Comparison of Yolk Fatty Acids in Market Available Black-bone Chicken Eggs and Ordinary Chicken Eggs
Comparison of Yolk Fatty Acids in Market Available Black-bone Chicken Eggs and Ordinary Chicken Eggs
Suhu Duan, Liuan Li*, Zaiqiang Li, Mengran Qin, Zhenzhen Fan, Keyan Zhang, Qian Wang, Zhongmou Zhang, Bailiang Yang
Tianjin Key Laboratory of Agricultural Animal Breeding and Healthy Husbandry, College of Animal Science and Veterinary Medicine, Tianjin Agricultural University, Tianjin 300384, P.R. China
ABSTRACT
The paper compares and analyses the content of fatty acids in yolk of the commercially available ordinary chicken eggs and Brand A and Brand B black-bone chicken eggs, thus providing theoretical and practical references for selective consumption of chicken eggs. The results have shown that a total of 18 types of fatty acids are detected in 3 types of chicken egg yolk, including 6 types of saturated fatty acids (SFA), 12 types of unsaturated fatty acids (UFA), 6 types of monounsaturated fatty acids (MUFA), and 6 types of polyunsaturated fatty acids (PUFA). There are no significant differences in content of total fatty acid (FA), SFA, MUFA, PUFA, ω-6 PUFA, and ω-3 PUFA among the three types of eggs (P>0.05). The content of C14:1 fatty acid in yolk of Brand A black-bone chicken eggs is significantly higher than that in Brand B black-bone chicken eggs (P<0.05). The contents of C16:1 fatty acid in yolk of ordinary chicken eggs and Brand A black-bone chicken eggs are significantly higher than that in Brand B black-bone chicken eggs (P<0.05). The contents of linoleic acid, (C18:2n6c), α-linolenic acid (C18:3n3), and DHA (C22:6n3) in yolk in Brand A and Brand B black-bone chicken eggs are higher than that in ordinary chicken eggs but the difference is not significant (P>0.05). The ratio of ω-6 PUFA/ω-3 PUFA in yolk of Brand A black-bone chicken eggs is somewhat lower than that in the ordinary chicken eggs and the ratio of ω-6 PUFA/ω-3 PUFA in yolk of Brand B black-bone chicken eggs is significantly lower than that in the ordinary chicken eggs (P<0.05). The results have indicated that the nutritive value of fatty acid in black-bone chicken eggs is higher than that in ordinary chicken eggs from the perspective of assessing the ω-6 PUFA/ω-3 PUFA ratio.
Article Information
Received 10 December 2018
Revised 10 June 2019
Accepted 01 July 2019
Available online 14 August 2019
Authors’ Contribution
LL designed this study. SHD, ZL and MRQ conducted the experiments. SHD, KYZh, ZZF, QW and ZMZ analyzed the data. SHD and LL wrote the manuscript. BLY provided the guidance.
Key words
Black-bone chicken eggs, Ordinary chicken eggs, Content of fatty acid
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.6.2107.2115
* Corresponding author: anliuli2003@163.com
0030-9923/2019/0006-2107 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
China always ranks the top countries in the world in production and consumption of chicken eggs. As a popular food of animal origin, chicken eggs have a series of advantages including high nutritive value, high absorbability, relatively low prices etc. (Ye et al., 2012; Surai et al., 2001; Khan et al., 2015; Duru et al., 2017). Currently, there are various types of commercially available chicken eggs. In addition to the ordinary chicken eggs, there are also special types of chicken eggs like black-bone chicken eggs etc. The price of black-bone chicken eggs is generally 2-4 times that of ordinary chicken eggs due to the special raising method for black-bone chickens and the special nutritive value of black-bone chicken eggs (Jia et al., 2017; Lv et al., 2017; Li et al., 2019).
Lipid components, dominated by fatty acids, account for more than 60% of the dry weight of chicken egg yolk (Mine 2008; Noble 1991). The fatty acids in eggs are mainly composed of saturated fatty acid (SFA) and unsaturated fatty acid (UFA). The UFA further includes monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid. PUFA primarily includes ω-3 PUFAs and ω-6 PUFAs. The content of UFA is one of the important bases for assessing the nutritive value of poultry eggs. Ingesting a certain amount of unsaturated fatty acid in the diet plays a positive role in regulating blood lipid, eliminating thrombi, boosting the brain, improving the vision, relieving arthritis symptoms, and improving the body immunological functions. The components and contents of fatty acids in eggs may be associated with various factors including breed, age, daily diet composition of animals (Sun et al., 2016; Kamely et al., 2016; Yang et al., 2017; Zhang et al., 2017). A previous study has found that there are differences in components and contents of fatty acids in both the albumen and yolk of duck eggs (Sun et al., 2016). The research has shown that the percentages of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in yolk increase after fish oil is added in the daily diet for quails of different ages (Kamely et al., 2016); the percentage of arachidonic acid (AA) and the ratio of n-6/n-3 fatty acids decrease; there are differences in content of fatty acid among eggs of quails of different ages. Yang et al. (2017) have studied the composition of fatty acids in yolk oil of chicken, duck, and quail eggs. Their research findings have shown that the fatty acids in the egg yolk oil main comprise oleic acid (C18:1), palmitic acid (C16:0), linoleic acid (C18:2) etc. The content of oleic acid in goose eggs is relatively high, that is to say, the composition of fatty acids is associated with types of poultry eggs. Zhang et al. (2017) have studied the effects of adding ALA, DHA, or EPA-enriched additives in the diet on the contents of fatty acids in chicken eggs. Their research findings have indicated that the dietary formula can significantly influence the components and contents of fatty acids in chicken eggs.
