
89 |
43 |
28 |
45 |
|
|
Orthopaedic wards (OW) |
OW1 |
162 |
52 |
13 |
51 |
149 |
69 |
18 |
94 |
|
OW2 |
90 |
27 |
12 |
12 |
84 |
19 |
17 |
45 |
|
|
OW3 |
99 |
25 |
17 |
32 |
96 |
59 |
17 |
41 |
|
|
OW4 |
82 |
24 |
17 |
39 |
77 |
20 |
13 |
53 |
|
|
OW5 |
85 |
40 |
27 |
32 |
72 |
45 |
25 |
70 |
|
|
OW6 |
75 |
26 |
23 |
37 |
54 |
21 |
21 |
48 |
|
|
Orthopaedic emergency rooms (OER) |
OER1 |
149 |
45 |
25 |
63 |
131 |
67 |
20 |
89 |
|
OER2 |
139 |
26 |
26 |
46 |
90 |
21 |
34 |
55 |
|
|
OER3 |
87 |
20 |
14 |
33 |
78 |
49 |
23 |
37 |
|
|
OER4 |
84 |
19 |
22 |
53 |
76 |
20 |
43 |
35 |
|
|
OER5 |
89 |
29 |
18 |
51 |
65 |
42 |
21 |
32 |
|
|
OER6 |
71 |
11 |
13 |
25 |
56 |
21 |
10 |
36 |
|
|
Mean |
113 |
31 |
20 |
37 |
85 |
39 |
21 |
51 |
|
|
Max |
163 |
72 |
55 |
63 |
149 |
69 |
43 |
94 |
|
|
Min |
71 |
11 |
10 |
12 |
54 |
19 |
10 |
32 |
|
|
Standard deviation |
32 |
15 |
10 |
11 |
23 |
18 |
08 |
20 |
|
*N, total number of samples from the hospitals during four sampling campaigns.
The Pb concentrations were specifically higher in the OOT’s as compared to the OW’s and OER’s (Figure 1a). Moreover, the levels were specifically higher in the hospitals indoors as compared to outdoors. Whereas, most of the studies have reported high levels of Pb outdoors (Wang et al., 2006a; Loupa et al., 2016). This was probably due to more pronounced sources of Pb in the current study e.g. tobacco smoke. The hospitals in the current study were not ETS free, as smoking indoors was not strictly controlled. Although according to the health ordinance 2002 of Pakistan smoking and other tobacco use in public areas specifically hospitals is prohibited (Tobacco Control Cell, Ministry of Health, GoP, 2010), but the implementation over the ban has been reported to be poor (Khan et al., 2016). A considerable number of doctors and paramedic staff in hospitals has been reported to be active smokers (Malik et al., 2010). Moreover, doctors have been observed to smoke during working hours, probably contributing to increase Pb levels (Piryani and Rizvi, 2004). The other indoor sources include timeworn lead-based paints which upon improper removal by sanding or dry scratching can cause harmful exposures, medical waste incinerators and dust infiltrating from outdoors (Yaffe et al., 1983). Indoor activities involving use of lead such as use of lead aprons and electrocautery in the operating rooms during surgeries can also be a potential source. The use of electrocautery has been reported to produce metal fumes during orthopaedic surgeries by Harkavy and Novak (2014) and Sah et al. (2017). Moreover, a recent study has reported the use of lead aprons during surgical procedures to be a major contributor towards indoor lead levels in hospitals with sixty-three percent on the tested aprons having quantifiable levels of lead dust on them which is consequently an occupational safety hazard (Burns et al., 2017). Outdoor Pb commonly originate from coal and diesel emissions, waste incineration and construction material (Janssen et al., 1997).
The monitored levels of Cd in ambient air (19- 69 ng/m3) were also higher and more than the reported levels in Iranian hospitals (5.97- 33.26 ng/m3) by Kermani et al. (2016). The higher concentration of Cd in outdoor environments (Figure 1b) is probably attributable to industrial and vehicular emissions, fertilizers, and combustion processes. The possible indoor sources of Cd within the hospital are medical waste incinerators. The bottom and fly ashes from hospital’s incinerators release heavy metal-laden solid particles along with inorganic and organic salts (Alvim-Ferraz and Afonso, 2003; Ibanez et al., 2000). Another source indoors is the ETS, which either in form of first or second hand smoke can be a potential threat to occupants specifically patients in hospitals (Willemsen et al., 2004). The people smoking in the corridors, outdoor entrances, cafeterias and parking lots of hospitals were observed signifying frequent policy breaching. Contrary, smoking is banned in European hospitals since 2000 with an effective compliance over the years. The enforcement of smoke free policies have been reported to decrease indoor air pollution (Connolly et al., 2009).
