Environmental Impact of Bollworms Infestation on Cotton, Gossypium hirsutum
Environmental Impact of Bollworms Infestation on Cotton, Gossypium hirsutum
Mujahid Niaz Akhtar* and Amjad Farooq
Institute of Pure and Applied Biology, Zoology Division, Bahauddin Zakaariya University, Multan
ABSTRACT
The present research work was conducted to evaluate the environmental changes on cotton bollworms like Pink Bollworm (PBW), Spotted Bollworm (SBW) and American bollworms (ABW) present in Multan (Southern Punjab), Pakistan. The experimental data was collected from spring 2014 to fall 2016. Eggs and adults count of these bollworms were counted throughout the cropping season. Statistix (Version 8.1) were used to analyzed the data statistically. The mean values were compared at the significance level of 5%. Bollworms (PBW, SBW, and ABW) population was lowest in April and May whereas the highest values were observed in September and October. Correlation between abiotic factor and bollworms infestation depicted non-significant negative correlation with rainfall and evening relative humidity while negative significant with minimum temperature. Linear regression equation is used to predict bollworm population to minimize economic losses.
Article Information
Received 07 August 2018
Revised 12 December 2018
Accepted 23 February 2019
Available online 14 August 2019
Authors’ Contribution
MNA conducted the experiments, compiled and interpret the results. AF provided technical support and helped in data analysis.
Key words
Chewing pests, Population densities, Correlation, Regression equation, Bollworms.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2099.2106
* Corresponding author: mujahidniaz81@gmail.com
0030-9923/2019/0006-2099 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
INTRODUCTION
Cotton, wheat, rice and sugarcane are cash crops produced by farmers of an agricultural country, Pakistan. In 2015-16, more than twenty-three lacs ton cotton were produced that offer 17% of agricultural and 1.5% of national GDP (GOP, 2016). Cotton (Gossypium hirsutum) fiber is soft, fragile and produce in cotton bolls. Naturally cotton plant is shrubby and cultivated generally in subtropical and tropical regions. From prehistoric time cotton is used to make fiber (Huckle and Lisa, 1993). Chinese are the main fabricator for the cotton production throughout world but they totally used this for domestic purpose only. While United states are the main exporter for many years (Moulherat et al., 2002). In Pakistan 5 million farmers are playing their role to take the country at 5th position in production and 3rd in export of cotton (GOP, 2010). It is an important cash providing crop, which are attacked by different insect pests belongs to chewing and sucking types (Agarwal et al., 1981). By applying, pesticide one can control the sucking pests, but chewing pests can cause severe damage including bollworm complex named as; Spotted bollworms, Earias insulana (Boisduval), Pink bollworm Pectinophora gossypiella (Saunders); Earias vittella (Fabricius) and American bollworm Helicoverpa armigera (Hubner) (Akhtar and Farooq, 2019). These bollworms are said to be notorious insect pests which can be the reason of up to 50 % loss (Dhawan et al., 1998). Bollworms are called as lepidopteran, attacked different plant’s fruiting bodies including cotton. They parasitized the cotton bolls and each bollworm is having different way of action on bolls, like as spotted bollworm, larvae get entry into the shoot which are the future floral bud and infestation has been observed when flower and buds are formed that results in falling of terminal shoots, squares and bolls, create a huge economic loss (Sarate et al., 2012; Sisterson and Tabashnik, 2004). American bollworm is polyphagous, larvae emerge from eggs after 3-4 days after that they attacked on leaves square and bolls. As for as the pink bollworms, after hatching they get entry into the bolls and completely damage the bolls inside (Reddy et al., 2015). Agronomic practices which are used in cotton farming enhances the probability of pest eruption, as well as ecological conditions are also playing a vital role in the population dynamics of different insect pests (Hasan et al., 2013; Idris and Hayat, 1997; Khaliq et al., 2014). A number of environmental factors are influenced on reproduction and survival of the bollworms, like as day length, humidity, rainfall and temperature. As insects are cold blooded organism, maximum and minimum temperature overwhelms the effect of environmental features (Afridi and Khan, 2015; Baloch et al., 1990; Reiter, 1989). Total number of eggs and Ovipositional behavior are highly influenced by the temperature (Cammel and Knight, 1992). Rainfall might have a damaging effect on the population of insect (eggs and neonate) due to dislocation or killed by rain. Metamorphosis time of insects prolonged during winter season, pigmentation changed with the change in humidity and temperature (Kadam and Kahaira, 1995; Schmutterer, 1990). Insects can survive only certain environmental conditions, it can be predicted the maximum activity of insects through a better understanding of environmental conditions. The present research work was undertaken to observe bollworms population and its association with abiotic factors such as maximum, minimum and average temperatures, rainfall (RF mm) and relative humidity (RH %) in cotton fields of Multan during growing to harvesting season from 2014 to 2016.