Currently, there is a great deal of research on the contents of fatty acids in chicken eggs while comparisons of contents of fatty acids in black-bone chicken eggs and ordinary chicken eggs are rarely reported. With black-bone chicken eggs and ordinary chicken eggs of different brands as the objects, the research determines the contents of fatty acids in yolk and compares the differences in contents of fatty acids in yolk between black-bone chicken eggs and ordinary chicken eggs, thus providing theoretical and practical references for people to make consumption choices.
Materials and Methods
Materials
Black-bone chicken eggs of Brand A and Brand B, ordinary chicken eggs with similar dates of production and packaging were purchased from a hypermarket in the city of Tianjin (Lv et al., 2019). 15 of each type of eggs were taken for detection. Mixed standard of fatty acids comprising 37 constituents and methyl ester and undecanoic acid triglyceride (C11:0) standard were purchased from U.S. Nu-Chek Company. Petroleum ether (boiling range 30-60°C), methanol, and hydrochloric acid were purchased from Tianjin Fengchuan Chemical Reagent Technologies Co., Ltd. Normal hexane was purchased from Tianjin Jinke Fine Chemicals Research Institute. 15% boron trifluoride methanol was purchased from Panjin Yanfeng Technology, Co., Ltd. Anhydrous ether was purchased from Li’an Longbohua (Tianjin) Medical Chemistry Co., Ltd. Pyrogallic acid was purchased from Tianjin Guangfu Fine Chemical Research Institute. Anhydrous sodium sulfate was purchased from Tianjin Bodi Chemical Co., Ltd. Sodium chloride was purchased from Tianjin Jizhun Chemical Reagents Co., Ltd. 95% ethanol was purchased from Tianjin Fuyu Fine Chemicals Co., Ltd. Sodium hydroxide was purchased from Booute (Tianjin) Chemical Trade Co., Ltd. 50mL centrifugal tubes and gas chromatographic sample bottles.
Apparatus and equipment
The gas chromatograph 7890B, from the U.S. Agilent Company. The capillary chromatographic column SP2560, from the U.S. Supelco Company. The thermostat water bath, from Shanghai Zhicheng Analytical Instruments Manufacturing Co., Ltd. The analytical balance, from Shanghai Shunning Hengping Scientific Instruments Co., Ltd. The above instruments and equipment were provided by the Laboratory of School of Animal Science and Animal Medicine, Tianjin Agricultural College.
Weighing and hydrolysis of samples
In this study, 5 were randomly selected from each type of chicken eggs and their yolks were well mixed. 0.5 g of the yolk mixture was poured into a 50 mL centrifugal tube. 2 mL of internal standard, 0.1 g of pyrogallic acid, 2 mL of 95% ethanol, and 4 mL of ultrapure water were well mixed. Then, 10 mL of hydrochloric acid (8.3 mol/L) was added. These substances were well mixed, heated for 40 min in a water bath at 75°C, vibrated once at an interval of 10 min, and cooled to room temperature for later use.
Extraction of fat
First, 10 mL of anhydrous ethanol was added to the above centrifugal tube and they were well mixed. Then, 15 mL of the mixed solution of anhydrous ethanol and petroleum ether of equal proportions. The tube was vibrated for 1-2 min and allowed to stand still for 10 min. The supernatant was pipetted to a 100 mL conical flask. The above steps were replicated three times. A conical flask was placed in a water bath for evaporation at 65°C until the conical flask nearly became dry. The residue was fat extract.
Fat saponification and fatty acid methyl esterification
First, 7 mL of methanol solution containing 2% sodium hydroxide was added to the fat extract. The flask was properly covered, heated in the water bath for 2-3 min at 80 °C. Then, 7 mL of methanol solution containing 15% boron trifluoride was quickly added. The flask was heated in the water bath for 2 min at 80 °C and cooled to room temperature quickly. The flask was vibrated for 2 min after the addition of 15 mL normal hexane and allowed to stand still for 10 min after the addition of saturated sodium chloride solution. 3 g of anhydrous sodium sulfate was added to a 10 mL test tube. The supernatant was pipetted into the test tube. The test tube was vibrated for 1 min and allowed to stand still for 5 min. The supernatant was pipetted into the gas chromatographic sample bottle and preserved at -20 °C for later use.
