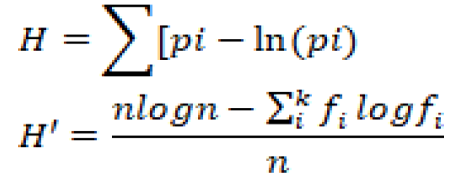Diversity and Abundance of Mite Species in Citrus Orchards of Sargodha, Pakistan
Diversity and Abundance of Mite Species in Citrus Orchards of Sargodha, Pakistan
Muhammad Afzal1, Muhammad Irfan Ullah1*, Muhammad Hamid Bashir2, Shah Najaf Mukhtar1, Muhammad Arshad1, Nimra Altaf1
1Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan.
2Department of Entomology, University of Agriculture, Faisalabad, 38000, Faisalabad, Pakistan.
Abstract | Mites are widely distributed throughout the world, and some species are important pests of fruit crops, field crops, vegetables, and weeds. In the present study, extensive sampling was done to assess mites’ biodiversity in different citrus orchards of Kinnow, Musambi, and Feutrell’s early at tehsil Sahiwal Sargodha, Sillanwali, and Bhalwal of district Sargodha. Sampling was done at fifteen days interval to record the population dynamics and identification of citrus mites during 2018. A total 37,963 mites specimens were collected in which nine species were identified from four families Cunaxa sp. (Cunaxidae); Amblyseious andersoni Chant, Euseius sp., Neoseiulus sp. (Phytoseiidae); Brevipalpus californicus Banks, B. phoenicis (Geijskes) (Tenuipalpidae), and Eutetranychus orientalis Klein, Panonychus citri (McGregor), Tetranychus urticae Koch (Tetranychidae). Brevipalpus sp., T. urticae and B. californicus were the dominant species. While Neoseiulus sp. and E. orientalis were less abundant species. Maximum numbers of mite specimens (12056 numbers) were collected from tehsil Sargodha, while minimum specimens (6499 numbers) were recorded from tehsil Sahiwal. The results are discussed concerning the abundance of mites that are the major citrus pests and dominant predacious mite species as candidates for biological control of various arthropods in Sargodha citrus.
Novelty Statement | To the best of our knowledge, no comprehensive study has been conducted before on mite diversity in Sargodha. The study would help run proper management practices to control the losses caused by mites.
Article History
Received: January 02, 2021
Revised: March 03, 2021
Accepted: March 28, 2021
Published: June 09, 2021
Authors’ Contributions
MA and MHB designed, supervised, and proof read the manuscript. SNM and MIU conducted research, recorded data, drafted manuscript and analyzed the data. MA and SNM executed the experiment, collected data and wrote the manuscript. NA helped in execution of the experiment and data collection.
Keywords
Mites, Biodiversity, Species richness, Species evenness
Corresponding author: Muhammad Irfan Ullah
muhammad.irfanullah@uos.edu.pk
To cite this article: Afzal, M., Ullah, M.I., Bashir, M.H., Mukhtar, S.N., Arshad, M. and Altaf, N., 2021. Diversity and abundance of mite species in citrus orchards of Sargodha, Pakistan. Punjab Univ. J. Zool., 36(1): 37-46. https://dx.doi.org/10.17582/journal.pujz/2021.36.1.37.46
Introduction
Citrus is one of the most produced fruit crop globally, and Pakistan ranked the sixth position in the world’s citrus production. Pakistan’s citrus production is mostly concentrated in the Sargodha district of Punjab State. Kinnow mandarins are the largest exportable commodity among all citrus cultivars contributing about 95% of Pakistan’s export (GOP, 2018).
Citrus orchards are attacked by several pests, including mites (Acari). Mites are responsible for causing yield reduction in citrus and lessen the quality of the fruit by forming blemishes, and ultimately, the export of citrus fruit decreases (da Encarnação Bobot et al., 2011). The phytophagous mites reduce citrus production by forming injuries substantially, especially when the environmental conditions become favorable (Dhooria and Butani, 2005). Seven thousand phytophagous mites species are well known worldwide, which fall under five important families; Tetranychidae, Tenuipalpidae, Tarsonemidae, Eriophyidae, Tuckerillidae (Childers et al., 2007). Tenuipalpidae is an essential family of mites, mostly referred to as the false spider mites or flat mites. Tropical and subtropical climates are mostly suited for these mites (Gerson, 2008). Spider mites are considered most important among this group due to their severe pest status and significant involvement in the spread of plant viruses serving as vectors of diseases (Chagas et al., 2003; Kitajima et al., 2003a, b; Kondo et al., 2003; Rodrigues et al., 2003). Another critical group of mites is the Tetranychids, and the members of this species suck the sap from the upper surface of leaves, which results in chlorosis and low growth of the plant. The damage by a two-spotted spider mite, Tetranychus urticae Koch, 1836 happens by developing the web and numerous colonies on the lower leaf surface (Aucejo et al., 2003; Martinez- Ferrer et al., 2006). Citrus rust mite, Phyllocoptruta oleivora (Ashmead, 1879) (Acari: Eriophyidae) is also an important pest of citrus crop globally. Excessive fruit damage by the feeding of rust mite results in severe russeting of the outer skin of fruits, which results in reduced quality and decreased yield of the crop (Qureshi et al., 2020).
Mite population can be significantly synchronized by the environmental conditions, especially the temperature and rainfall. Low rainfall and high temperature encourage mite production (Choudhury et al., 2006). Other than climatic conditions, crop management could also affect the diversity of mite species. For example, pesticides may negatively affect mites’ diversity (Meyer et al., 2009). Damage and yield losses by mites can be predicted based on their regular scouting to develop efficient control strategies (Qureshi et al., 2020). The density of mites depends significantly upon the infestation, season, and available hosts’ nature (Di Sabatinol et al., 2010).
In Pakistan, citrus growers are unaware of these minute creatures, and limited research is available on the biodiversity of mite species in citrus orchards of Pakistan. There is a dire need to identify mite species associated with citrus cultivars so that proper management practices can be developed to control the losses and to improve the quality and quantity of citrus fruits. So, the main objective of this study was to evaluate the diversity of mite species in different citrus cultivars from four tehsils of district Sargodha, Pakistan.