Materials and Methods
Current research study was carried out in order to evaluate the environmental impact of bollworm infestation on squares and bolls in five different varieties of cotton during the crop season 2014-2016. Out of five varieties four were Bt (Sitara-009, MNH-988, Lalazar, IUB-33) and one Non Bt (Niab). These are the approved cotton varieties by PSC (Punjab Seed Council) Pakistan.
Experimental layout
Randomized Complete Block design was used to carry out the experiment. Five Bt and non Bt varieties were randomly assigned in three blocks of 1 Kanal (20 Marlas) plot size. Nine inch raised beds with five feet distance in each bed were managed for the seed sowing. The width of each bed in each block was 2.50 ft and length were 49.4 ft. Agronomic practices recommended by the Department of agriculture, Punjab, Pakistan were accomplished.
Field conditions
Fields were selected while keeping in mind the soil fertility status, suitable insect pressure, availability of field inputs and meteorological conditions of research area.
Seed collection
Seeds of Bt cotton were collected from cotton research institute, Multan. While Non Bt cotton seed was obtained from local seed supplier.
Seed sowing
In April of each year seed sowing was started. Squares and bolls were collected from haphazardly selected plants and bollworms infestation was noted.
Collection of data
Data about infested bolls by bollworms were noted after every ten days of interval. Percentage damaged bolls from bollworms were calculated by using following formula:
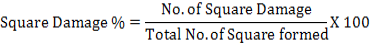
Following formula were used to calculate the multiple regression as well:
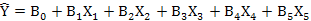
Meteorological data
Meteorological data was calculated from the meteorology department, Multan to correlate the weather parameters with the bollworm infestation.
Data analysis
Recorded data during the research duration was computed for ANOA (Analysis of variance by using the computer-oriented software i.e. Statistix (Version 8.1). The means were calculated for significant differences by LSD (least standard deviation).
RESULTS
Population dynamics of bollworms on different cotton genotypes were examined periodically.
Cotton squares and bollworm infestation
Square of different cotton varieties irrespective of Bt and non Bt, infested by Bollworms are shown in Table I.
Table I.- Bollworm infestation on square of Bt and non Bt cotton varieties.
|
Genotype |
Bollworm infestation on squares (Standard Meteorological Week) |
||||||||||||
|
30 |
31 |
32 |
33 |
34 |
35 |
36 |
37 |
38 |
39 |
40 |
41 |
Mean |
|
|
Sitara-009 |
0.20 |
0.48 |
0.62 |
0.68 |
0.87 |
1.10 |
1.31 |
1.01 |
1.21 |
3.39 |
3.37 |
3.27 |
1.45 |
|
MNH-988 |
0.70 |
0.71 |
1.13 |
0.95 |
1.28 |
1.37 |
2.83 |
3.10 |
2.42 |
4.21 |
4.61 |
3.42 |
2.22 |
|
Lalazar |
0.37 |
0.53 |
0.80 |
1.02 |
1.24 |
1.30 |
2.06 |
3.29 |
2.21 |
4.73 |
4.54 |
3.39 |
2.12 |
|
IUB-33 |
0.39 |
0.88 |
1.31 |
1.25 |
1.60 |
1.60 |
3.17 |
3.39 |
2.75 |
6.10 |
5.60 |
4.52 |
15.18 |
|
Niab non Bt |
3.33 |
3.53 |
4.11 |
6.88 |
7.55 |
10.44 |
14.66 |
18.53 |
23.31 |
33.2 |
29.87 |
26.76 |
23.68 |
|
Mean |
5.83 |
1.22 |
1.59 |
2.15 |
2.5 |
3.16 |
4.8 |
5.86 |
6.38 |
10.32 |
9.59 |
8.27 |
23.68 |
|
SD |
1.315 |
1.297 |
1.432 |
2.648 |
2.830 |
4.072 |
5.555 |
7.147 |
9.481 |
12.82 |
11.359 |
10.347 |
5.855 |
|
SEM (±) |
0.588 |
0.580 |
0.640 |
1.184 |
1.265 |
1.821 |
2.484 |
3.196 |
4.240 |
5.735 |
5.080 |
4.627 |
|
Table II.- Means (±SEM) number of adult bollworms for different periods during 2014-16.