Gaseous phase conditions
Gas chromatographic column: SP2560 (100 m×250 μm×0.2 μm); chromatographic column flow: 1 mL/min;H2 flow: 30 mL/min; carrier gas: He; split ratio: 20:1; FID detector; detector temperature: 280°C; column oven temperature: 250°C; temperature rise procedures for column oven: initial temperature 140°C, kept for 5 min, rising to 220°C at a rate of 4°C/min, rising to 230°C at a rate of 0.5°C/min, rising to 240°C at a rate of 4°C/min, kept for 15 min; make-up gas flow: 25 mL/min; injection volume: 5 μL.
Computational method
The method for computing the contents of fatty acid methyl esters in the samples is as follows:
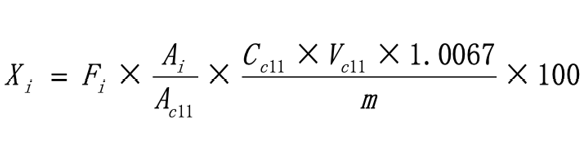
where Xi is the contents of fatty acid methyl esters in the samples in g/100g; Fi is the response factor of the fatty acid methyl ester; Ai is the peak area of fatty acid methyl ester in the sample; Ac11 is the peak area of the internal standard of undecanoic triglyceride added to the sample; Cc11 is the concentration of the undecanoic triglyceride in mg/mL; Vc11 is the volume of the internal standard added to the sample in mL; 1.0067 is the coefficient of conversion from undecanoic triglyceride to undecanoic methyl ester; m is the sample mass in mg; 100 is the coefficient of conversion from the content to the content per 100g of sample.
See GB5009.168—2016 (National health and family planning commission of the People’s Republic of China. 2017. National Food Safety Standard Determination of Fatty Acids in Food: GB5009.168-2016) for the methods for computing the contents of various fatty acids in the samples.
Statistical analysis
The SPSS 20.0 software was used. The one-way ANOVE analysis (LSD) was utilized for multiple comparisons among mean values and significance tests. All data were expressed as mean ± standard deviation. The difference was considered significant when P<0.05.
RESULTS
Total fatty acids
As can be seen in Figure 1, the content of total fatty acid in yolk of the commercially available black-bone chicken eggs of two brands and the content of total fatty acid in yolk of ordinary chicken eggs roughly approximated. There were no significant differences in content of total fatty acid among the three types of yolks (P>0.05).
Saturated fatty acids
As can be seen in Figure 2, the content of saturated fatty acid in the yolk of black-bone chicken eggs of Brand A was somewhat higher than that in black-bone chicken eggs of Brand B and ordinary chicken eggs. The content of saturated fatty acid in the yolk of black-bone chicken eggs of Brand B was somewhat lower than that in ordinary chicken eggs. There were no significant differences among the three types of eggs (P>0.05).
As can be seen in Table 1, neither the saturated fatty acid C4:0~C13:0 nor C21:0~C24:0 was detected in the yolk of the commercially available black-bone chicken eggs and ordinary chicken eggs. The content of pentadecanoic acid (C15:0) in the yolk of the black-bone chicken eggs of Brand B was significantly higher than that in the ordinary chicken eggs (P<0.05). The content of margaric acid (C17:0) in the yolk of the black-bone chicken eggs of Brand B was significantly higher than that in the black-bone chicken eggs of Brand A (P<0.05).
Table I.- Saturated fatty acid content in commercially available black-bone chicken eggs and ordinary chicken egg yolks (g/100g).
|
Common name |
Saturated fatty acid |
Ordinary eggs |
A brand black-bone chicken eggs |
B brand black-bone chicken eggs |
|
|
Butyric acid |
C4:0 |
ND |
ND |
ND |
|
|
Capric acid |
C6:0 |
ND |
ND |
ND |
|
|
Caprylic acid |
C8:0 |
ND |
ND |
ND |
|
|
Decanoic acid |
C10:0 |
ND |
ND |
ND |
|
|
Undecanoic acid |
C11:0 |
ND |
ND |
ND |
|
|
Lauric acid |
C12:0 |
ND |
ND |
ND |
|
|
Tridecylic acid |
C13:0 |
ND |
ND |
ND |
|
|
Myristic acid |
C14:0 |
0.050±0.002 |
0.059±0.006 |
0.043±0.009 |
|
|
Pentadecanoic acid |
C15:0 |
0.005b±0.002 |
0.007ab±0.001 |
0.014a±0.003 |
|
|
Hexadecanoic acid |
C16:0 |
3.784±0.173 |
3.981±0.259 |
2.913±0.464 |
|
|
Margaric acid |
C17:0 |
0.025ab±0.002 |
0.019b±0.006 |
0.046a±0.009 |
|
|
Stearic acid |
C18:0 |
1.215±0.076 |
1.288±0.102 |
0.993±0.157 |
|
|
Arachidic acid |
C20:0 |
ND |
0.004±0.004 |
0.013±0.001 |
|
|
N-heneicosanoic acid |
C21:0 |
ND |
ND |
ND |
|
|
Docosanoic acid |
C22:0 |
ND |
ND |
ND |
|
|
Tricosanoicacid |
C23:0 |
ND |
ND |
ND |
|
|
Carnaubic acid |
C24:0 |
ND |
ND |
ND |
|
Notes: ND indicates that no result was detected.