Materials and Methods
Citrus orchards
The research work was conducted in four tehsils (Sargodha, Sahiwal, Bhalwal, and Sillanwali) of district Sargodha (Figure 1). The survey was conducted during March-December 2018 for the collection and identification of mites. From each location, one orchard of about 1 acre of each cultivar was selected. The average plants per acre of Kinnow were 104.8 plants; Musambi 89.4 plants and Feutrell’s early 96.9 plants.
Sampling of mites
A sampling of mites was done randomly through each citrus orchard. Three plants of about the same age and height were selected randomly to collect the mite population from each orchard. Sampling was done at biweekly interval. Sampling was carried out using two methods: (i) branch shaking/spot shaking and (ii) randomly collected leaves and fruits. Each plant was divided into four quadrates, and one branch from each quadrate was trembled. A white paper was placed under the branch to see the mites on the paper after shaking. After a few seconds, the mite specimen starts to move, which were easily observed under bright sunlight. The mite specimens were collected and preserved in tubes containing 70% ethyl alcohol. The specimens on the page were collected with a moist camel hair brush, and magnifying glass was used to view the minute creatures. The second sampling method was done randomly collecting twenty leaves and 3-5 damaged fruits of the selected tree from each quadrate. Mites on fruits were individually removed with a brush under a stereomicroscope and placed in 70% alcohol. The samples were kept in plastic bags and transported to the laboratory of Department of Entomology, University of Sargodha. The samples were stored at 4 oC temperature for further identification.
Identification of mites
The permanent slides of collected mite samples were prepared by using Hoyer’s Medium. The collected specimens were mounted using the prepared medium on the glass slides and kept for drying in the slides racks for fifteen days. The mites were observed under a stereomicroscope and identified using taxonomic keys (Tuttle and Baker, 1964; Bashir and Afzal, 2005; Vacante, 2009, 2010). Specimens were also deposited as a reference collection at the laboratory of China-Pakistan Joint Research Center, Department of Entomology, University of Sargodha and Department of Entomology, University of Agriculture, Faisalabad.
Data analysis
Data for species identity and the total number of mites found on each citrus cultivar at four different locations were analyzed. Following formulas were used to calculate the species richness and evenness, dominance, Simpson’s diversity index, and Shannon Wiener diversity:
Shannon’s diversity index was calculated as;
Where; H= Shannon’s diversity index; Pi= Fraction of total population belonging to species i; S= Number of species encountered; Σ= Sum of species from 1 to species N. Maximum diversity was calculated as;
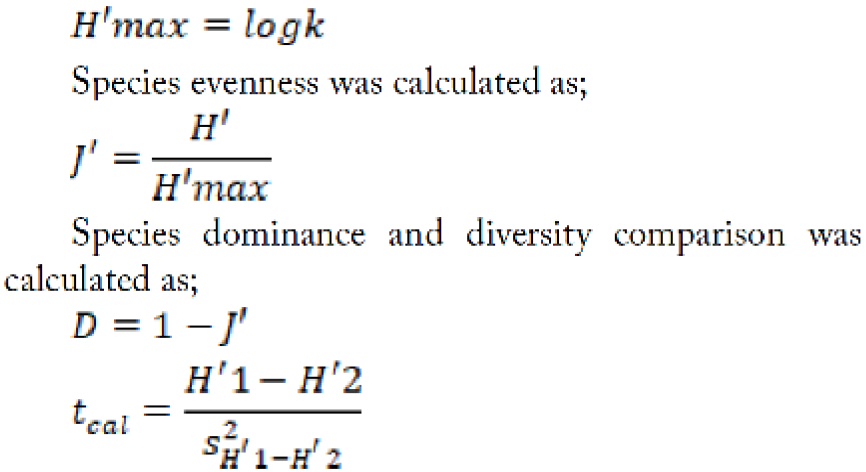
Results and Discussion
Identification of mites species
Overall, nine mite species from four different families; Cunaxa sp. (Cunaxidae); A. andersoni, Euseius sp., Neoseiulus sp. (Phytoseiidae); B. californicus B. phoenicis (Tenuipalpidae), and E. orientalis, P. citri, T. urticae (Tetranychidae) were identified (Table 1).
Table 1: Detail of families and species of mites identified in this study.
|
Family |
Species |
|
Cunaxidae |
Cunaxa sp. |
|
Phytoseiidae |
Amblyseious andersoni |
|
Euseius sp. |
|
|
Neoseiulus sp. |
|
|
Tenuipalpidae |
Brevipalpus californicus |
|
Brevipalpus phoenicis |
|
|
Tetranychidae |
Eutetranychus orientalis |
|
Panonychus citri |
|
|
Tetranychus urticae |
Family cunaxidae baker and hoffmann, 1948
Diagnostic features
Cunaxidae includes more than 330 species that belongs to 27 genera (Den Heyer, 2011). They have five segmented padipalps, spine-like setae on telofemur, attenuate tarsi, and dorsal shield not reticulated. In gnathosoma, five segmented palps are present. Trochanter having no setae, basifemur with simple dorsomedial seta, inner anterior surface of telofemur having one uncinated apophyses and outer surface with simple dorsolateral seta. It has a small claw (Den Heyer and Sergeyenko, 2009; Skvarla et al., 2014). Their family members are free-living, and most of them are a predator in nature and feed on nematodes, scale insects, spider mites, and other small arthropods (Skvarla et al., 2014). Some of their species also feed on the honeydew produced by their host plants. Heyden first defined genus Cunaxa in 1826 with one type of species, Scirus setirostris Hermann 1804 (Heyden, 1826).
Only Cunaxa sp. were identified from the Cunaxidae family
Cunaxa sp. (Figure 2a)
Description: The body length is 637 µm in length (without gnathosoma) and 401 µm in width. Gnathosoma is 352 µm long. Hypostome cone-shaped at distal side having four pairs of hypognathal setae and two pairs of adoral setae. Palp 350 µm and five segmented. Chelicera terminates in a claw and 150 µm long with lobes present on its dorsal and ventral sides and having one simple dorsolateral seta. Sub-rectangular shield present on propodosoma originated from gnathosoma base and extended towards anterior region of hysterosoma and dotted striae separate both. Venters have dotted striations. Dotted lobes present on legs and I-IV tarsi slender, attenuated, and long (Bashir et al., 2005).