Error bars are the 95% confidence interval, the bottom and top of the box are the 25th and 75th percentiles, the line inside the box is the 50th percentile (median), and any outliers are shown as filled red circles
While Pb was found to be higher in concentration indoors, Ni levels were higher in ambient samples (Figure 1a, 1b). This in agreement with Wang et al. (2006b) who reported higher levels of Ni in the PM fractions outdoors (28-52 ng/m3) as compared to indoors (23-39 ng/m3) of hospitals. This is understandable, as Ni is majorly contributed to ambient air by combustion processes and sewage and waste incineration processes (Radulescu et al., 2015). However, the indoor sources in hospitals could be medical waste incinerators and ETS. As concentrations (10-55 ng/m3) however, remained almost the same in all samples. Still, these levels were significantly higher (0.12-3.65 ng/m3) than those reported by Kermani et al. (2016). However, another study related to the elemental composition of hospital air reported As levels between 49 -126 ng/m3 in PM10 and between 40-140 ng/m3 in PM2.5 size fractions (Slezakova et al., 2012). However, it is necessary to be careful while comparing the trace metals levels with other studies because of different sampling equipment, filter types and PM fraction. As is generally not an indoor pollutant but can be found in the indoor areas with tobacco smoking (Slezakova et al., 2009). Ambient levels of As are generally related to metal industries combined with the combustion of fossil fuels (Tanner et al., 2008) and since Lahore is a highly urbanized and industrialized metropolis, these sources cannot be neglected. Overall, the metal concentration indoors and outdoors dust were significantly different for Pb (p=0.000) and Ni (p=0.005) but not for the Cd (p=0.124) and As (p=0.608) respectively.
Indoor-outdoor ratio (I/O) is an important indicator to differentiate between the indoor and outdoor concentrations of pollutants. The mean I/O ratio of Pb (1.34) was indicative of indoor sources of Pb (Figure 2). Similarly, I/O ratio of 0.99 and 0.94 for As and Cd respectively indicate almost similar contributions from both indoor and outdoor sources. In addition, for Ni, a strong influence of ambient sources was identified with a mean I/O ratio of 0.79. According to Hassan (2012) and Habil et al. (2013), two major sources of toxic metals in indoor air is infiltration from ambient sources along with indoor sources including wall paint and different types of equipment (Paoletti et al., 2006; Kefeni and Okonkwo, 2013; Tanner et al., 2008). Moreover, as discussed earlier, the use of lead aprons in orthopaedic surgeries is also a contributing factor (Burns et al., 2017).
It is noteworthy that while Pb levels were comparatively elevated at all sites, they are still within the threshold limits defined by WHO and European Commission for 1-year as seen in Table 4; while levels of Cd, Ni and As were above the suggested limits of 5, 20 and 6 ng/m3 respectively at all sites. Such high levels reflect the importance of identifying the contributing agents in health care facilities and to replace or eliminate them for a safe environment for the patients and staff.
The airborne metals recovered from particulate matter PM indicated a positive correlation between Pb and Cd (r = 0.64) while correlation among other metals was statistically insignificant (Table 3). These results suggest that Pb and Cd probably originated from similar sources, while other metals probably had a different origin. Pb and Cd have been reported to be released in the environment simultaneously (Brender et al., 2006; Walker et al., 2007).
Table 3: Spearmen correlation coefficient matrix(r) between selected airborne trace metals.
|
Pb |
Cd |
As |
Ni |
|
|
Pb |
1 |
.646** |
-.131 |
.281 |
|
Cd |
.646** |
1 |
-.228 |
.160 |
|
As |
-.131 |
-.228 |
1 |
.009 |
|
Ni |
.281 |
.160 |
.009 |
1 |
Values in bold with ** Correlation is significant at the 0.01 level (2-tailed).