The content of arachidic acid (C20:0) in the yolk of black-bone chicken eggs of Brand B was significantly higher than that in the yolk of black-bone chicken eggs of Brand A and ordinary chicken eggs (P<0.05). There were no significant differences in content of myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0) in yolk between the black-bone chicken eggs of Brand A and Brand B and the ordinary chicken eggs (P>0.05).
Unsaturated fatty acids
As can be seen in Table II, there were significant differences in contents of oleic acid (C14:1) and palmitoleic acid (C16:1) in yolk between black-bone chicken eggs and ordinary chicken eggs (P<0.05). The content of C14:1 in the yolk of the black-bone chicken eggs of Brand A was significantly higher than that in the black-bone chicken eggs of Brand B (P<0.05). The contents of C16:1 in the yolk of the ordinary chicken eggs and the black-bone chicken eggs of Brand A were significantly higher than that in the black-bone chicken eggs of Brand B (P<0.05). None of pentadecenic acid (C15:1), eicosapentaenoic acid (EPA), C20:2, γ linolenic acid (C20:3n6), erucic acid (C22:1n9), arachidic triolefinic acid (C20:3n3), docosadienoic acid (C22:2), EPA (C20:5n3), and nervonic acid (C24:1) was detected in the yolk of the three types of chicken eggs. The contents of oleic acid (C18:1n9t), and arachidonic acid (C20:4n6) in the yolk of black-bone chicken eggs of Brand A were higher than that in ordinary chicken eggs. The contents of C18:1n9t and C20:4n6 in the yolk of black-bone chicken eggs of Brand B were lower than that in ordinary chicken eggs but the differences were not significant (P>0.05). The contents of linoleic acid (C18:2n6c), α-linolenic acid (C18:3n3), and DHA (C22:6n3) in the yolk of the black-bone chicken eggs of Brand A and Brand B were higher than that in ordinary chicken eggs but the differences were not significant (P>0.05).
As can be seen in Figure 3, there were no significant differences in contents of UFA, MUFA, PUFA, ω-6 PUFA, and ω-3 PUFA among the three types of chicken eggs (P>0.05).
As can be seen in Figure 4, the ratio of ω-6 PUFA/ω-3 PUFA in the yolk of black-bone chicken eggs of Brand B was significantly lower than that in the ordinary chicken eggs (P<0.05). The ratio of ω-6 PUFA/ω-3 PUFA in the yolk of black-bone chicken eggs of Brand A was somewhat lower than that in the ordinary chicken eggs but the differences were not significant (P>0.05).
Table II.- Unsaturated fatty acid content in commercially available black-bone chicken eggs and ordinary chicken egg yolks (g/100g).
|
Common name |
Unsaturated fatty acid |
Ordinary eggs |
A brand black-bone chicken eggs |
B brand black-bone chicken eggs |
|
Butyric acid |
C14:1 |
0.006ab±0.003 |
0.012a±0.001 |
0.002b±0.002 |
|
Capric acid |
C15:1 |
ND |
ND |
ND |
|
Caprylic acid |
C16:1 |
0.494a±0.022 |
0.454a±0.034 |
0.240b±0.039 |
|
Decanoic acid |
C17:1 |
0.020±0.005 |
0.027±0.004 |
0.031±0.009 |
|
Undecanoic acid |
C18:1n9t |
0.022±0.001 |
0.015±0.005 |
0.026±0.006 |
|
Lauric acid |
C18:1n9c |
5.962±0.250 |
6.023±0.364 |
4.405±0.806 |
|
Tridecylic acid |
C18:2n6t |
0.106±0.077 |
0.115±0.015 |
0.106±0.080 |
|
Myristic acid |
C18:2n6c |
1.789±0.152 |
1.895±0.176 |
2.014±0.335 |
|
Pentadecanoic acid |
C18:3n6 |
0.013±0.001 |
0.017±0.002 |
0.015±0.003 |
|
Hexadecanoic acid |
C20:1 |
0.008±0.004 |
ND |
0.004±0.004 |
|
Margaric acid |
C18:3n3 |
0.047±0.009 |
0.087±0.012 |
0.105±0.025 |
|
Stearic acid |
C20:2 |
ND |
ND |
ND |
|
Arachidic acid |
C20:3n6 |
ND |
ND |
ND |
|
N-heneicosanoic acid |
C22:1n9 |
ND |
ND |
ND |
|
Docosanoic acid |
C20:3n3 |
ND |
ND |
ND |
|
Tricosanoicacid |
C20:4n6 |
0.325±0.007 |
0.331±0.024 |
0.260±0.041 |
|
Carnaubic acid |
C22:2 |
ND |
ND |
ND |
|
Butyric acid |
C20:5n3 |
ND |
ND |
ND |
|
Capric acid |
C24:1 |
ND |
ND |
ND |
|
Caprylic acid |
C22:6n3 |
0.096±0.050 |
0.130±0.008 |
0.179±0.035 |
DISCUSSION
Fatty acid plays a significant role in vital activities of organisms. The fatty acids that can be synthesized by human body are called non-essential fatty acids. The fatty acids, essential to vital activities of organisms, that cannot be synthesized by the organisms themselves and must be supplied by food are called essential fatty acids, such as polyunsaturated fatty acids including DHA, EPA, ALA etc. (Liu, 2010). In contrast, less attention is paid to the total fatty acid. Considering the content of total fatty acid alone also has some disadvantages. Thus, components, contents, and properties of various fatty acids, notably their biological functions, have been becoming one of the hot areas of research by scholars (Song et al., 2015). There are no significant differences in content of total fatty acid among the three types of yolk in the research.