Economic importance: This mite species is a predator in nature and feed on many other harmful mites. It also feeds on nematodes, scale insects, spider mites, and other small arthropods and it is being produced and commercially available as biological control of insect pests world-wide (Bashir et al., 2005).
Distribution: It is distributed towards Africa, Asia and European regions (Den Heyer et al., 2011).
Species examined: This species was observed from all cultivars of sampling sites except tehsil Sargodha in Musambi cultivar.
Family Phytoseiidae, Berlese 1916
Diagnostic features
Their bodies are highly sclerotized and pyriform and color vary in different species from white to brown. Males of this family are slightly smaller than their females, with a body length of 300 µm. Their body is divided into two parts; gnathosoma and idiosoma. Gnathosoma has a dual purpose as it is used for censoring and helps prey capture and ingestion. It bears chelicerae, stylophore and sensorial palps. Chelicerae constituted a variable number of teeth. Idiosoma is covered with three dorsal and ventral shields in females and two in males. Diagnostic of Phytoseiidae species is based on their female morphological characters, i.e., legs, setal shape, cheliceral dentition, and spermatheca shape (McMurtry et al., 1992).
Following species of Phytoseiidae were identified:
1. Amblyseious andersoni Chant, 1957 (Figure 2b)
Description: Its dorsum length is about 0.3-0.4 mm, and the body is smooth with few reticulations. It has 17 pairs of serrated setae, which are longer and have one pair of lateral setae. Three pairs of setae present on the sternal plate and three macrosetae on the fourth leg. It lay about 30-40 eggs and completes its generation in just 2-3 weeks (Swirski et al., 1967).
Economic importance: It is a predatory mite and feeds on pest mites, whiteflies, thrips and citrus psyllids. It can also survive on non-living diets and is unaffected by insect growth regulators applied to control other pests (Juan-Blasco et al., 2012).
Distribution: This species was found in the Middle Eastern part of the world. It is now cosmopolitan due to its commercial use.
Species examined: This predatory species was observed from cultivars of tehsil Sargodha and Bhalwal only.
2. Euseius sp. (Figure 2c)
Description: Its dorsum is reticulated and about 0.3 mm in length. Two pairs of setae present on the lateral body side, and 17 pairs of sub-equal setae are also present; the fourth leg with single macroseta. The optimal temperature for this mite is 25 °C, and at this temperature, its female lay about 40 eggs, and generation completes in 3-4 weeks (Abbassy et al., 2012).
Economic importance: It has great economic importance because of its predatory nature. It feeds on many pest species of mite like T. urticae, P. citri, E. orientalis and also feed on whiteflies (Kasap and Sekeroglu, 2004).
Distribution: It is distributed in Turkey, Egypt, Australia, Morroco, America and some parts of South Asia (Abbassy et al., 2012).
Species examined: This species was observed from all cultivars of selected sampling sites.
3. Neoseiulus sp. (Figure 2d)
Description: It is a predatory mite, and the body color of non-feeding mites is yellow and feeding mites often contains the color of their prey. Its dorsum is 0.5-1.0 mm long and mostly smooth or little sclerotized. There are some reticulations on the body and it has 17 pairs of short and nude setae. Single macroseta on the fourth leg and three pairs of setae on the sternal plate. The optimum temperature is 25 °C, and the female lays about 30 eggs. It completes generation in 1-2 weeks (Sarwar, 2016).
Economic importance: These mites feed on several major pests like thrips, citrus psyllid and other pest species of mites. It is being produced and sold worldwide by many companies (Zhang et al., 2001; Arthurs et al., 2009).
Distribution: It is distributed from European to the Middle Eastern parts of the world (Jafari et al., 2012).
Species examined: This predatory species was observed from all cultivars of sampling sites except Tehsil Sargodha and Bhalwal in Feutrell’s early cultivar.
Family tenuipalpidae, Berlese 1913
Diagnostic features
Macroscopically these are confused with the mites belongs to Tetranychidae because of their orange to red body color. They are sometimes yellowish-red, green, reddish-green, or reddish-black formed by gnathosoma and an idiosoma (Childers et al., 2003). Their body is somewhat dorso-ventrally flattened and 200-400 μm in length with small ridges and reticulations on the body. They are slow-moving mites. They do not produce silk webbing. That is why called as “false spider mites”. Few species have damaging effect to citrus orchards of the world, such as Central and Southern America (Childers et al., 2001). The tenuipalpids are mostly regarded as secondary plant pests but recent studies also exploited these acts as a vector of plant viruses (Gerson, 2008).
Following species of Teuipalpidae were identified:
1. Brevipalpus californicus Banks, 1904 (Figure 2e)
Description: These are commonly known as a flat citrus mite. These phytophagous mites have more than 300 host plants and responsible for transmitting diseases pathogen. It is a carrier of citrus leprosis disease. These minutes species are red, about 0.25-0.30 mm long, have an oblong, flattened body covered with various reticulation dorsally. It also consists of short setae on opisthosoma laterally and sensory setae on tarsus (Childers et al., 2003).
Economic importance: These are the pest of sweet oranges, valencia oranges, mandarins, specifically lime, and bitter oranges. Feeding causes corky blemishes, yellowish-brown scab like spots on the fruit peel, which degrade the fruit quality. In severe attack, leaf drop occurs. These phytophagous mites have more than 300 host plants and responsible for transmitting viral pathogen diseases. It is a vector of citrus leprosis disease.
Distribution: It is widely distributed throughout the world so-called as cosmopolitan species.
Species examined: This species was observed from all cultivars of selected sampling sites except Musambi plants in tehsil Sahiwal.