Table 4: Guidelines for the vinyl-based trace metals by WHO and European commission.
|
Trace metal |
Proposed limits |
Averaging period |
Source |
|
Lead (Pb) |
0.5 µg/m3 |
1-year |
|
|
Cadmium (Cd) |
5 ng/m3 |
1-year |
|
|
Nickel (Ni) |
20 ng/m3 |
1-year |
|
|
Arsenic (As) |
6 ng/m3 |
1-year |
Conclusion
The mean indoor levels of trace metals (Cd, As, Ni) were less than those monitored outdoors, suggesting more pronounced outdoor emission sources. Whereas, the higher levels of Pb indoors suggests an indoor origin of the pollutant. The Cd, As and Ni levels were almost 3-8 times higher than the recommended limits of 5,6 and 20 ng/m3 respectively by WHO, European commission. However, the Pb concentration remain within the recommended levels. Based on the findings, it can be concluded that indoor air of hospitals is not satisfactory. The use of environmental tobacco smoke (ETS), different equipment such as lead aprons and electrocautery poses a health risk as prolonged exposure to trace metals, most of which are carcinogenic in nature, and may result in adverse health effects especially in immune-compromised patients and health care workers. The regional and national laws of European countries implements complete ban over smoking in the hospital premises for twenty years. Whereas, the hospitals in Pakistan are still not ETS free and struggling for the compliance of tobacco control policy. This probably also results in increased levels of trace metals indoors. This calls for strict implementation of the ETS free hospital policy in Pakistan. Moreover, the long-term potential exposure risk to trace metals is required when assessing health risks in hospitals.
Acknowledgments
A part of this study was funded by the Higher Education Commission, Pakistan under International Research Support Initiative Program (IRSIP) granted to Afzal Nimra for her doctoral research.
Conflict of interest
The authors have declared no conflict of interest.
References
Alvim-Ferraz, M.C. and Afonso, S.A., 2003. Incineration of different types of medical wastes: emission factors for particulate matter and heavy metals. Environ. Sci. Techol., 37: 3152-3157. https://doi.org/10.1021/es026209p
Aziakpono, O.M., Ukpebor, E. and Ukpebor, J., 2013. Baseline, spatial and temporal variation of respirable (PM2. 5) particulate matter in Isoko Land. Greener J. Phys Sci., 3: 247-254.
Brender, J.D., Suarez, L., Felkner, M., Gilani, Z., Stinchcomb, D., Moody, K., Henry, J. and Hendricks, K., 2006. Maternal exposure to arsenic, cadmium, lead, and mercury and neural tube defects in offspring. Environ. Res., 101: 132-139. https://doi.org/10.1016/j.envres.2005.08.003
Brown, K.W., Sarnat, J.A. and Koutrakis, P., 2012. Concentrations of PM 2.5 mass and components in residential and non-residential indoor microenvironments: The Sources and Composition of Particulate Exposures study. J. Expo. Anal. Environ. Epidemiol., 22: 161-172. https://doi.org/10.1038/jes.2011.41
Burns, K.M., Shoag, J.M., Kahlon, S.S., Parsons, P.J., Bijur, P.E., Taragin, B.H. and Markowitz, M., 2017. Lead aprons are a lead exposure hazard. J. Am. Coll. Radiol., 14: 641-647. https://doi.org/10.1016/j.jacr.2016.10.024
Chattopadhyay, G., Lin, K.C.-P. and Feitz, A.J., 2003. Household dust metal levels in the Sydney metropolitan area. Environ. Res., 93: 301-307. https://doi.org/10.1016/S0013-9351(03)00058-6
Connolly, G.N., Carpenter, C.M., Travers, M.J., Cummings, K.M., Hyland, A., Mulcahy, M. and Clancy, L., 2009. How smoke-free laws improve air quality: a global study of Irish pubs. Nicotine Tob. Res., 11: 600-605. https://doi.org/10.1093/ntr/ntp038
Dreher, K.L., Jaskot, R.H., Lehmann, J.R., Richards, J.H., Ghio, J.K.M.A.J. and Costa, D.L., 1997. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Environ. Hlth., 50: 285-305. https://doi.org/10.1080/009841097160492
Fang, G.-C., Huang, Y.-L. and Huang, J.-H., 2010. Study of atmospheric metallic elements pollution in Asia during 2000–2007. J. Hazard. Mater., 80: 115-121. https://doi.org/10.1016/j.jhazmat.2010.03.120
Habil, M., Massey, D.D. and Taneja, A., 2013. Exposure of children studying in schools of India to PM levels and metal contamination: sources and their identification. Air. Qual. Atmos. Hlth., 6: 575-587. https://doi.org/10.1007/s11869-013-0201-3
Harkavy, L.M. and Novak, D.A. 2014. Clearing the air: Surgical smoke and workplace safety practices. Or Nurse., 8: 1-7. https://doi.org/10.1097/01.ORN.0000453446.85448.2f
Hassan, S.K.M., 2012. Metal concentrations and distribution in the household, stairs and entryway dust of some Egyptian homes. Atmos. Environ., 54: 207-215. https://doi.org/10.1016/j.atmosenv.2012.02.013
Health Ordinance, 2002. (Ordinance No. LXXIV of 2002), Tobacco Control Cell, Ministry of Health, Government of Pakistan, 2010., avaiable at: http://www.tcc.gov.pk/
Hu, X., Zhang, Y., Ding, Z., Wang, T., Lian, H., Sun, Y. and Wu, J., 2012. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ., 57: 146-152. https://doi.org/10.1016/j.atmosenv.2012.04.056
IARC, 1972. IARC monographs on the evaluation of carcinogenic risk of chemicals to man. IARC Monogr. Eval. Carcinog. Risk Chem. Man, pp. 1.