|
2014 |
2015 |
2016 |
|||||||
|
ABW± SEM |
PBW± SEM |
SBW± SEM |
ABW± SEM |
PBW± SEM |
SBW± SEM |
ABW± SEM |
PBW± SEM |
SBW ±SEM |
|
|
April |
0.13±0.09 |
0.00 ±0.00 |
0.00± 0.00 |
0.66± 0.066 |
0.00± 0.00 |
0.00± 0.00 |
0.00± 0.00 |
0.00± 0.00 |
0.00± 0.00 |
|
May |
1.40±0.78 |
0.06± 0.05 |
0.06± 0.05 |
12.73± 4.974 |
10.13± 4.75 |
2.46± 1.32 |
0.00± 0.00 |
0.00± 0.00 |
0.00± 0.00 |
|
June |
14.00 ±2.19 |
2.80± 1.61 |
1.93± 2.17 |
12.66± 4.126 |
9.66± 4.08 |
4.06± 2.40 |
2.53± 2.11 |
0.80± 0.73 |
0.86± 0.79 |
|
July |
15.40±3.29 |
14.13± 4.31 |
7.26± 3.33 |
12.53± 4.97 |
11.40 ±3.70 |
3.06± 1.42 |
6.53± 3.20 |
3.86± 2.41 |
4.53± 2.38 |
|
August |
15.66 ±3.82 |
18.93± 5.67 |
11.80± 5.39 |
13.20± 5.34 |
15.20± 3.76 |
2.93± 1.47 |
10.13± 4.83 |
7.06± 3.70 |
7.46± 3.33 |
|
Sept. |
17.13 ±2.90 |
21.33± 3.68 |
7.13± 2.57 |
16.46± 4.75 |
24.06± 4.77 |
2.73± 1.32 |
15.33± 4.18 |
13.66± 6.89 |
14.8± 6.00 |
|
October |
16.60 ±2.63 |
22.47± 3.13 |
8.93± 3.40 |
14.60± 4.25 |
25.13± 4.35 |
2.13± 1.03 |
19.93± 4.56 |
13.26± 4.62 |
10.40± 4.77 |
ABW, American bollworm; PBW, pink bollworm; SBW, spotted bollworm; SEM, standard error mean
Table III.- Means (±SEM) number of bollworm eggs for different periods during 2014-2016.
|
2014 |
2015 |
2016 |
|||||||
|
ABW± SEM |
PBW± SEM |
SBW± SEM |
ABW ±SEM |
PBW± SEM |
SBW± SEM |
ABW± SEM |
PBW± SEM |
SBW ±SEM |
|
|
April |
0.33± 0.15 |
0.00± 0.00 |
0.00± 0.00 |
0.26± 0.16 |
0.00± 0.00 |
0.00 ±0.00 |
0.00± 0.00 |
0.00± 0.00 |
0.00 ±0.00 |
|
May |
1.8± 0.23 |
0.00± 0.00 |
0.00± 0.00 |
4.60± 1.81 |
2.00± 0.65 |
4.73± 2.300 |
0.00± 0.00 |
0.00± 0.00 |
0.00 ±0.00 |
|
June |
1.93± 0.54 |
1.80± 1.19 |
0.13± 0.09 |
9.80± 2.97 |
6.200± 2.49 |
6.26± 3.03 |
13.73 ±9.03 |
13.53 ±9.23 |
14.4 0 ±9.82 |
|
July |
5.80± 1.94 |
2.40± 1.29 |
0.20± 0.10 |
11.21 ±3.47 |
17.33± 4.09 |
7.06± 3.21 |
25.73 ±13.06 |
23.53± 12.55 |
21.40 ±11.10 |
|
August |
11.40 ±3.46 |
7.53± 2.92 |
1.20± 0.61 |
10.66 ±3.29 |
13.73± 3.75 |
7.46± 3.12 |
27.13± 12.26 |
23.40± 12.11 |
25.46 ±10.06 |
|
Sept. |
14.20 ±4.55 |
15.00 ±3.144 |
0.40± 0.19 |
8.66± 2.33 |
12.60± 2.14 |
7.73± 2.71 |
16.46± 5.47 |
24.66± 11.64 |
32.00 ±11.77 |
|
October |
9.46± 3.12 |
19.26± 2.55 |
0.26± 0.15 |
3.12± 12.8 |
20.46± 4.07 |
9.33± 3.83 |
8.66± 4.49 |
17.53± 6.24 |
21.80 ±3.10 |
For abbreviations, see Table II.