2. Brevipalpus phoenicis Geijskes, 1936 (Figure 2e)
Description: It is commonly known as a reddish-black flat mite. Its body is oblong, about 0.25- 0.30 mm long, and red. Dorsum show variability and is covered with irregular, dark red patterns and rounded striae with no reticulations. The second tarsus has two sensory setae and opisthosoma bears six pairs of setae on the lateral side. The cycle completes in three weeks at 27°C. Its developmental rate depends upon the host plant. They prefer to aggregate on the lower sides of the mature leaves around the pedicle, the button of fruits, and invaginations of leaves (Beard et al., 2015).
Economic importance: During their feeding on plants, they inject toxins inside host tissues that causes a variety of losses to the plant. In citrus, their damage causes loss in weight of fruit and overall 50 % loss in yield. Affected oranges show reddish color round patches. At high infestation, it causes brownish leaves and they become dry and drop. It also acts as a vector of many plant viruses such as leprosis. It causes greater economic losses by reducing the plant yield and plants may also die due to high infestation (Childers and Derrick, 2003).
Distribution: It is distributed in Egypt, South-Eastern Asia, the Mediterranean region, East Asia, Brazil, Africa, and some Middle East parts (Childers et al., 2003).
Species examined: This species was observed from all cultivars of selected sampling sites.
Family tetranychidae donnadieu, 1875
Diagnostic features
Mites of this family tend to make webs and are mostly known as spider mites. These mites typically are phytophagous in nature and cause damage to cultivated plants and other economic important crops. Most of their species are polyphagous. This family includes 1250 species under 73 genera and is distributed throughout the world (Migeon and Flechtmann, 2004). Their body is soft and round with 350-1000 μm in length. They may be red, green, orange, or yellow formed from gnathosoma and idiosoma. About 60 species of Tetranychidae have been recorded on citrus plants from different world regions and had damaging effects (Vacante, 2010).
Following species of Tetranychidae were identified:
1. Eutetranychus orientalis Klein, 1936 (Figure 2g)
Description: It is commonly known as oriental spider mite or spider mite. These microscopic specimens are phytophagous in nature and have long legs and an oval body. Females are reddish-brown, about 0.5 mm, while the males are smaller with pointed hyteronotum and slightly lighter in physical appearance and short dorsal setae on the body. It completes 10-12 generations per annum (Khanjani et al., 2017).
Economic importance: Cell death in patches on leaves observes and loss of pigmentation on fruits due to high population.
Distribution: This mite specimen is reported from eastern Mediterranean countries, in Asia from mostly from Pakistan, India and China, and also, from Africa.
Species examined: This species was observed from all cultivars of sampling sites except tehsil Sillanwali and Feutrell’s early cultivar in Bhalwal.
2. Tetranychus urticae Koch, 1836 (Figure 2h)
Description: It is commonly known as two-spotted spider mites due to two darken exact dorso-lateral spots. The body color of this phytophagous species is greenish which mostly darkens after feeding. The species body size ranges from 0.4-0.5mm. The male specimens are smaller and narrower in size than females and some females are reddish, commonly known as red spider mite or carmine spider mite. It has 9-11 generations recorded per annum.
Economic importance: Chlorosis on leaves and fruits, brown spots and webbing appeared due to high infestation of mite attack (Auger et al., 2013).
Distribution: This species is distributed throughout the world because it has various host plants, including citrus, vegetables, cotton and tomatoes (Hazan et al., 1973).
Species examined: This species was observed from all cultivars of sampling sites except Kinnow cultivar in tehsil Sahiwal.
3. Panonychus citri McGregor, 1916 (Figure 2i)
Description: It is commonly known as red mite because of red appearance throughout the life cycle. Its body is covered with setae. Claw like empodium is present on tarsi with ventrally directed three pairs of hairs. There are 13 pairs of long and prominent setae present on its dorsum. Female lays about 40 eggs at 26°C and generation completes in two weeks. Its population becomes numerous during early winter and less abundant in summer (Jeppson et al., 1975).
Economic importance: It’s feeding causes stippling spots on leaves that give silvery or yellow appearance. At high infestation, the leaves may drop, loss in fruit quality, twigs dieback, and sometimes, the tree also dies due to its damage (McMurtry et al., 1979).
Distribution: Red mite occurs wherever citrus is grown. It is a major pest in South Africa, California, and Japan. It is distributed worldwide.
Species examined: This species was observed from all cultivars of sampling sites except tehsil Sillanwali and Feutrell’s early cultivar in Tehsil Bhalwal.
Biodiversity of citrus mites in district Sargodha
Maximum richness (9) on Kinnow mandrins was recorded in two tehsils; Sargodha and Bhalwal, while minimum richness (6) was observed in Sillanwali. Similarly, a maximum Shannon Wiener diversity index (2.108) was observed in Sargodha, while Sillanwali showed a minimum diversity index (1.310). Evenness (J’) was higher (0.960) in the Sargodha location, followed by Bhalwal (0.906) and minimum evenness was found (0.731) in Sillanwali. Full dominance (D’) was observed in Sillanwali which was 0.269 and minimum (0.040) dominance was found in Sargodha. Sargodha showed higher Simpson’s diversity (0.868) while Sillanwali showed least value of Simpson’s diversity index (0.794) in Kinnow orchard. Maximum richness (9) of mite species on Musambi plantations was recorded in Tehsil Bhalwal, while minimum richness (6) was observed in tehsil Sillanwali. Shannon Wiener diversity index was recorded maximum (1.92) in Sargodha. Sargodha also showed maximum (0.927) evenness (J’), followed by Bhalwal. Maximum dominance (D’) of species was found in Sillanwali, which was 0.814 and minimum (0.073) dominance was shown by Sargodha. Sargodha showed maximum Simpson’s diversity (1.94) while Sillanwali showed minimum index (1.59) in Musambi plantations. In Feutrell’s early, maximum richness (8) was recorded in tehsil Sargodha, while minimum richness (6) was observed in both tehsil Sillanwali and Bhalwal. Shannon Wiener diversity index was recorded maximum (1.94) in Sargodha. Bhalwal showed maximum (0.987) evenness (J’), while minimum (0.89) evenness was observed in Sahiwal.