IARC, 2006. Working group on the evaluation of carcinogenic risks to humans: Inorganic and organic lead compounds. IARC Monogr. Eval. Carcinog. Risks Hum., 87:1–471
IARC, 2012. Working group on the evaluation of carcinogenic risks to humans: Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum., 100 (Pt C): 11–465
Ibanez, R., Andres, A., Viguri, J., Ortiz, I. and Irabien, J., 2000. Characterisation and management of incinerator wastes. J. Hazard. Mater., 79: 215-227. https://doi.org/10.1016/S0304-3894(00)00268-5
Janssen, N.A., Van Mansom, D.F., Van Der Jagt, K., Harssema, H. and Hoek, G., 1997. Mass concentration and elemental composition of airborne particulate matter at street and background locations. Atmos. Environ., 31: 1185-1193. https://doi.org/10.1016/S1352-2310(96)00291-9
Kefeni, K.K. and Okonkwo, J.O., 2013. Trace metals, anions and polybromodiphenyl ethers in settled indoor dust and their association. Environ. Sci. Pol. Res., 20: 4895-4905. https://doi.org/10.1007/s11356-013-1469-4
Kermani, M., Arfaeinia, H., Nabizadeh, R., Alimohammadi, M. and Aalamolhoda, A., 2016. Levels of PM2. 5-associated heavy metals in the ambient air of Sina hospital district, Tehran, Iran. J. Air. Pollut. Hlth., 1: 1-6.
Khan, J.A., Sohail, A.H., Malik, A., Maan, A. and Arslan, M., 2016. Tobacco control laws in Pakistan and their implementation: a pilot study in Karachi. J. Pak. Med. Assoc., 66: 875.
Khillare, P., Balachandran, S. and Meena, B.R., 2004. Spatial and temporal variation of heavy metals in atmospheric aerosol of Delhi. Environ. Monit. Assmnt., 90: 1-21. https://doi.org/10.1023/B:EMAS.0000003555.36394.17
Lawrence, A.J. and Khan, T., 2020. Quantification of Airborne Particulate and Associated Toxic Heavy Metals in Urban Indoor Environment and Allied Health Effects: Measurement, analysis and remediation of environmental pollutants (ed. A.K. Agarwal), Springer, Singapore, pp. 7-58. https://doi.org/10.1007/978-981-15-0540-9_2
Li, R., Fu, H., Hu, Q., Li, C., Zhang, L., Chen, J. and Mellouki, A.W., 2017. Physiochemical characteristics of aerosol particles in the typical microenvironment of hospital in Shanghai, China. Sci. Total Environ., 580: 651-659. https://doi.org/10.1016/j.scitotenv.2016.12.011
Lomboy, M.F.T.C., Quirit, L.L., Molina, V.B., Dalmacion, G.V., Schwartz, J.D., Suh, H.H. and Baja, E.S., 2015. Characterization of particulate matter 2.5 in an urban tertiary care hospital in the Philippines. Build. Environ., 92: 432-439. https://doi.org/10.1016/j.buildenv.2015.05.018
Loupa, G., Zarogianni, A.-M., Karali, D., Kosmadakis, I. and Rapsomanikis, S., 2016. Indoor/outdoor PM2. 5 elemental composition and organic fraction medications, in a Greek hospital. Sci. Total Environ., 550: 727-773. https://doi.org/10.1016/j.scitotenv.2016.01.070
Malik, A.K., Chaudhry, A., Karamat, A., Arif, N., Cheema, M.A. and Rauf, A., 2010. Cigarette smoking and health care professionals at Mayo Hospital, Lahore, Pakistan. J. Pak. Med. Assoc., 60: 509-512.