Bollworm infestation was started from 30th standard meteorological week (SMW) to 41 SMW i.e. July to October. Bt varieties shows significantly lower infestation than non-Bt. Sitara-009 shows significantly lowest infestation on 30th SMW (0.20%) and it was followed by lalazar (0.37%), IUB-33 (0.39%), MNH-988 (0.70%), while Niab non Bt showed significantly higher infestation (3.33) and it was more vulnerable for bollworm infestation. Infestation by bollworms in square was increased during 35 SMW that continue to increase till harvesting i.e. 41 SMW. On the basis of mean value, it was observed that minimum infestation calculated during 30th SMW and reached to maximum during 39th SMW; after that decrease was recorded.
Population count of bollworm eggs and adult
In April 2014-16 lowest population of ABW was detected and highest in October 2014-16. Population counts varied abruptly in September 2015 and reaches to its maximum. Overall it was observed that lowest and highest adult population was found in 2015 and 2016, respectively. Egg count of ABW varied through out the cropping season. It was lowest in June 2014 and highest in September 2014. In October and April 2015, July and May 2016, highest and lowest ABW counts were noted correspondingly. On the other hand, adult count of PBW showed highest and lowest number in September, April from 2014-2016 in each year (Table II). Lowest and Highest egg count of PBW were observed in April and September in each year. Adult population of SBW was found to be highest in October 2014, July 2015, September 2016 and lowest in April 2014-2016. SBW egg count showed lowest and highest figures in September, May 2014, October, April 2015 and September, April 2016 respectively in study area (Table III).
Table IV.- Correlation of Bollworms infestation with weather parameters.
|
Weather parameter |
Correlation co-efficient (r-value) |
|
Damage by Bollworm |
|
|
Minimum temperature (oC) |
-0.739 |
|
Maximum temperature (oC) |
-0.308 |
|
Evening relative humidity (%) |
-0.424 |
|
Morning relative humidity (%) |
0.122 |
|
Rain fall (mm) |
-0.073 |
|
Wind speed (Km/h) |
-0.327 |
Correlation with weather parameters
Environment has played a vital role in fluctuation of bollworms population. Impact of abiotic factors on bollworms infestation was computed as depicted in Table IV and Figure 1. It was observed that bollworm infestation showed significant negative correlation with minimum temperature (r=-0739), while with evening relative humidity (r=-0.424), maximum temperature (r=-0.308), with rainfall (r=-0.073) showed non-significant negative correlation, respectively. Morning relative humidity (r=0.122) exhibit positive correlation with the bollworm infestation.
Table V.- Multiple regression analysis between meteorological factor and bollworms infestation in squares on cotton genotypes.
|
Regression |
R2 |
|
|
Square infested by Bollworms |
Y1=61-0.53B1-1.45B2-0.136B3+ 0.147B4+0.275B5 |
0.79 |
|
Y2=38-1.54B2+0.09B4 |
0.73 |
|
|
Y3=36-1.25B2 |
0.62 |
B1, minimum temperature (oC); B2, maximum temperature (oC); B3, evening relative humidity (%); B4, morning relative humidity (%); B5, wind speed (Km/h).
Regression equation
Relationship between weather parameters and bollworms infestation were analyzed by using multiple regression equation. For this purpose, Bollworm infestation (Y) is used as dependent variable while weather parameters are independent variable as described in Table V. Changes in weather parameters and amount of change in bollworms damage clearly indicated that these factors are significantly affected i.e. 79% regression equation Y1. Out of this, 73% variability accounted for evening relative humidity and minimum temperature (regression equation Y2), while minimum temperature contributed 62% (regression equation Y3).
DISCUSSION
Among insect’s pest chewing worm can cause large financial loss to cotton crop. There are different types of bollworms, but most dangerous one is Army, Pink and Spotted bollworms. Maggots of these pests enter into cotton bolls, spoil the bolls by chewing fiber inside and became a reason of excessive loss to the crop. Insect population may fluctuate with the growing crops and with varying season. As the seed was sown, these insect moths visit field area and lay eggs. As for as crop grow, eggs hatch to larvae and get entry into bolls. Insect and egg densities vary throughout different stages of crop, which are related to moth’s visit, eggs laying and hatching into worms, and then into adult stage. Environmental factors show significant variation on eggs hatching and other stages. Some difference might happen due to regional land and environment condition. In southern part of Punjab versatile climatic conditions were observed ranging from wet dry to harsh dry. It is difficult to predict weather in this area as precipitation rate is very low, some part totally depends upon rain, while in some area riverine water is supplemented with rain water.