Maximum dominance (D’) of species was found in Sahiwal which was 0.111 and minimum (0.067) dominance was recorded in Sargodha. Sahiwal showed maximum Simpson’s diversity (0.853), and minimum Simpson’s diversity index (0.77) was recorded in the Sillanwali location (Table 2).
Table 2: Diversity index of mite species in three citrus cultivars from different locations of district Sargodha during March-December 2018.
|
Location R Hʹ Hʹ max. Jʹ Dʹ Simpson’s |
|||||||
|
Kinnow |
|||||||
|
Sahiwal |
7 |
1.678 |
1.946 |
0.863 |
0.137 |
0.835 |
|
|
Sargodha |
9 |
2.108 |
2.197 |
0.960 |
0.040 |
0.868 |
|
|
Sillanwali |
6 |
1.310 |
1.792 |
0.731 |
0.269 |
0.794 |
|
|
Bhalwal |
9 |
1.989 |
2.197 |
0.906 |
0.094 |
0.836 |
|
|
Musambi |
|||||||
|
Sahiwal |
7 |
1.331 |
1.946 |
0.684 |
0.316 |
1.729 |
|
|
Sargodha |
8 |
1.927 |
2.079 |
0.927 |
0.073 |
1.940 |
|
|
Sillanwali |
6 |
0.332 |
1.792 |
0.186 |
0.814 |
1.598 |
|
|
Bhalwal |
9 |
1.813 |
2.197 |
0.825 |
0.175 |
1.767 |
|
|
Feutrell’s early |
|||||||
|
Sahiwal |
7 |
1.729 |
1.946 |
0.889 |
0.111 |
0.853 |
|
|
Sargodha |
8 |
1.941 |
2.079 |
0.933 |
0.067 |
0.847 |
|
|
Sillanwali |
6 |
1.599 |
1.792 |
0.892 |
0.108 |
0.770 |
|
|
Bhalwal |
6 |
1.768 |
1.792 |
0.987 |
0.013 |
0.825 |
|
Hʹ= Shannon’s diversity index, Hʹ max. = maximum diversity, Dʹ = species dominance, Jʹ = species evenness.
Citrus orchards are highly infested with citrus mites that are considered severe pests worldwide (Xiao and Fadamiro, 2010). They damage citrus fruit and leaves and form blemishes responsible for export loss (Vacante, 2010). Export of citrus in Pakistan has been declining due to various factors in which mites were considered a potential pest (Bakar et al., 2016). Their presence is much annoying and always causes a threat to citrus production. Diversity and distribution of citrus mites were determined on citrus
Table 3: List of citrus mites in relation to different tehsils of district Sargodha.
|
Sr# |
Species |
Sahiwal |
Sargodha |
Sillanwali |
Bhalwal |
All Sites |
|
1 |
Amblyseious andersoni Pr |
0 |
1585 |
0 |
951 |
2536 |
|
2 |
Brevipalpus californicus Pe |
713 |
1530 |
1357 |
2426 |
6026 |
|
3 |
Brevipalpus phoenicis Pe |
1886 |
2004 |
1923 |
1727 |
7540 |
|
4 |
Cunaxa sp. Pr |
621 |
797 |
391 |
765 |
2574 |
|
5 |
Eutetranychus orientalis Pe |
142 |
577 |
0 |
340 |
1059 |
|
6 |
Neoseiulus sp. Pr. |
256 |
594 |
597 |
517 |
1964 |
|
7 |
Panonychus citri Pe |
743 |
1147 |
0 |
396 |
2286 |
|
8 |
Phyllocoptruta oleivora Pe |
1191 |
1276 |
956 |
1322 |
4745 |
|
9 |
Tetranychus urticae Pe |
304 |
1888 |
1919 |
2935 |
7046 |
|
10 |
Un-Identified |
643 |
658 |
307 |
579 |
2187 |
|
|
N |
6499 |
12056 |
7450 |
11958 |
37963 |
varieties, including Kinnow, Feutrell’s early and Musmabi in tehsils Sahiwal, Sargodha, Sillanwali and Bhalwal.
During field visits, a total of 37,923 specimens of citrus mites were collected. Nine species (Cunaxa spp; Cunaxidae), (A. andersoni, Euseius spp., Neoseiulus spp.; Phytoseiidae), (B. californicus, B. phoenicis; Tenuipalpidae), and (E. orientalis, P. citri, T. urticae; Tetranychidae) of mites from four different families were identified in the laboratory. Mite species occurrence can be correlated with Denmark’s (1984) documentation who reported three species of mites, including B. californicus, B. phoenicis and B. obovatus found abundantly in citrus fields. These mite species are also abundant in citrus fields in different regions of Asia (Jeppson et al., 1975). Results can also be compared by the findings of Ledesma et al. (2011), whose work showed the presence of T. urticae, P. citri and E. orientalis abundantly on different citrus cultivars.
Our findings showed that B. phoenicis was most abundant citrus mite species in district Sargodha while E. orientalis was found to be the least abundant in this region. Among different tehsils, all of the identified species (9) were found in Sargodha, while the least number of species (6) was found in tehsil Sillanwali. Population dynamics of different mite species were recorded from tehsils of district Sargodha. The maximum population of mites were collected in Sargodha (12056) followed by Bhalwal (11958) and Sillanwali (7450), while the minimum population was found in Sahiwal (6499) (Table 3). The most important species of Brevipalpus reported on citrus plants globally are; B. amicus Chaudhri, B. californicus (Banks), B. chilensis Baker, B. lewisi (McGregor), B. obovatus Donnadieu, B. mcgregori Baker, and B. phoenicis (Geijskes) (Baker and Suigong, 1988). Brevipalpus mites are parthenogenetic (thelytokous). B. phoenicis is an efficient in vectoring leprosis disease (Chiavegato and Salibe, 1984). The transmission efficiency is higher in the larval stage than adult stages (Chagas et al., 1984). Once a mite infects the virus, it continues vectoring the disease in successive instars (transstadial transmission) (Rodrigues, 2000). Tetranychus urticae was also abundant in all selected locations of Sargodha district. Both immature and adult stages feed on older leaves of citrus plants inserting their stylets into the tissue, tiny silvery spots or yellowing of leaves occur which turn brown later (Hoque et al., 2010). In severe infestations, the leaf becomes curl, reduction in chlorophyll and photosynthesis occurs, and plant vigor decreases. Phyllocoptruta oleivora also known as citrus rust mite infests plants of genera Citrus and Fortunella. It infests leaves, branches and fruits, causing fruit rind russeting, which leads to yield losses and deteriorates the fruit quality making them unfit for export (Sarada et al., 2018).