Nimra, A., Ali, Z., Khan, M.N., Gulshan, T., Sidra, S., Gardezi, J.R., Tarar, M.R., Saleem, M., Nasir, Z.A. and Colbeck, I., 2015. Comparative ambient and indoor particulate matter analysis of operation theatres of government and private (trust) hospitals of Lahore, Pakistan. J. Anim. Plt. Sci., 25: 628-635.
Okuda, T., Katsuno, M., Naoi, D., Nakao, S., Tanaka, S., He, K., Ma, Y., Lei, Y. and Jia, Y., 2008. Trends in hazardous trace metal concentrations in aerosols collected in Beijing, China from 2001 to 2006.Chemosphere, 72: 917-924. https://doi.org/10.1016/j.chemosphere.2008.03.033
Pandey, P., Patel, D., Khan, A., Barman, S., Murthy, R. and Kisku, G., 2013. Temporal distribution of fine particulates (PM2. 5, PM10), potentially toxic metals, PAHs and Metal-bound carcinogenic risk in the population of Lucknow City, India. J. Environ. Sci. Hlth., 48: 730-745. https://doi.org/10.1080/10934529.2013.744613
Panne, U., Neuhauser, R., Theisen, M., Fink, H. and Niessner, R., 2001. Analysis of heavy metal aerosols on filters by laser-induced plasma spectroscopy. Spectrochim. Acta Part B: At. Spectrosc., 56: 839-850. https://doi.org/10.1016/S0584-8547(01)00209-9
Paoletti, L., De Berardis, B., Arrizza, L. and Granato, V., 2006. Influence of tobacco smoke on indoor PM10 particulate matter characteristics. Atmos. Environ., 40: 3269-3280. https://doi.org/10.1016/j.atmosenv.2006.01.047
Piryani, R.M. and Rizvi, N., 2004. Smoking habits amongst house physicians working at Jinnah Postgraduate Medical Center, Karachi, Pakistan. Trop. Doct., 34: 44-45. https://doi.org/10.1177/004947550403400123
Radulescu, C., Iordache, S., Dunea, D., Stihi, C. and Dulama, I., 2015. Risks assessment of heavy metals on public health associated with atmospheric exposure to PM2. 5 in urban area. Rom. J. Phys., 60: 1171-1182.
Sah, S., Bikash, K., Dangi, S.J., Khanal, K. and Basnet, R., 2017. Risk for the surgical team during orthopaedic surgeries. J. Soc. Anesthesiol. Nepal, 4: 29-34. https://doi.org/10.3126/jsan.v4i1.17439
Sella, S.M., Netto, A.D.P., Da Silva Filho, E.V. and Araújo, M.T., 2004. Short-term and spatial variation of selected metals in the atmosphere of Niteroi City, Brazil. Microchem. J., 78: 85-90. https://doi.org/10.1016/j.microc.2004.03.015
Shah, M.H. and Shaheen, N., 2008. Annual and seasonal variations of trace metals in atmospheric suspended particulate matter in Islamabad, Pakistan. Water Air Soil Pollut., 190: 13-25. https://doi.org/10.1007/s11270-007-9575-x
Slezakova, K., Da Conceição Alvim-Ferraz, M. and Carmo Pereira, M., 2012. Elemental characterization of indoor breathable particles at a Portuguese urban hospital. J. Toxicol. Environ. Hlth., 75: 909-919. https://doi.org/10.1080/15287394.2012.690707
Slezakova, K., Morais, S. and Do Carmo Pereira, M., 2014. Trace metals in size-fractionated particulate matter in a Portuguese hospital: Exposure risks assessment and comparisons with other countries. Environ. Sci. Pollut. Res., 21: 3604-3620. https://doi.org/10.1007/s11356-013-2316-3
Slezakova, K., Pereira, M. and Alvim-Ferraz, M., 2009. Influence of tobacco smoke on the elemental composition of indoor particles of different sizes. Atmos. Environ., 43: 486-493. https://doi.org/10.1016/j.atmosenv.2008.10.017
Shirazi, S.A. and Kazmi, S.J.H., 2014. Analysis of population growth and urban development in Lahore-Pakistan using geospatial techniques: Suggesting some future options. South Asian Stud., 29: 269-280.