Up to now, people have different opinions on the understanding of saturated fatty acids. Partial saturated fatty acids have some effects on the physiological functions of organisms, which is expected to be further studied and demonstrated (Xie, 2011; Song et al., 2017; Zhang et al., 2012; Chen et al., 2008). Some research has indicated that the intake of saturated fatty acids is not directly associated with cardiovascular diseases. The U.S. government has also adjusted the intake of saturated fatty acids in its dietary guideline. However, the intake of saturated fatty acids is still considered one of the factors inducing cardiovascular diseases by most people due to the fact that the relationship between the intake of saturated fatty acids and organism health is not well popularized (Yang et al., 2017; Virtanen et al., 2016). The research by Trumbo et al. (2002) has indicated that the saturated fat in the diet can often increase the concentrations of cholesterol in blood of animals and humans (Trumbo et al., 2002). The research by Forouhi et al. (2014) has shown that different saturated fatty acids have different odd and even chains, thus leading to different effects on Type II diabetes. Saturated fatty acids with an odd chain may somewhat inhibit the occurrence of Type II diabetes while the saturated fatty acid with an even chain may somewhat promote the occurrence of Type II diabetes (Forouhi et al., 2014).
In the test, neither the saturated fatty acid C4:0-C13:0 nor C21:0-C24:0 was detected in the three types of yolk. Based on an analysis, this may be because the content in the yolk is little. There were significant differences in contents of C15:0, C17:0, and C20:0 in the yolk of commercially available ordinary chicken eggs and black-bone chicken eggs, which may be largely associated with different daily diets as well as a range of factors like breed, age, growth and development etc. In this test, the contents of C16:0 and C18:0 in SFA are high, which is similar to the results of the research by Xie et al. (2011) Some research has indicated that the two types of fatty acids play a significant role in improving the physiological functions of organisms. The research by Sundram et al. (1992) has indicated that the content of palmitic acid (C16:0) has no adverse effect on the lipoprotein and cholesterol metabolism of organisms and can on the contrary reduce the content of cholesterol. A certain level of stearic acid (C18:0) may regulate the synthesis of organism cholic acid and inhibit the capacity of the intestine to absorb the cholesterol, thus reducing the content of cholesterol in blood and internal organs (Cowles et al., 2002; Zhang et al., 2017).
MUFA and PUFA in UFA play a role in accelerating growth and development of organisms, alleviating diseases, and regulating immunity. The research by Strandvik et al. (2016) has shown that the oleic acid in MUFA can benefit growth and development and neural activities of infants and the infants need supplementation of a certain amount of oleic acid. The research by Matsumoto et al. (2017) has shown that MUFA may be one of the key factors that inhibit the occurrence of rheumatoid arthritis of organisms. The research by Joris et al., has shown that the cis-form MUFA may reduce the risk of coronary heart disease but its long-term effects are expected to be further studied (Joris et al., 2016). The test conducted by Ralston et al. (2017) has shown that MUFA may somewhat alleviate the dysfunction of the organism pancreas. In the test, the content of C14:1 in MUFA is low and the contents of C16:1 and C18:1n9c are high. The results are similar to the results obtained by Wu et al. (2015) and Xue et al. (2017).