Amblyseious andersoni was present in only Sargodha and Bhalwal tehsils. However, no specimen was recorded from Sahiwal and Sillanwali (Supplementary Table S1). Amblyseious andersoni is a generalist predator and can feed on various mite and insect species (Jaworski, 2000; Duso and Pasini, 2003). Cunaxa sp. and Neoseiulus sp. were also present in all localities of the Sargodha district. The members of both Cunaxa and Neoseiulus are important predators on a variety of prey, including spider mites, scale insects, and other small arthropods (Döker et al., 2017; Fonseca et al., 2020).
The geographical distribution plays a vital role in the occurrence of economically important pests. Different predator and pest population include in Acarina fauna of citrus trees. Biodiversity parameters for Kinnow concluded that maximum richness (9) of citrus mites was recorded in tehsil Sargodha and Bhalwal with minimum richness (6) in Sillanwali. Similarly, other parameters, including Shannon Wiener diversity index, evenness, and Simpson’s diversity, were recorded maximum in Sargodha tehsil. From nine different citrus orchards, 29 species of mites were identified belonging to 9 different families by Fadamiro et al. (2009). Variation in abundance of mite species across different localities could be due to climatic conditions and types and numbers of insecticides application in the orchard (Meena et al., 2013).
Conclusions and Recommendations
Conclusively, our study reported 9 species of mites from four different families in Sargodha citrus. Among the predatory mites, Cunaxa sp. from the family Cunaxidae, A. andersoni, and Neoseiulus sp from the family Phytoseiidae were recorded. The B. californicus and B. phoenicis from Tenuipalpidae family, and E. orientalis, P. citri and T. urticae from Tetranychidae family were identified that are important pests of citrus plants. Overall, mite species were more abundant in tehsil Sargodha, while the lowest abundance was recorded in tehsil Sahiwal.
Acknowledgments
The authors acknowledge the provision of funds under the UOS-ORIC project (project no. UOS/ORIC/2016/09) for this research work.
Conflict of interests
The authors have declared no conflict of interest.
References
Abbassy, M.R., Hendy, H.H., Mowafi, M.H., and Nawar, M.A., 2012. Biology of Euseius scutalis (Acari: Phytoseiidae) on Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae) at different temperatures. Acarines., 6: 15-19. https://doi.org/10.21608/ajesa.2012.163618
Arthurs, S., McKenzie, C.L., Chen, J., Dogramaci, M., Brennan, M., Houben, K., and Osborne, L., 2009. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol. Cont., 49: 91-96. https://doi.org/10.1016/j.biocontrol.2009.01.002
Aucejo, S., Foo, M., Gimeno, E., Gomez, A., Monfort, R., Obiol, F., and Tirado, V., 2003. Management of Tetranychus urticae in citrus in Spain: acarofauna associated to weeds. IOBC Press Bull., 26: 213-220.
Auger, P., Migeon, A., Ueckermann, E. A., Tiedt, L., and Navarro, M.N., 2013. Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): Review and new data. Acarologia, 53: 383-415. https://doi.org/10.1051/acarologia/20132102
Bakar, M.A., Aqueel, M.A., Sohail, M., Raza, A.B.M., Afzal, M., Tayyab, M., and Arshad, M., 2016. Influence of weather factors on the seasonal abundance of citrus mite Eutetranychus orientalis (Klein) on different citrus cultivars. J. Entomol. Zool. Stud., 4: 105-111.
Baker, E.W., and Suigong, Y., 1988. A catalog of the false spider mites (Tenuipalpidae: Acari) of the United States. Int. J. Acarol., 14: 143-155. https://doi.org/10.1080/01647958808683507.
Bashir, M.H., Afzal, M., and Ali, S., 2005. Description of a new cunaxid mite Cunaxa reticulatus (Acari) from Pakistan. Pak. Entomol., 27: 57-60.
Bashir, M.H., and Afzal, M., 2005. New cunaxid mites of the genus Armascirus from Punjab, Pakistan. Pak. J. Agric. Sci., 42: 117-122
Beard, J.J., Ochoa, R., Braswell, W.E., and Bauchan, G.R., 2015. Brevipalpus phoenicis Geijskes species complex (Acari: Tenuipalpidae) a closer look. Zootaxa, 3944: 1-67. https://doi.org/10.11646/zootaxa.3944.1.1
Chagas, C.M., Kitajima, E.W., and Rodrigues, J.C.V., 2003. Coffee rings pot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) in coffee. Exp. Appl. Acarol., 30: 203-213. https://doi.org/10.1023/B:APPA.0000006549.87310.41
Chagas, C.M., Rossetti, V., and Chiavegato, L.G., 1984. Effectiveness of the different life stages of Brevipalpus phoenicis (Geijskes) on leprosis transmission. Proc. 9th Conf. Int. Org. Citrus Virol. Riverside, CA, USA. pp. 211-214.
Chiavegato, L.G., and Salibe, A.A., 1984. Transmissibility of leprosis symptoms by Brevipalpus phoenicis to young citrus plants under laboratory conditions. Int. Org. Citrus Virol. Conf. Proc., 9: 1957-2010.
Childers, C.C., and Derrick, K.S., 2003. Brevipalpus mites as vectors of unassigned rhabdoviruses in various crops. Exp. App. Acarol., 30: 1-3. https://doi.org/10.1023/B:APPA.0000006542.96404.63
Childers, C.C., Kitajima, E.W., Welbourn, W.C., Rivera, C., and Ochoa, R., 2001. Brevipalpus mites on citrus and their status as vectors of citrus leprosis. Manejo Integrado de Plagas (Costa Rica)., 60: 66-70.