The crops that required excess water, totally depend upon bed water for irrigation. In such harsh environmental condition only, certain insect pests can survive and flourish. The experimental site, which is center of Punjab (Pakistan), dry weather was observed round the year, winter are of quite short duration while summers are very long. In winter diurnal temperature is noticeable and minor changes occurred during summer. Proliferation of some kind of cotton chewing pests favored by such weather conditions in the study area. Pests density increases during the summer and touching to its maximum when temperature became normal. Adult of chewing Insect pests lay eggs on newly sown crop which hatch into maggots that ultimately transform into bollworms. The insect’s densities and their eggs change accordingly. April or May was the month when the cotton crop was sown in each year. As seedling grew in size, eggs of these pests can be found there in the field. Obvious trend is observed when we look deeper into results. At the start of cropping season, low adult density was detected. As the more food and space for the insect available their number gradually rises. In September and/or October but sometimes in August, highest population density was seen. Eggs count depend upon number of insects visiting crop. The difference in egg counts were observed because egg hatching take place after some days. Reduction in egg counting was due to hatching of eggs. In April of every year, bollworms i.e. PBW, SBW, and ABW had lowest and nearly same count. ABW population was found to be higher in 2014 but reduced in next two years which became higher again in October 2016. Climatic condition and pesticide spray might be the reason for this decline. In 2014 and 2015 adult ABW population enhanced abruptly, but in next months of the cropping season gradual decrease was seen. In September 2014, 2015 and 2016 highest number of PBW was observed, before and after this month their number was not significant. This picture depicted that adult number depend upon different stages of cotton plant like as appearance of flower and formation of bolls, which are extremely correlated with density of pests. SBW adults show lower count than PBW and ABW from 2014-2016. August 2014, July 2015 and September 2016 were more favored for SBW adult populations. Bollworms moth’s population show fluctuation but remained active throughout the year (Glick and Graham, 1965; Qureshi et al., 2009; Zafar et al., 2013). In May- July which are the summer months decreased of bollworms were observed but become higher in October the long night month. March, April was the month where virtually no emergence of moths detected (Qureshi et al., 2009; Reddy et al., 2015). Population of larvae also show same fluctuation pattern round the cropping season. Like as pink bollworm found to be highest in August (Glick and Graham, 1965). Spotted bollworm infestation was noted during August to October (Ali et al., 2016; Qureshi and Ahmad, 1991) while emergence of army bollworm reached to its maximum from May to September (Ragab et al., 2014). Moderate and warmer areas are the best sites for the growth of Insect population than the colder regions. Precipitation rate and temperature could enhance pest populations quickly (Kavitam et al., 2015). Impact of temperature on development of bollworm eggs depicted that increase in temperature might disturb the development of larvae and insect pupae (Barteková and Praslička, 2006; Prasad and Bambawale, 2010; Aziz et al., 2011; Satti, 2012; Akram, 2013; Kumar et al., 2016). Environmental impact could influence on pest population and have valued correlation with each other. Pests show positive correlation with predator population while negatively correlated with temperature and rain fall (Parajulee et al., 2006; Izumi et al., 2005; Pratheepa et al., 2010; Lepage et al., 2012; Pazhanisamy and Deshmukh, 2011; Ghosh et al., 2014; Pan et al., 2014; Kumar et al., 2016). Population of pink bollworm depicted negative correlation with rain fall and temperature (Khan et al., 2002, Wu et al., 2008; Tripathi, 2008; Chen et al., 2014; Sharma et al., 2016; Ali et al., 2016). Bollworm population had been favored by Monsoon and predators favored by winter season (Pratheepa et al., 2010; Hussain et al., 2014; Pan et al., 2014; Reddy et al., 2015). Bollworms infestation on squares was started last week of July i.e. 30th standard meteorological week in each year, and continue till the end of the cropping season. On bollworm infestation, environment played a vital role. In present research work, the infestation by bollworms is negative significant correlated with minimum temperature. Non-significant negative correlation was seen with evening relative humidity, maximum temperature and with rain fall. Same observation was reported (Kalkal et al., 2014; Purohit et al., 2006). Regarding the multiple regression analysis, it was noted that environmental factors contribute 81% variability in infestation. The prediction rate of pink and spotted bollworm population was 84% and 44% (Babu and Meghwal, 2014). That support the present study as well. The regression and correlation clearly indicated the importance of environmental factors in bollworm square infestation in cotton crop. Affective role of environmental factors was observed on the insect population round the year during cropping season. Proliferation and survival of these insect required suitable environmental conditions otherwise their survival, mortality and propagation are inevitable (Venette et al., 2000; Mironidis and Soultani, 2008).