Within the body, MUFA can generate PUFA under the action of catalytic conversion of carbon-chain elongase and desaturase. PUFA has a wide range of functions like anticancer, antioxidant, promoting genetic expression, improving immunological regulation, promoting growth and development, reducing blood glucose, alleviating hyperactivity in children, and dementia in the elderly people (Jordan et al., 2011; Behesht Moghadam and Cherian, 2017). In the test, the contents of UFA and MUFA in the yolk of black-bone chicken eggs of Brand A are higher than that in the yolk of ordinary chicken eggs while the contents of UFA and MUFA in the yolk of black-bone chicken eggs of Brand B are lower than that in ordinary chicken eggs. This may be associated with a series of factors like different components of fodder for black-bone chickens of different brands and their ages. MUFA is a monoenoic acid with a stable structure, making it difficult to oxidize. PUFA is a polyenoic acid with many double bonds, making it susceptible to oxidative metabolism (Wang and Huo, 2001). The research by Hu et al. (2015) has shown that the contents of PUFA in fodder are different if the fodder for layer chickens has different formulas. As a result, the contents of PUFA in chicken eggs also vary.
In this study, the total content of PUFA in the yolk of black-bone chicken eggs and the contents of ω-3 PUFA and ω-6 PUFA are higher than that in the ordinary chicken eggs but there are no significant differences. C18:2n6c has the highest content in PUFA; no C20:5n3 is detected; the contents of C20:4n6 and C22:6n3 are high. The results are similar to that obtained by Bruneel (2013). This may be due to different breeds and ages of layer chickens. In other words, the chickens of different breeds at different ages may have different metabolisms of fatty acids within the body and the enrichment modes of PUFA in eggs. There are no significant differences, which is associated with the insufficient quantity of samples. The research by Wu et al. (2018) has indicated that the small intestines of the layer chickens of different ages have different levels of capacity to absorb the fatty acid ingested, thus influencing the content of fatty acid deposited in eggs in form of lipoprotein via the blood circulation system.
The ratio of ω-6 PUFA/ω-3 PUFA in the yolk of black-bone chicken eggs of Brand B is significantly lower than that in the ordinary chicken eggs. This may be caused by the differences in ratio of ω-6 PUFA/ω-3 PUFA in daily diet between the two types of layer chickens. The research by Cachaldora et al. (2008) has found that a large amount of ω-6 PUFA in the daily diet would intensify the competition against desaturase, thus leading to a decline in the ALA conversion efficiency. Therefore, the ratio of ω-6 PUFA/ω-3 PUFA is one of the major factors that influence the ratio of ω-6 PUFA/ω-3 PUFA in eggs. The ratio of ω-6 PUFA/ω-3 PUFA is one of the important indicators for assessing the nutrition intake balance. The research by Simopoulos a decrease in the ratio of ω-6 PUFA/ω-3 PUFA helps reduce the morbidities of a variety of chronic diseases (Simopoulos 2002). The recommended ratio of ω-6 PUFA/ω-3 PUFA is 4~6:1 in China and 4~10:1 in the globe.
Conclusion
A total of 18 fatty acids are detected in the yolk of three types of chicken eggs, including 6 types of saturated fatty acids and 12 unsaturated fatty acids. There are no significant differences in contents of total FA, SFA, MUFA, PUFA, ω-6 PUFA, and ω-3 PUFA among the three types of chicken eggs (P>0.05). The ratio of ω-6 PUFA/ω-3 PUFA in the yolk of the black-bone chicken eggs of Brand A is slightly lower than that in ordinary chicken eggs. The ratio of ω-6 PUFA/ω-3 PUFA in the yolk of the black-bone chicken eggs of Brand B is significantly lower than that in the ordinary chicken eggs (P<0.05). In terms of assessing the ratio of ω-6 PUFA/ω-3 PUFA, the nutritive value of fatty acids in the black-bone chicken eggs is higher than that in the ordinary chicken eggs.
Acknowledgement
We acknowledge the Tianjin “131” Innovative Talent Team (20180318), the Major Science and Technology and Engineering Project in Tianjin (18ZXBFNC00310), Innovative Talents Training plan of Young and Middle-Aged Backbone in Tianjin City University, Tianjin Enterprise Science and Technology Commissioner Project (2019187).
Statement of Conflicts of interest
We declare no conflicts of interest in this study.
References
Beheshti Moghadam, M.H. and Cherian, G., 2017. Use of flaxseed in poultry feeds to meet the human need for n-3 fatty acids. World’s Poult. Sci. J., 73: 803–812. https://doi.org/10.1017/S0043933917000721
Bruneel, C., Lemahieu, C., Fraeye, I., Ryckebosch, E., Muylaert, K., Buyse, J. and Foubertab, I., 2013. Impact of microalgal feed supplementation on omega-3 fatty acid enrichment of hen eggs. J.Funct. Fds., 5: 897-904. https://doi.org/10.1016/j.jff.2013.01.039
Cachaldora, P., García-Rebollar, P., Alvarez, C., Blas, J.C.D. and Méndez, J., 2008. Effect of type and level of basal fat and level of fish oil supplementation on yolk fat composition and n-3 fatty acids deposition efficiency in laying hens. Anim. Feed Sci. Technol., 141: 104-114. https://doi.org/10.1016/j.anifeedsci.2007.05.024
Chen, Y.J., Ju, X.R. and Zhou, G.H., 2008. Classification and physiological function of saturated fatty acids. China Oils Fats, 33: 35-39.