Susaya, J., Kim, K.-H., Ahn, J.-W., Jung, M.-C. and Kang, C.-H., 2010. BBQ charcoal combustion as an important source of trace metal exposure to humans. J. Hazard. Mater., 176: 932-937. https://doi.org/10.1016/j.jhazmat.2009.11.129
Taner, S., Pekey, B. and Pekey, H., 2013. Fine particulate matter in the indoor air of barbeque restaurants: Elemental compositions, sources and health risks. Sci. Total Environ., 454: 79-87. https://doi.org/10.1016/j.scitotenv.2013.03.018
Tanner, P.A., Ma, H.-L. and Yu, P.K., 2008. Fingerprinting metals in urban street dust of Beijing, Shanghai, and Hong Kong. Environ. Sci. Tech., 42: 7111-7117. https://doi.org/10.1021/es8007613
Thomson, E.M., Breznan, D., Karthikeyan, S., Mackinnon-Roy, C., Vuong, N.Q., Dabek-Zlotorzynska, E., Celo, V., Charland, J.-P., Kumarathasan, P. and Brook, J.R., 2016. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part Fibre Toxicol., 13: 65. https://doi.org/10.1186/s12989-016-0176-y
US EPA, 2011. National primary and secondary ambient air quality standards. Appendix G to Part 50 - Reference Method for the Determination of Lead in Suspended Particulate Matter Collected From Ambient Air. Available at: http://www.epa.gov/osw/hazard/testmethods/sw846/pdfs/6020a.pdf.
Valavanidis, A., Fiotakis, K. and Vlachogianni, T., 2008. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Hlth., 26: 339-362. https://doi.org/10.1080/10590500802494538
Walker, L., Simpson, V., Rockett, L., Wienburg, C. and Shore, R., 2007. Heavy metal contamination in bats in Britain. Environ. Pollut., 148: 483-490. https://doi.org/10.1016/j.envpol.2006.12.006
Wang, Bi, X., Chen, D., Sheng, G. and Fu, J., 2006a. Hospital indoor respirable particles and carbonaceous composition. Build. Sci., 41: 992-1000. https://doi.org/10.1016/j.buildenv.2005.04.024
Wang, X., Bi, X., Sheng, G. and Fu, J., 2006b. Hospital indoor PM10/PM2. 5 and associated trace elements in Guangzhou, China. Sci. Total Environ., 366: 124-135. https://doi.org/10.1016/j.scitotenv.2005.09.004
Willemsen, M., Görts, C., Van Soelen, P., Jonkers, R. and Hilberink, S., 2004. Exposure to environmental tobacco smoke (ETS) and determinants of support for complete smoking bans in psychiatric settings. Tob. Control, 13: 180-185. https://doi.org/10.1136/tc.2003.004804
World Health Organization. 2000. Air quality guidelines for Europe, 2000. WHO Reg. Publ. Eur. Ser., pp. 91.
Xia, T., Kovochich, M. and Nel, A.E., 2007. Impairment of mitochondrial function by particulate matter (PM) and their toxic components: implications for PM-induced cardiovascular and lung disease. Front. Biosci., 12: 1238. https://doi.org/10.2741/2142
Yaffe, Y., Flessel, C.P., Wesolowski, J.J., Rosario, A.D., Guirguis, G.N., Matias, V., Degarmo, T.E., Coleman, G.C., Gramlich, J.W. and Kelly, W.R., 1983. Identification of lead sources in California children using the stable isotope ratio technique. Arch Environ. Hlth. An. Int. J., 38: 237-245. https://doi.org/10.1080/00039896.1983.10545809
Yang, K.X., Swami, K. and Husain, L., 2002. Determination of trace metals in atmospheric aerosols with a heavy matrix of cellulose by microwave digestion-inductively coupled plasma mass spectroscopy. Spectrochim. Acta Part B: At. Spectrosc., 57: 73-84. https://doi.org/10.1016/S0584-8547(01)00354-8
To share on other social networks, click on any share button. What are these?