CONCLUSIONS
Present study showed that Bollworms population density vary as seasonal changes occur. It became higher in summer season. Initial sprays could have some influenced but afterward rapidly increasing trend were observed. Population density reduced to lowest level by using control measures paralleling the seasonal change. Destroying eggs could be a fruitful tool to minimize the bollworm’s population. Regression analysis predicted that environmental factor had significant effect on bollworm damage. Different weather parameters show negative correlation with bollworm infestation.
Statement of conflict of interest
The authors declare no conflict of interest.
REFERENCES
Afridi, R.H. and Khan, M.A., 2015. Comparative effect of water of Parthenium hysterophorus, Datura alba, Pragmites australis and Oryza sativa on weeds and wheat. Sains Malays., 44: 693-699. https://doi.org/10.17576/jsm-2015-4405-08
Agarwal, R.A., Gupta, A.P. and Garg, D.O., 1984. Cotton pest management research. Co-Publications, East Azad Nagar, Delhi, pp. 91.
Akhtar, M.N. and Farooq, A., 2019. Predator prey interaction between lepidopteran pests and coccinellids insects of cotton in southern Punjab, Pakistan. Pakistan. J. Zool., 51: 583-589.
Akram, M., Hafeez, F., Farooq, M., Arshad, M., Hussain, M., Ahmad, S., Zia, K. and Khan, H.A.A., 2013. A case to study population dynamics of bemisia tabaci and thrips tabaci on Bt and non-Bt cotton genotypes. Pakistan J. agric. Sci., 50: 617-623.
Ali, A., Shah, Z., Saleem, M., Hafeez, F., Ullah, Z., Abbas, M., Farooq, M. and Ghaffar, A., 2016. Influence of weather factors on the trapped population of spotted bollworm (E. vittella f and E. insulana b) under Bahawalpur agro-ecosystem. J. agric. Res., 54: 477-485.
Aziz, M.A., Hasan, M. and Ali, A., 2011. impact of abiotic factors on incidence of fruit and shoot infestation of spotted bollworms Earias spp. on Okra (Abelmoschus esculentus L.). Pakistan J. Zool., 43: 863-868.
Babu, S.R. and Meghwal, M.L., 2014. Population dynamics and monitoring of sucking pests and bollworms on bt cotton in humid zone of Southern Rajasthan. The Bioscan, 9: 629-632.
Baloch, A.A., Soomroo, B.A., Laghari, M.A. and Sanjrani, M.W., 1990. Studies on economic injury levels of insect pests of cotton. Turk. Entomol. Derg., 14: 131-148.
Barteková, A. and Praslička, J., 2006. The effect of ambient temperature on the development of cotton bollworm (Helicoverpa armigera Hübner, 1808). Pl. Protec. Sci., 42: 135–138. https://doi.org/10.17221/2768-PPS
Cammel, M.E. and Knight, J.D., 1992. Effects of climatic change on the population dynamics of crop pests. Adv. Ecol. Res., 22:117-162. https://doi.org/10.1016/S0065-2504(08)60135-X
Chen, C., Xia, Q. W., Fu, S. and Wu, X. F., 2014. Effect of photoperiod and temperature on the intensity of pupal diapause in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Bull. entomol. Res., 104:12-18. https://doi.org/10.1017/S0007485313000266
Dhawan, A.K, Sindhu, A.J. And Simwat, G.S., 1998. Monitoring the seasonal abundance of cotton bollworms with pheromone traps. Indian J. Ecol., 23:129-131.
Ghosh, K., Rajavel, M., Samui, R.P. And Karmakar, C., 2014. Forewarning incidence of American boll worm (Heliothis armigera H.) of cotton at Akola in Vidarbha region of Maharashtra. Mausam, 65:73-82.
Glick, P.A. and Graham, H.M., 1965. Seasonal light-trap collections of lepidopterous cotton insects in South Texas. J. econ. Ent., 58: 880-882. https://doi.org/10.1093/jee/58.5.880
GoP, 2010. Pakistan economic survey 2008-2009. Federal Bureau of Statistics, Government of Pakistan.
GoP, 2016. Pakistan economic survey 2014-2015. Federal Bureau of Statistics, Government of Pakistan.