Cowles, R.L., Lee, J.Y., Gallaher, D.D., Stueferpowell, C.L. and Carr, T.P., 2002. Dietary stearic acid alters gallbladder bile acid composition in hamsters fed cereal-based diets. J. Nutri., 132: 3119-3122. https://doi.org/10.1093/jn/131.10.3119
Duru, M., Duru, A.A., Karadaş, K., Eyduran, E., Cinli, H. and Tariq, M.M., 2017. Effect of carrot (Daucus carota) leaf powder on external and internal egg characteristics of hy-line white laying hens.Pakistan J. Zool., 49: 129-137
Forouhi, N.G., Koulman, A., Sharp, S.J., Imamura, F., Kröger, J., Schulze, M.B. and Wareham, N.J., 2014. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diab. Endocrinol., 2: 810–818. https://doi.org/10.1016/S2213-8587(14)70146-9
Hu, Q.Q. and Yang, G.M., 2015. Comparison of polyunsaturated fatty acids in several feeds and their application in animal husbandry. Swine Produc., 6:14-16.
Jia, J.M. and Zhang, L.N., 2017. Comparison of egg quality and nutrient composition of different varieties of eggs. Jiangsu agric. Sci., 45: 152-155.
Jordan, R.G., 2011. Prenatal omega-3 fatty acids: Review and recommendations. J. Midwif. Womens Hlth., 55: 520-528. https://doi.org/10.1016/j.jmwh.2010.02.018
Joris, P.J. and Mensink, R.P., 2016. Role of cis-monounsaturated fatty acids in the prevention of coronary heart disease. Curr. Atheroscl. Rep., 18:1-7. https://doi.org/10.1007/s11883-016-0597-y
Kamely, M., Torshizi, M.A.K., Khosravinia, H., 2016. Omega-3 enrichment of quail eggs: age, fish oil and savory essential oil. J. agric. Sci. Technol., 18: 347-359.
Khan, S.A., Khan, A., Khan, S.A., Beg, A. and Damanhouri, G., 2015. Comparative study of fatty-acid composition of table eggs from the jeddah food market and effect of value addition in omega-3 biofortified eggs. Saudi J. biol. Sci., 24: 929-935. https://doi.org/10.1016/j.sjbs.2015.11.001
Li, L., Li, L.A., Zhang, R.B., Deng, Z.C., Jin, T.M. and Du G.M., 2019. Effects of dietary supplementation of selenium enriched yeast on egg selenium content and egg production of north china hens. Pakistan J. Zool., 51: 49-55.
Liu, F., 2010. Simultaneous determination of 34 kinds of fatty acids in food by gas chromatography. Fujian Analy. Test., 19: 15-20.
Lv, L., Li, L.A., Zhang, R.B., Deng, Z.C., Du, G.M., Jin, T.M., Yu, X.X., Zhang, W. and Jiao, X.L., 2018. Effects of dietary supplementation of nano-selenium on the egg selenium content and egg production rate of North China hens. Nanosci. Nanotechnol. Lett., 10: 1567-1571
Lv, L., Li, L.A, Deng, Z.C. Jin, T.M., Yu, X.X., Zhang, W. and Jiao, X.L., 2019. Using hydride generation-atomic fluorescence spectrometry to study the selenium content of Astragalus mongholicus produced in different habitats. J. Biobased Mater. Bioen., 13: 274-278.
Matsumoto, Y., Sugioka, Y., Tada, M., Okano, T., Mamoto, K. and Inui, K., 2017. Monounsaturated fatty acids might be key factors in the mediterranean diet that suppress rheumatoid arthritis disease activity: the tomorrow study. Clin. Nutri., 37: 675-680. https://doi.org/10.1016/j.clnu.2017.02.011
Mine, Y., 2008. Egg bioscience and biotechnology. John Wiley & Sons, Inc., Hoboken, New Jersey, USA. https://doi.org/10.1002/9780470181249
National health and family planning commission of the People’s Republic of China. 2017. National food safety standard determination of fatty acids in food. GB5009.168-2016. China Standard Press, Beijing, 3-4.
Noble, R.C., 1991. Comparative composition and utilisation of yolk lipid by embryonic birds and reptiles. Egg Incub., 10: 17-28. https://doi.org/10.1017/CBO9780511585739.003
Ralston, J.C., Nguyen, T.M.S., Lyons, C.L., Murphy, A.M., Cooke, A.A., Falvey, A., Finucane, O.M., Rutter, G.A. and Roche, H.M., 2017. Pancreatic islet dysfunction is partially attenuated by replacement of dietary saturated fatty acids with monounsaturated fatty acids. Proc.Nutri. Soc., 76(OCE3), E55. https://doi.org/10.1017/S0029665117001288
Simopoulos, A., 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother., 56: 365–379. https://doi.org/10.1016/S0753-3322(02)00253-6
Song, W.G., Wang, G.Y., Cheng, Z.B., Gu, D.H., Zhu, R.J. and Liao G.Z., 2017. Study on the contents of fatty acids and mineral from five yunnan local chicken breeds eggs. Fd. Indust., 8: 177-180.