Childers, C.C., McCoy, C.W., Nigg, H.N., Stanly, P.A., and Rogers, M.E., 2007. Florida citrus pest management guide: Rust mites, spider mites and other phytophagous mites. ENY-603, UF, University of Florida, IFAS Extension.
Childers, C.C., Rodrigues, J.C.V., and Welbourn, W.C., 2003. Host plants of Brevipalpus californicus, B. obovatus, and B. phoenicis (Acari: Tenuipalpidae) and their potential involvement in the spread of viral diseases vectored by these mites. Exp. App. Acarol., 30: 29-105. https://doi.org/10.1023/B:APPA.0000006544.10072.01
Choudhury, P., Duttal, B.K., and Bhattacharjee, P.C., 2006. Some ecological factors on population dynamics of red spider mite (Oligonychus coffeae Nietner) and their control in the tea agro-ecosystem of Barak Valley, Assam (India). Int. J. Tehnol. Sci., 5: 29-39.
da Encarnação Bobot, T., Franklin, E., Navia, D., Gasnier, T.R.J., Lofego, A.C., and de Oliveira, B., 2011. Mites (Arachnida, Acari) on Citrus sinensis L. Osbeck orange trees in the State of Amazonas, Northern Brazil. Acta Amaz., 41: 557-566. https://doi.org/10.1590/S0044-59672011000400013
Den Heyer, J., 2011. Some statistics on the taxonomy of the family Cunaxidae (Acari: Prostigmata). Zoosymposia, 6: 34-38. https://doi.org/10.11646/zoosymposia.6.1.6
Den Heyer, J., and Sergeyenko, A.L., 2009. Neotype designation for Cunaxa setirostris (Hermann, 1804) (Acari: Prostigmata: Cunaxidae). Zootaxa, 2106: 61-68. https://doi.org/10.11646/zootaxa.2106.1.5
Den Heyer, J., Ueckermann, E.A., and Khanjani, M., 2011. Iranian Cunaxidae (Acari: Prostigmata: Bdelloidea): Part 2. Subfamily Cunaxinae. J. Nat. Hist., 45: 1667-1678. https://doi.org/10.1080/00222933.2011.559602
Denmark, H.A., 1984. Brevipalpus mites found on Florida citrus (Acarina: Tenuipalpidae), 69: 1-4.
Dhooria, M.S., and Butani, D.K., 2005. Seasonal incidence of citrus mites, Eutetranychus orientalis and its pradator. Ind. J. Acarol., 7: 59-62.
Di Sabatinol, A.R., Gerecke, R., Gledhill, R., and Smit, T.H., 2010. The taxonomic status of the water mite genera Todothyas Cook and Parathyas Lundblad. Supplement to Di Sabatino et al. (2009). Zootaxa, 2361: 68. https://doi.org/10.11646/zootaxa.2361.1.6
Döker, I., Stathakis, T.I., and Kazak, C., 2017. Cunaxa capreolus (Berlese, 1889), (Acari: Prostigmata: Cunaxidae): A new record for predatory mite fauna of Turkey. N. Western J. Zool., 13: 360-362.
Duso, C., and Pasini, M., 2003. Distribution of the predatory mite Amblyseius andersoni Chant (Acari: Phytoseiidae) on different apple cultivars. Anzeiger für Schädlingskunde. J. Pest Sci., 76: 33-40. https://doi.org/10.1046/j.1439-0280.2003.03003.x
Fadamiro, H.Y., Xiao, Y., Nesbitt, M., and Childers, C.C., 2009. Diversity and seasonal abundance of predacious mites in Alabama Satsuma citrus. Ann. Entomol. Soc. Am., 102: 617-628. https://doi.org/10.1603/008.102.0406
Fonseca, M.M., Pallini, A., Marques, P.H., Lima, E., and Janssen, A., 2020. Compatibility of two predator species for biological control of the two-spotted spider mite. Exp. App. Acarol., 80: 409-422. https://doi.org/10.1007/s10493-020-00472-8
Gerson, U., 2008. The Tenuipalpidae: An under-explored family of plant-feeding mites. Syst. App. Acarol., 13: 83-102. https://doi.org/10.11158/saa.13.2.1
GOP, 2018. Economics Survey of Pakistan, 2017-18. Economic advisory wing, finance division, Islamabad, Pakistan.
Hazan, A., Gerson, U., and Tahori, A.S., 1973. Life history and life table of the carmine spider mite. Acarologia, 15: 414-440.
Heyden, C.V., 1826. Versuch einer systematischen Eintheilung der Acariden. Isis, Oken, 1: 607-613.
Hoque, M.F., Khalequzzaman, M., and Islam, W., 2010. Population dynamics of Tetranychus urticae Koch and Phytoseiulus persimilis Athias-Henriot on three host plants. Pak. Entomol., 32: 6-11.
Jafari, S., Fathipour, Y., and Faraji, F., 2012. Temperature-dependent development of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) at seven constant temperatures. Insect Sci., 19: 220-228. https://doi.org/10.1111/j.1744-7917.2011.01444.x
Jaworski, S., 2000. Occurrence of phytoseiid mites (Acari Phytoseiidae) on blackcurrent plantations and in surrounding vegetation in southern Poland. IOBC WPRS Bull., 23: 57-62.
Jeppson, L.R., Keifer, H.H., and Baker, E.W., 1975. Mites injurious to economic plants. University of California Press Berkeley, pp. 614. https://doi.org/10.1525/9780520335431
Juan-Blasco, M., Qureshi, J.A., Urbaneja, A., and Stansly P.A., 2012. Predatory mite, Amblyseius swirskii (Acari: Phytoseiidae), for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol., 95: 543-552. https://doi.org/10.1653/024.095.0302
Kasap, İ., and Şekeroğlu, E., 2004. Life history of Euseius scutalis feeding on citrus red mite Panonychus citri at various temperatures. BioControl, 49: 645-654. https://doi.org/10.1023/B:BICO.0000046733.53887.2b
Khanjani, M., Khanjani, M., and Seeman, O.D., 2017. New spider mites (Acari: Tetranychidae) of the genera Paraplonobia and Eurytetranychus from Iran, and a description of all life stages of Eutetranychus orientalis (Klein). Acarologia, 57: 465-491.