Hasan, M., Sagheer, M., Khaliq, A., Khan, F.Z.A., Gul, H.T., Ahmad, K., Manzoor, S.A., Yasir, M., Javed, M. and Nadeem, M., 2013. Assessment of relative resistance in advanced rice genotypes in response to variation in abiotic factors and development of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Int. J. Biosci., 3: 33-38. https://doi.org/10.12692/ijb/3.12.33-38
Huckell and Lisa, W., 1993. Plant remains from the Pinaleño cotton cache, Arizona, Kiva. J. Southw. Anthropol. Hist., 59: 147–203. https://doi.org/10.1080/00231940.1993.11758236
Hussain, M., Akram, M., Abbas, Q., Ahmad, S., Babar, T.K. and Karar, H., 2014. Impact of environmental factors on the population dynamics of leaf hopper Amrasca biguttula biguttula Ishida (Homoptera: Jassidae) on various transgenic cotton genotypes in Multan. Acad. J. Ent., 7: 27-31.
Idris, A.B., and Hayat, F.S., 1997. Biological control of ∂iamondback moth, Plutella xylotella (L.), using parasitoids and Bacteria. Sains Malays., 26:79-94.
Izumi, Y., Anniwaer, K., Yoshida, H., Sonoda, S., Fujisaki, K. and Tsumuki, H., 2005. Comparison of cold hardiness and sugar content between diapausing and nondiapausing pupae of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Physiol. Ent., 30: 36-41. https://doi.org/10.1111/j.0307-6962.2005.00422.x
Kadam, J.R. and Khaire, V.M., 1995. Raining and relative humidity: key factors to suppress Earias vittella (Fabricius) infestation on ‘okra’ crop. J. entomol. Res., 19: 201-205.
Kalkal, D., Lal. R., Dahiya, K.K., Jat, B.L. and Kumar, A., 2014. Effect of abiotic factors on population dynamics of pink bollworm in relation to genetically modified cotton. J. Cotton Res. Develop., 28: 280-285.
Kavitam, B., Manisha, M., Nazaneen, S. and Nerendra, K., 2015. Impact of climate change on insect pests. Trends Biosci., 8:597-600.
Khaliq, A., Javed, M., Sohail, M. and Sagheer, M., 2014. Environmental effects on insects and their population dynamics. J. Ent. Zool. Stud., 2: 1-7.
Khan, B.S., Afzal, M. and Murtaza, M.A., 2002. Effect of abiotic factors against the infestation of pink bollworm (pectinophora gossypiella) on different nectarid and nectariless cotton varieties under unsprayed conditions. Pakistan J. agric. Sci., 39:338-340.
Kumar, D., Yadav, S.S., Saini, V.K. and Dahiya, K.K., 2016. Impact analysis of genetically modified (Bt) cotton genotypes on economically important natural enemies under field conditions. Adv. Ent., 4: 61-74. https://doi.org/10.4236/ae.2016.42008
Kumar, J. D., Singh, D. K. and Kumar, N. S., 2016. Impact of climate change on insect diversity and various other aspects of insect-pest interaction with host-plant (critical review). Adv. Life Sci., 5: 2009-2018.
Lepage, M.P., Bourgeois, G., Brodeur, J. and Boivin, G., 2012. Effect of soil temperature and moisture on survival of eggs and first-instar larvae of Delia radicum. Environ. Ent., 41: 159-165. https://doi.org/10.1603/EN10313
Mironidis, G.K. and Soultani, M.S., 2008. Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environ. Ent., 37:16-28. https://doi.org/10.1093/ee/37.1.16
Moulherat, C., Tengberg, M., Haquet, J.R.M.F. and Mille, B.T., 2002. First evidence of cotton at neolithic Mehrgarh, Pakistan: Analysis of mineralized fibres from a copper bead. J. Archaeol. Sci., 29: 1393. https://doi.org/10.1006/jasc.2001.0779
Pan, H., Liu, B., Lu, Y. and Desneux, N., 2014. Identification of the key weather factors affecting overwintering success of apolyguslucorum eggs in dead host tree branches. PLoS, 9:1-9. https://doi.org/10.1371/journal.pone.0094190
Parajulee, M.N., Shrestha, R.B. and Lesr, J.F., 2006. Influence of tillage, planting date, and Bt cultivar on seasonal abundance and within-plant distribution patterns of thrips and cotton fleahoppers in cotton. Int. J. Pest Manage., 52: 249–260. https://doi.org/10.1080/09670870600774240
Pazhanisamy, M. and Deshmukh, S.D., 2011. Influencing of weather parameters on pheromone trap catches of cotton bollworms. Rec. Res. Sci. Tech., 3: 136-139.