Song, Z.T., Wang, H.L., Yang, R., Han, C.W. and Cai, R.X., 2015. Research progress on the function of fatty acids and application in pig production. China Anim. Husb.Vet. Med., 42: 3253-3260.
Strandvik, B., Ntoumani, E., Lundqvist-Persson, C. and Sabel, K.G., 2016. Long-chain saturated and monounsaturated fatty acids associate with development of premature infants up to 18 months of age. Prostagl. Leukotr. Essent. Fatty Acids, 107: 43-49. https://doi.org/10.1016/j.plefa.2016.01.002
Sun, H.Z., Qiu L.Q. and Song S.F., 2016. Analysis of fatty acid content in different varieties of eggs. J. Fd Safe. Qual., 7: 2459-2464.
Sundram, K., Hornstra, G., Houwelingen, A.C.V. and Kester, A.D.M., 1992. Replacement of dietary fat with palm oil: effect on human serum lipids, lipoproteins and apolipoproteins. Br. J. Nutri., 68: 677-692. https://doi.org/10.1079/BJN19920125
Surai, P.F. and Sparks, N.H.C., 2001. Designer eggs: from improvement of egg composition to functional food. Trends Fd. Sci. Technol., 12: 7-16. https://doi.org/10.1016/S0924-2244(01)00048-6
Trumbo, P., Schlicker, S., Yates, A.A. and Poos, M., 2002. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Dietet. Assoc., 102: 1621-1630. https://doi.org/10.1016/S0002-8223(02)90346-9
Virtanen, J.K., Mursu, J., Virtanen, H.E., Fogelholm, M., Salonen, J.T., Koskinen, T.T., Voutilainen, S. and Tuomainen, T.P., 2016. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein e phenotype in men: the kuopio ischaemic heart disease risk factor study. Am. J. clin. Nutri., 103: 895-901. https://doi.org/10.3945/ajcn.115.122317
Wang, L.H. and Huo, G.C., 2001. The biologic functions of ω-3 unsatrated fatty acid. J. Northeast agric. Univ., 1: 100-104
Wu, Y.B., Li, L., Wen, Z.G., Yang, P.L. and Yan, H.J., 2018. Research progress on metabolism and physiological function of very-long-chain polyunsaturated fatty acids in animals. Chinese J. Anim. Sci., 54: 20-26.
Wu, Y.B., Yang, L.Y., Yan, H.J., Cai, H.Y., Wu, S.G., Yu, H.M., Yue, H.Y. and Zheng S.N., 2015. Comparative study of increasing ω-3 polyunsaturated fatty acids content in yolk by dietary microalgae and flaxseed supplementation. Chinese J. Anim. Nutri., 27: 3188-3197.
Xie, L.L., Studies on egg yolk lipids fatty acid composition. WU HAN, CHINA, Huazhong Agricultural University, 2011.
Xue, B., Zhang, H., Liu, Z.D., Lin, Z.X. and Jia, F.C., 2017. Nutrition value evaluation of fatty acids in Tibetan chicken egg and ordinary egg. Fd. Res. Develop., 38:140-144.
Yang, M., Liang, S.H., Wang, M.Y. and Zhang, M., 2017. Physicochemical propeertie and fatty acid compositions of eggyolk oils from four avian species. J. Henan Univ. Technol. (Nat. Sci. Ed.), 38: 47-51.
Yang, X.S., He, Y.H., Jin, C.J. and He, J.G., 2017. The nutrition and food processing safety in palm oil. J. Chinese Inst. Fd. Sci. Technol., 17: 191-198.
Ye, T., Zhao, S.J. and Jing, P., 2012. Enrichment of polyunsaturated fatty acid on egg yolks. Fd. Indust, 33: 90-92
Zhang, F., Bai, Y.A. and Lu H.L., 2012. Research advances in saturated fatty acid and health. China Oils Fats, 37: 29-33.
Zhang, P., Tang, C., Ding, Z., Huang, H. and Sun, Y., 2017. Effects of simultaneous supplementation of laying hens with alpha-linolenic acid and eicosapentaenoic acid/docosahexaenoic acid resources on egg quality and n-3 fatty acid profile. Asian-Australas. J. Anim. Sci., 30: 973-978. https://doi.org/10.5713/ajas.15.0850
Zhang, X.X., Yin, P.P., Yang, L.G., Fan, H., Sun, L.W. and Liu Y.J., 2017. Oil contents in flaxseeds from different origins and fatty acid compositions of flaxseed oils. China Oils Fats, 42:142-146.
To share on other social networks, click on any share button. What are these?