Kitajima, E.W., Chagas, C.M., and Rodrigues, J.C.V., 2003a. Brevipalpus-transmitted plant virus and virus-like diseases: cytopathology and some recent cases. Exp. App. Acarol., 30: 135-160. https://doi.org/10.1023/B:APPA.0000006546.55305.e3
Kitajima, E.W., Rezende, J.A.M., and Rodrigues, J.C.V., 2003b. Passion fruit green spot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) on passion fruit in Brazil. Exp. App. Acarol., 30: 225-231. https://doi.org/10.1023/B:APPA.0000006551.74604.84
Kondo, H., Maeda, T., and Tamada, T., 2003. Orchid fleck virus: Brevipalpus californicus mite transmission, biological properties and genome structure. Exp. App. Acarol., 30: 215-223. https://doi.org/10.1023/B:APPA.0000006550.88615.10
Ledesma, C., Wong, M.E., Vela, J.M., Jacas, J.A., and Boyero, J.R., 2011. Population dynamics of the citrus oriental mite, Eutetranychus orientalis (Klein) (Acari: Tetranychidae), and its mite predatory complex in southern Spain. IOBC/WPRS Bull., 62: 83-92.
Martínez-Ferrer, M.T., Jacas, J.A., Ripollés-Moles, J.L., and Aucejo-Romero, S., 2006. Approaches for sampling the two spotted spider mite (Acari: Tetranychidae) on clementines in Spain. J. Econ. Entomol., 99: 1490-1499. https://doi.org/10.1093/jee/99.4.1490
McMurtry, J.A., Morse, J.G., and Johnson, H.G., 1992. Studies of the impact of Euseius species (Acari: Phytoseiidae) on citrus mites using predator exclusion and predator release experiments. Exp. App. Acarol., 15: 233-248. https://doi.org/10.1007/BF01246565
McMurtry, J.A., Shaw, J.G., and Johnson, H.G., 1979. Citrus red mite populations in relation to virus disease and predaceous mites in southern California. Environ. Entomol., 8: 160-164. https://doi.org/10.1093/ee/8.1.160
Meena, N.K., Barman, D., and Medhi, R.P., 2013. Biology and seasonal abundance of the two-spotted spider mite, Tetranychus urticae, on orchids and rose. Phytoparasitica, 41: 597-609. https://doi.org/10.1007/s12600-013-0320-2
Meyer, G.A., Kovaleski, A., and Valdebenito-Sanhueza, R.M., 2009. Pesticide selectivity used in apple crops Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). Rev. Brasil. Frut., 31: 381-387. https://doi.org/10.1590/S0100-29452009000200011
Migeon, A., and Flechtmann, C.H., 2004. First additions and corrections to the world catalogue of the spider mite family (Acari: Tetranychidae). Int. J. Acarol., 30: 143-152. https://doi.org/10.1080/01647950408684383
Qureshi, J., Stelinski, L.L., Martini, X., and Diepenbrock, L.M., 2020. 2020–2021 Florida citrus production guide: Rust mites, spider mites, and other phytophagous mites. EDIS. IFAS extension. https://doi.org/10.32473/edis-cg002-2020
Rodrigues, J.C.V., 2000. Relacoes patogeno-vetor-planta no sistema leprose dos citros. Ph.D. thesis Piracicaba, Brasil, Centro de Energia Nuclear na Agricultura, da Universidade de Sao Paulo (in Portuguese).
Rodrigues, J.C.V., Kitajima, E.W., Childers, C.C., and Chagas, C.M., 2003. Citrus leprosis virus vectored by Brevipalpus phoenicis (Acari: Tenuipalpidae) on citrus in Brazil. Exp. App. Acarol., 30: 161-179. https://doi.org/10.1023/B:APPA.0000006547.76802.6e
Sarada, G., Nagalakshmi, T., Gopal, K., and Yuvaraj, K.M., 2018. Citrus rust mite (Phyllocoptruta oleivora Ashmead): A Review; J. Entomol. Zool. Stud., 6: 151-158.
Sarwar, M., 2016. Comparative life history characteristics of the mite predator Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on mite and pollen diets. Int. J. Pest Manage., 62: 140-148. https://doi.org/10.1080/09670874.2016.1146806
Skvarla, M.J., Fisher, J.R., and Dowling, A.P., 2014. A review of Cunaxidae (Acariformes, Trombidiformes): Histories and diagnoses of subfamilies and genera, keys to world species, and some new locality records. ZooKeys, 418: 1-103. https://doi.org/10.3897/zookeys.418.7629
Swirski, E., Amitai, S., and Dorzia, N., 1967. Laboratory studies on the feeding, development and reproduction of the predaceous mites Amblyseius rubini Swirski and Amitai and Amblyseius swirskii Athias (Acarina: Phytoseiidae) on various kinds of food substances. Israel J. Agric. Res., 17: 101-109.
Tuttle, D.M., and Baker, E.W., 1964. The spider mites of Arizona. University of Arizona, Tech. Bull., 158: 1-41.
Vacante, V., 2009. Citrus mites: Identification, bionomy and control. CABI. https://doi.org/10.1079/9781845934989.0000
Vacante, V., 2010. Review of the phytophagous mites collected on citrus in the world. Acarologia, 50: 221-241. https://doi.org/10.1051/acarologia/20101969
Xiao, Y., and Fadamiro, H.Y., 2010. Functional responses and prey-stage preferences of three species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus citri (Acari: Tetranychidae). Biol. Contr., 53: 345-352. https://doi.org/10.1016/j.biocontrol.2010.03.001
Zhang, Y.X., Zhang, Z.Q., Chen, C.P., Lin, J.Z., and Chen, X., 2001. Amblyseius cucumeris (Acari: Phytoseiidae) as a biocontrol agent against Panonychus citri (Acari: Tetranychidae) on citrus in China. Syst. App. Acarol., 6: 35-45. https://doi.org/10.11158/saa.6.1.6
To share on other social networks, click on any share button. What are these?