Prasad, Y.G. and Bambawale, O.M., 2010. Effects of climate change on natural control of insect pests. Indian J. Dry Land Agric. Res. Dev., 25: 1-12.
Pratheepa, M., Meena, K., Subaramaniam, K.R.M., Venugopalan, R. and Bheemanna, H., 2010. Seasonal population fluctuations of cotton bollworm, Helicoverpa armigera (Hübner) in relation to biotic and abiotic environmental factors at Raichur, Karnataka, India. J. Biol. Contr., 24: 47-50.
Purohit, D., Ameta, O.P. and Sarangdevot, S.S., 2006. Seasonal incidence of major insect pests of cotton and their natural enemies. Pestology, 12: 24-29.
Qureshi, Z.H., Bughio, A.R., Siddqui, Q.H. and Ahmad, N., 2009. Seasonal population fluctuation of pink bollworm, Pectinophora gossypiella (Saund.) (Lep., Gelechiidae) as monitored by gossyplure. J. appl. Ent., 98: 43-46. https://doi.org/10.1111/j.1439-0418.1984.tb02681.x
Qureshi, Z.A. and Ahmed, N., 1991. Monitoring seasonal population fluctuation of spotted and spiny bollworms by synthetic sex pheromones and its relationships to boll infestation in cotton. J. appl. Ent., 112: 171-175. https://doi.org/10.1111/j.1439-0418.1991.tb01043.x
Ragab, M.G., El-Sayed, A.A.A. and Nada, M.A., 2014. The effect of some biotic and abiotic factors on seasonal fluctuations of Helicoverpa armigera (hub.). Egypt. J. agric. Res., 92: 101-118.
Reddy, G.V.P., Shi, P., Hui, C., Cheng, X., Ouyang, F. and Ge, F., 2015. The seesaw effect of winter temperature change on the recruitment of cotton bollworms Helicoverpa armigera through mismatched phenology. Ecol. Evol., 5: 5652–5661. https://doi.org/10.1002/ece3.1829
Reiter, P.W., 1989. Vector biology and arborial recrudescence. In: The Arboiruses: Epidemiology and ecology, Vol. 1 (ed. T.P. Monath), CRC Press Inc., Boca Raton, Fl. pp. 245.
Sarate, P.J., Tamhane, V.A., Kotkhar, H.M., Ratnakaran, N., Susan, N., Gupta, V.S. And Giri, A.P., 2012. Developmental and digestive flexibilities in the midgut of a polyphagous pest, the cotton bollworm, Helicoverpa armigera. J. Insect Sci., 12: 1-16. https://doi.org/10.1673/031.012.4201
Satti, A., 2012. combating agricultural pests and diseases through cultural means. The Experiment, 5: 304-314.
Schmutterer, H., 1990 Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Annu. Rev. Ent., 35:271-297. https://doi.org/10.1146/annurev.ento.35.1.271
Sharma, H.C., 2016. Climate change vis-a-vis pest management conference on national priorities in plant health management. February 4-5.
Sisterson, M.S. and Tabashnik, B.E., 2004. Arthropod abundance and diversity in Bt and Non-Bt cotton fields. Environ. Ent., 33: 921-929. https://doi.org/10.1603/0046-225X-33.4.921
Tripathi, C.P.M. and Pandey, A.K., 2008. Effect of temperature on the development, fecundity, progeny sex ratio and life-table of Campoletis chlorideae, an endolarval parasitoid of the pod borer, Helicoverpa armigera. Bio-Control, 53: 461. https://doi.org/10.1007/s10526-007-9083-3
Venette, R.C., Naranjo, S.E. and Hutchison, W.D., 2000. Implications of larval mortality at low temperatures and high soil moistures for establishment of pink bollworm (Lepidoptera: Gelechiidae) in Southeastern United States cotton. Environ. Ent., 29: 1018-1026. https://doi.org/10.1603/0046-225X-29.5.1018
Wu, K.M., Lu, Y.H., Feng, H.Q., Jiang, Y. and Zhao, J., 2008. Suppression of cotton bollworm in multiple crops in china in areas with Bt toxin–containing cotton. Science, 321: 1676-1678. https://doi.org/10.1126/science.1160550
Zafar, K., Sohail, A., Arshad, M. and Arif, J., 2013. Impact of weather factors on population fluctuation of H. armigera on sunflower. Pakistan J. Nutr., 1: 50-54. https://doi.org/10.3923/pjn.2013.50.54
To share on other social networks, click on any share button. What are these?










